1. Introduction
Aquaculture is the fastest growing food production sector in the world [
1] and is therefore responding to the demands of ensuring sustainable global food security. Global aquaculture production has grown by nearly 7% per year. These demands are expected to continue to rise in response to projections of increased global population and the associated need for new protein sources. The marine fish aquaculture industry in Europe is dominated by Atlantic salmon (
Salmo salar) in the north and by gilthead seabream (
Sparus aurata) and European seabass (
Dicentrarchus labrax) in the south.
Since the publication of the European Commission’s 2013 Strategic Guidelines on the sustainable development of European aquaculture [
2], the Commission has worked closely with Member States to address the barriers hampering the development of the sector while launching several campaigns to promote sustainable and competitive fish farming. A large part of this expansion and species diversification is expected to take place in land-based aquaculture facilities or intensive systems where water is reused in some form, such as recirculating aquaculture systems (RAS).
RAS are currently operating for many different species and have recently been developed to the large industrial scale. The highly technological RAS allows a very high degree of water reuse, normally in the range of 95–99% when compared to the water consumption in a flow-through system. Such facilities need to be designed for safe operation in the long-term, involving continuous and close monitoring of several critical parameters to safeguard fish welfare and profitability. The benefits of RAS are the reduced fresh and saltwater usage, reduced land requirement due to the high stocking density, reduced wastewater effluent volume, increased biosecurity by effectively treating disease outbreaks, and independence from weather and variable environmental conditions. Indeed, RAS satisfy the objectives of European Union for sustainable aquaculture by producing food while sustaining natural resources with a minimum ecological impact. However, RAS are complex systems where fish biomass and water chemistry/quality interact and where small variations may result in suboptimal conditions. These variations may cause stress and reduced feed intake and may ultimately result in reduced growth performance or even mortalities [
3].
Ammonia is a limiting factor for producing fish in RAS [
4,
5], but the tolerance affecting mortality and growth varies [
6]. Different water quality parameters affect the kinetics of the RAS biological filters (BF) [
7], causing variation in ammonia removal. This gives rise to the dilemma of how to control and limit ammonia levels in order to optimize production under different conditions. Tolerance to ammonia varies between fish species and within different life stages and physiological status (starved, stresses, and health status) of the same species [
8,
9]. Furthermore, the type of rearing conditions may play a part. Other water quality parameters like temperature, carbon dioxide, oxygen, and salinity may also interact and influence the fish response to water quality and stress [
8], which again can vary between different fish farms, and even throughout a production cycle. In addition, ammonia tolerance depends on how the fish acclimate to ammonia [
10] and to changing water quality [
11]. Not least, the different efficiency in which different RAS system convert ammonia to less toxic N-compounds varies depending on the RAS-design [
12]. Acute levels of ammonia lead to acute mortality, while chronic levels of ammonia lead to suboptimal conditions for the fish. These suboptimal conditions are difficult to monitor but may result in reduced appetite and growth and eventually increased mortality [
13]. Even in well-managed RAS, this is probably a common issue due to the many and intricate interactions between the fish and the other water quality parameters mentioned. The Food and Agriculture Organization (FAO) of the United Nations has advised that the ammonia levels should cover a range of at least 1-100 ng/mL [
14,
15].
In this way, water quality and toxicity levels in recirculation systems are critical, and ammonia is an important parameter which is closely linked to the fish excretion and efficacy of the BF. Growth and stocking density (biomass production and loading) are the main factors affecting the ammonia fluctuations and load in RAS [
7]. The existing sensors for ammonia are generally used in sewage treatment plants and do not give consistent values in saltwater systems [
9]. Moreover, there is a lack of available technology to add sensing elements before and after the BF and degassing column of a RAS.
In this respect, suitable multipoint monitoring elements for ammonia are essential. The use of optical fiber sensor-based devices offers many well-known and desirable features for label-free methods when compared with conventional electrical transducers. Examples are size, immunity to electromagnetic interference, cost, light path control, remote sensor deployment, high transmission rate, multiple sensors just on a single fiber and the use of biocompatible and biofunctionalized materials, and intrinsic safety and inert nature, which enables them to have minimal impact on the environment [
16]. These advantages motivate the use of optical fiber sensors in medicine [
17], environmental monitoring [
18], and antibody detection [
19]. Optical fiber sensors are selected for such applications due to their operational safety in aqueous environments and ease of introduction into the tanks, which eliminates the need to take samples to be tested in external instruments. Additionally, sensing can be performed with either a handheld probe or as a set of remotely operated devices along an optical fiber cable.
