1. Introduction
Blooms of cyanobacteria are generally considered a harbinger for anthropogenic eutrophication [
1]. Potentially massive blooms can have long durations and detrimental environmental and human health impacts [
2]. Cyanobacterial blooms are often toxic and can contribute to hypoxia when the blooms senesce and increase the biological oxygen demand. These blooms have a particular affinity for warm stratified water, and as such, blooms of cyanobacteria may become more prevalent in the context of a warming climate [
3]. Cyanobacteria can produce various toxins (such as microcystins, anatoxins, and saxitoxin) that pose a health risk and cause mortalities in domestic and wild animal populations [
4]. Additionally, several species have compounds such as geosmin that can cause taste and odor issues in drinking water. As a result of these detrimental influences, cyanobacterial blooms are classified as Harmful Algal Blooms (HAB) and should be monitored to reduce potential deleterious impacts.
Perhaps the most effective monitoring, detection, and assessment tool for describing cyanobacterial bloom dynamics is synoptic remote sensing [
5,
6]. In order to detect any significant increase in cyanobacterial bloom concentration and frequency, there must be an effective and robust algorithm to estimate the extent and severity of these blooms. After an effective algorithm is determined, there needs to be a way to merge the detection technique across different sensors as new ones are deployed and older ones cease operations. These satellite intercalibrations work under the basic premise that two instruments should make identical observations over the same place and time with consistent spatial and spectral responses and identical view angles [
7]. Obviously, this is impossible in a real-world situation. However, it is possible to compensate for any error effect by applying a correction if biases are small and the relationship between the two remotely sensed parameters is linear. Many intercalibration studies have been conducted using consistent, bright targets as the intermediary. For example, Yu et al. [
8] use the Sonoran Desert, a consistently bright target that is visible to both the GOES-East and GOES-West satellites, to perform an intercalibration between the two satellites based on getting the same reflectance from each sensor. Bouvet et al. [
9] describe an important new calibration network (The Radiometric Calibration Network; RadCalNet) that can be used for vicarious calibration based on a set of reference sites of relatively stable surface reflectance in different parts of the world. This network is an important advancement in calibration for land targets. The aquatic environment is far too dynamic for a similar correction technique (and also, the MODIS ocean color bands saturate over bright land targets). A more typical approach for aquatic applications is a point-by-point or pixel-to-pixel matchup [
10,
11]. The pixel-to-pixel matchup has some caveats. Spatial heterogeneity in water bodies can be substantial and subject to change. Instrument characteristics (ground field-of-view, sensor response functions and others) can all introduce mismatches between sensors even when viewing the same point on the water. The result is noise and biases in the resulting comparison. Here, we propose a technique using pixel integration across the cyanobacterial blooms detected within a basin as a means of reducing analytical error. We compare this technique with the more traditional pixel-to-pixel intercalibration analysis.
1.1. CI Algorithm Usage
Since 2009, the National Oceanic and Atmospheric Administration (NOAA) has released short-term (<1 week) forecasts of cyanobacterial blooms in the western basin of Lake Erie [
12]. The primary product used is a satellite proxy for cyanobacterial biomass called the cyanobacterial index (CI). The CI has been used extensively in various freshwater systems throughout the United States [
13,
14,
15,
16]. While the algorithm was initially developed by Wynne et al. [
17,
18] for the MERIS sensor on-board the Envisat spacecraft, it has since been successfully applied to MODIS [
10,
11]. This was necessary as the Envisat spacecraft ceased operation in April 2012. In early 2016, ESA launched the follow-on mission to MERIS, the Ocean and Land Color Imager (OLCI) on the Sentinel 3A spacecraft that is now operated by the European Organization for the Exploration of Meteorological Satellites (EUMETSAT). A second OLCI sensor was launched on the Sentinel 3B satellite in 2018 (
Figure 1). The MODIS sensor is deployed on two spacecraft: Terra (launched December 1999) and Aqua (launched May 2002). Both MODIS sensors are well past their designed mission life and could become inoperable at any time. The OLCI and MERIS sensors are less noisy and have a higher spatial resolution (for MERIS, high-resolution images were only available when specifically recorded or captured by direct broadcast [
16]) and would generally be preferred to MODIS for monitoring cyanobacterial blooms [
19]. As a result, it is advantageous to develop a way to calibrate the CI algorithm among the three sensors to create a seamless time-series blending the MODIS, MERIS, and OLCI sensors. Having a method for comparing the CI values between sensors is imperative as it could help direct monitoring of cyanobacterial blooms over decadal timescales and address the efficacy of management decisions designed to decrease the detrimental impacts of cyanobacterial blooms. Furthermore, having a technique to compare algorithms across remotely sensed platforms satisfactorily could be beneficial to intercalibrating other biogeophysical algorithms between sensors. Having interoperability between sensors allows for increased observational frequency, as well as an increase in spatial coverage. This will assist in overcoming data gaps due to clouds, orbital patterns, or sunglint.
The process described here has three steps: first, to intercalibrate the CI product between MERIS and MODIS; second, to intercalibrate MODIS-to-OLCI CI; third, to use MODIS as a bridge to establish a relationship between OLCI-to-MERIS CI (
Figure 1).
1.2. Characteristics of the MODIS, OLCI, and MERIS Sensors
The characteristics of the three sensors that are relevant to this study are described in
Table 1. The OLCI, MERIS, and MODIS Terra sensors all have overpass times of approximately 10:30 a.m. local time. The MODIS Aqua sensor has an overflight time in the afternoon, approximately 3 h later. This time lag is sufficiently long to allow bloom locations to shift, introducing a significant bias when scenes from this sensor are compared to comparable ones collected during the earlier OLCI and MERIS overpasses. To ensure the greatest similarity in data collection in space and time [
7], only the MODIS Terra data will be used in this study for intercalibrating the different satellite sensors. Consequently, any reference to the MODIS