The optical fiber sensors approach for ammonia assessment is generally related to the use of nanoparticles or dyers that interact with the dissolved ammonia, resulting in intensity [
20] or wavelength [
21] variations in the optical fiber probe. An optical fiber interferometer was proposed by the authors of [
21] for the ammonia detection. Using a nanocomposite coating, the sensor was able of detecting ammonia concentrations as low as 14 ppm, which is higher than the lower bound of ammonia detection in RAS [
14]. In addition, wavelength-based sensors need an optical spectrum analyzer, which is generally a high-cost equipment. In order to obtain a low-cost sensor system, intensity variation-based systems have been proposed, such as the one presented by the authors of [
22], where the luminescence from the nanoparticles upconversion was used on the sensor development. In this case, the sensor was tested in a range of 100-10000 ppm, i.e., far greater than the ammonia levels recommended in water. Similar techniques employed dyers in the lateral section of an optical fiber to evaluate the differences in the fluorescence as function of the ammonia levels [
23]. The authors of [
24] proposed a porous waveguide with a combination of copolymers as pH indicator, where ammonia concentrations as low as 10 ppm were measured. Then, with further improvements in the Oxazine 170 perchlorate deposition in polymer optical fibers (POFs), the authors of [
20] proposed a highly sensitive ammonia sensor with detection limit of 1.4 ppm, the sensor was tested in stagnated and dynamic water. However, the approach was based on the transmission mode, which, for field applications, can lead to practical disadvantages on the sensor positioning. Moreover, intensity variation-based sensors are also sensitive to light source power deviations, and self-compensation techniques for these deviations need to be addressed for a higher resolution of the sensors.
Considering this background, this paper presents a low-cost POF sensor to cover the ammonia levels required by RAS and suitable to add in critical locations of a RAS, in which the proposed sensor was based on the reaction between the POF cladding with Oxazine 170 perchlorate and the water-dissolved ammonia. Such reaction led to variations in the evanescent field, resulting in optical power variations. In addition, a reflector was manufactured in the optical fiber tip in order to operate in the reflection mode, which brought important advantages regarding to the sensor positioning inside the water tank. Another advantageous feature was the possibility of using unclad POFs recycled from the LCD monitors (as depicted by the authors of [
25]) for the sensor manufacturing, where the cladding was based on the Oxazine 170 perchlorate solution.
2. Sensors Development and Experimental Setup
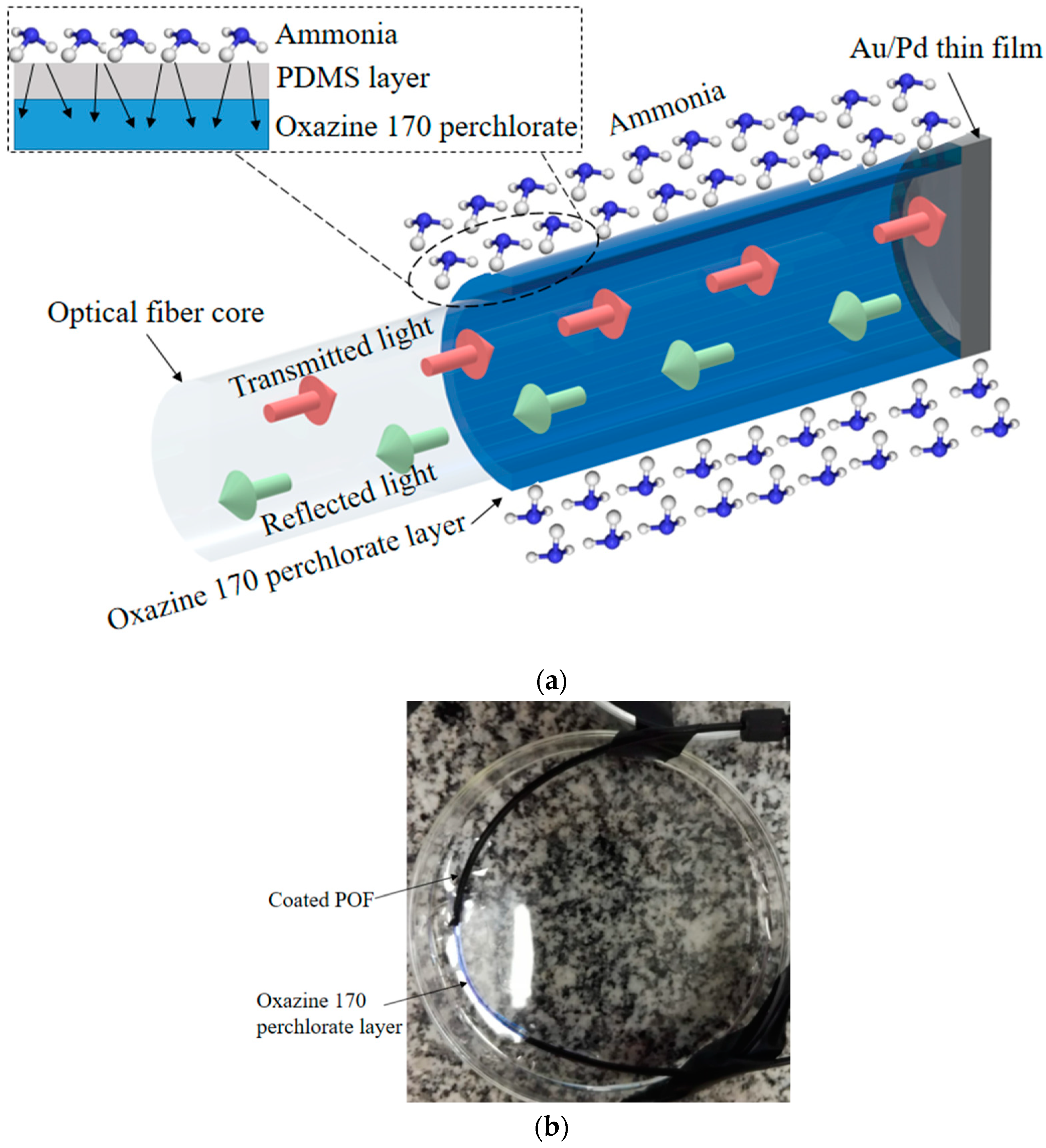
An uncladed multimode POF with polymethyl methacrylate (PMMA) core was used on the fabrication of the optical fiber probe with a 980-µm core (ESKA Mitsubishi, Japan). The fiber diameter was about 1 mm and the fabrication of the proposed ammonia sensor occurred in a few steps, as summarized in
Figure 1. The first step involved the gold-palladium (Au/Pd) thin layer deposition using a sputter coater. The sputter target was composed of 20% palladium and 80% gold. The POF end facet was positioned on the sputter target and the Au/Pd thin layer was created, where this layer acted as a mirror and reflects the optical signals, which enabled the use of the proposed sensor in the reflection mode.
Then, in the second step, the Oxazine 170 perchlorate was deposited on the fiber uncladed region by dip coating. The Oxazine 170 perchlorate deposition followed the methods discussed by the authors of [
20], where a dye solution was prepared using 1 mg of Oxazine dissolved in 100 mL of distillated water though a temperature-controlled magnetic stirrer. The uncladed region of the POF was dipped in the Oxazine solution (without dipping the fiber end facet, which had the Au/Pd thin layer) for about 2 hours at 70 °C, as shown in Step 2 of
Figure 1, where an u-bend was made on the fiber to position it without dipping its end facet. Thereafter, the fiber was removed, and the temperature of the solution was decreased to 20 °C. After the solution reached the input temperature (20°C), the fiber was dipped again for 10 hours. The Oxazine 170 perchlorate dipping step was the one in which the sensor element was fabricated. The operation principle of the proposed sensor was based on the chemical reactions between the dissolved ammonia and the Oxazine layer on the uncladed POF. Such reactions led to differences in the evanescent field, which resulted in the optical power variation in the POF. The operation principle of the proposed sensor is depicted in
Figure 2a, whereas a photograph of the sensor (Sample 3) is shown in
Figure 2b.
The chemical reaction between the Oxazine and the ammonia occurs at aqueous solution or in the presence of moisture, which is in accordance with the proposed application, i.e., ammonia sensing on water tanks. However, the direct contact between the sensor and the water can result in a lower lifespan of the proposed sensor if the water can remove the Oxazine layer. For this reason, in the third step (after the Oxazine 170 perchlorate deposition), a polydimethylsiloxane (PDMS) layer was applied. The PDMS layer was also applied by dip coating the optical fiber in the region where the Oxazine was deposited. As proposed by the authors of [
20], the optical fiber was dipped in a water container before the application of the PDMS layer in order to create a region with entrapped water, which was needed for the chemical reactions between the Oxazine and ammonia. Then, the fiber was dipped in the PDMS curing agent solution with 24 hours for the layer curing. In this case, there was an additional mechanism in the sensor operation principle, which was the ammonia diffusion in the PDMS layer due to the pressure difference between the layer and the solution containing ammonia [
20]. A schematic representation of the ammonia diffusion is presented in
Figure 2a inset, where the pressure gradients in conjunction with the ammonia concentration resulted in higher ammonia diffusion into the PDMS layer. This additional mechanism can lead to higher response times for the sensor when compared with the one without the PDMS layer. However, as presented in previous works [
20], the ammonia diffusion was fast (of a few seconds), and it was expected that the sensors with and without the PDMS layer would present similar response times. Nevertheless, the ammonia diffusion was also related with its concentration. For this reason, in the tests with variation of ammonia concentration, the sensor with the PDMS layer presented lower linearity than the one without this layer, which can be attributed to the concentration dependency of the ammonia diffusion.
In the last step, a thermal annealing was performed in the fiberoptic probe, where the sensor was positioned inside a climatic chamber at about 50 °C for 6 hours in order to reduce residual stresses created in the fiber during its manufacturing or during the previous steps of the sensor fabrication. In addition, the thermal annealing also accelerated the PDMS curing and provided a better adhesion of the Oxazine coating layer on the uncladed POF.
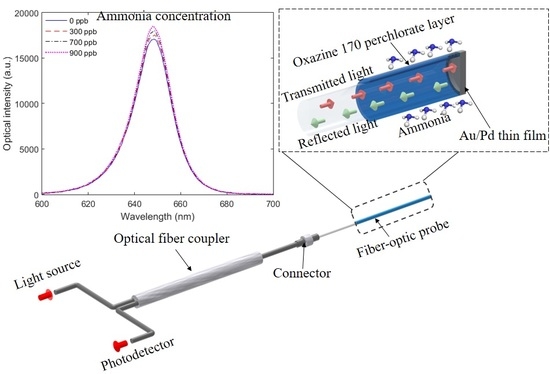
For the fiberoptic probe interrogation unit, an optical fiber coupler 2x1 IF 562 (Industrial Fiber Optics, USA) with 50:50 coupling ratio was used. The light source, comprising of a red laser (Phywe, Germany) centered at 650 nm (optical power of 4 mW) and a photodetector IF-D92 (Industrial Fiber Optics, USA), was positioned in the two input ports, whereas the other port was positioned in the fiberoptic probe. The photodetector was connected to a microcontroller with a 16-bit analog-to-digital converter FRDM KL25Z (NXP, Netherlands). The proposed probe was positioned inside a container filled with distillated water in which different concentrations of ammonium hydroxide solutions were injected. In this case, the ammonia concentration in the solutions ranged from 100 ppb to 900 ppb. In addition, the sensors were tested in sodium chloride solutions to verify its capability of detecting ammonia in high-salinity conditions, which are commonly found in fish farms.
In order to evaluate each of the steps for the fiberoptic probe fabrication, different set of samples (with three samples) were fabricated using different combinations of the steps.
Figure 3 shows the experimental setup and the indication of which steps were used in each set of samples. It is worth mentioning that all samples were subjected to Step 2, in which the sensing layer was created, which resulted in similar Oxazine 170 perchlorate thickness of 35±5 µm for all samples. The comparison between samples was based on the sensitivity and linearity as a function of the ammonia concentration. In the first analysis, the sensors operating at reflection and transmission modes (with and without Step 1, respectively) were compared (Samples 2 and 4). Thereafter, the influence of the PDMS layer on the sensor responses was analyzed (Samples 1 and 2). Finally, annealed and nonannealed samples were compared, i.e., Samples 2 and 3. In the last test, the differences between the proposed fiberoptic probe spectral responses at high-salinity water were obtained with a spectrometer at the wavelength range of 400 nm to 900 nm USB4000 (Ocean Optics, USA) in order to verify if there were any variations in the spectral responses due to the water salinity.
3. Results and Discussion
First, a statistical analysis was performed for each sample, where 15 consecutive measurements of each ammonia concentration were analyzed. In this case, one-way ANOVA was applied for each sample result to verify if there were significant statistical differences between measurements for a specific sample set. The one-way ANOVA tests perform for each sample and ammonia concentrations showed a p-value lower than 0.05 in all cases. These results indicate that there were no significant statistical differences in 15 measurement for each sample in different ammonia concentrations.
Figure 4a shows the mean and standard deviation results of the sensors with and without the PDMS layer for six ammonia concentrations in distillated water, from 0 ppb to 500 ppb in 100-ppb steps. It is worth mentioning that the responses were not in the same plot due to the larger differences in the sensors’ sensitivities. In addition, the linearity of the sensors was presented as the determination coefficient (R
2) between the sensors’ responses and a linear regression. The results in
Figure 4a show that the PDMS layer led to lower linearity of the sensor when compared with the samples without the PDMS layer. Although some differences in the sensors’ sensitivities were expected due to some minor differences in the Oxazine layer thickness, there was a high difference in the sensitivity of the sensors with and without the PDMS layer. The sensors without the additional PDMS layer had a sensitivity of 0.025 ppm
−1, which was seven-times higher than the one obtained from the sensor with the PDMS layer (0.0036 ppm
−1). Therefore, concerning the sensors’ performance, the sensor without the PDMS layer was preferable for the proposed application. It is worth noting that the fiberoptic probe without the PDMS layer can have lower lifespan than the one with the PDMS protection. This feature can be verified in
Figure 4a by the lower standard deviation of the probe with PDMS layer. However, the sensors were fabricated with low-cost methods (a few U
$ per sensor) and with easy assembly that enabled a scalable production and the replacement of probes in field applications.
In order to verify the sensor repeatability and reusability of the sensor without the PDMS layer (since it presented a better performance when compared with the one with PDMS layer),
Figure 4b shows the results of the sensor as a function of time for two consecutive cycles, one in 300-ppb concentration, where the sensor was kept in this concentration for about 10 seconds, and the other in 600-ppb concentration. It is possible to observe in both cases that the sensor presented a response reversibility, since the normalized optical power returned to its initial value when the sensor was not immersed in ammonia solutions, where response times close to 5 seconds were found for both increasing and decreasing concentrations of ammonia. As another performance analysis of the sensor, its hysteresis was analyzed in cycles of ammonia concentration. As shown in
Figure 4c, the sensor presented low hysteresis of 2.48%, which indicates the suitability of the sensor in detecting ammonia concentrations in dynamic cycles with low errors.
Regarding to the thermal treatment effects on the sensors’ performance, Samples 2 and 3 were tested in a larger range of ammonia concentrations (from 0 ppb to 900 ppb) (see
Figure 5). The results show that the nonannealed samples presented a nonlinear behavior that could be accurately represented by an exponential regression. Nevertheless, in concentrations below 60 ppb, the nonannealed samples showed lower sensitivity than the annealed ones. The annealed samples showed a high linearity, with R
2 as high as 0.98. However, the sensitivity of nonannealed samples was higher than the annealed one for ammonia concentrations higher than 600 ppb. For early ammonia detection, a sensor with higher linearity in low concentrations of ammonia is desirable. For this reason, the annealed samples were preferable for the proposed application, but it is worth noting that in environments with high ammonia concentrations, the nonannealed samples may lead to a sensor system with higher resolution in the ammonia detection.
The tests performed in the fiberoptic probes operating at transmission and reflection modes are presented in
Figure 6, where both sensors presented similar sensitivities, i.e., 0.035 ppm
−1 and 0.041 ppm
−1 for transmission and reflection fiberoptic probes, respectively. Regarding to the sensors’ linearity, the transmission mode presented higher linearity than the one of the reflection mode. However, the R
2 values of both sensors were close to each other, which indicates compatibility between both operation modes. Therefore, due to the necessity of regular substitution of the probes and to facilitate their installation, the probe in the reflection mode was the preferable one, since both modes had similar performance.
Then, the fiberoptic probe in the reflection mode with annealing and without the PDMS layer (Samples 1 and 2 in
Figure 3) was tested in a sodium chloride solution to simulate an environment with high-salinity water as observed in RAS.
Figure 7a presents the spectra of the sensors at each ammonia concentration to verify if the water salinity leads to other variations in the reflected spectrum (such as shift in the wavelength or spectral width) besides the intensity variation. However, the results in
Figure 7a show only an amplitude (or intensity) variation on the reflected spectra, which indicate a similar performance of the sensor when compared with the results in distillated water. The
Figure 7a inset shows the magnified view of the peaks, where the differences in the peak intensities can be observed. In addition, the proposed sensor was able to detect ammonia concentrations as low as 100 ppb with a straightforward and low-cost sensor approach, which can be used in many RAS industries for early detection of ammonia.
Figure 7b shows the comparison between the sensor responses at distillated water and saltwater, where it is possible to observe that the sensor presented similar responses with a slight sensitivity reduction in saltwater. However, considering the standard deviations between measurements, the upper limit of the sensitivity in saltwater was 0.041 ppm
−1, whereas the lower limit of the sensitivity in distilled water was 0.040 ppm
−1. This analysis indicates that there were no significant sensitivity differences in sensors responses in distilled water and saltwater.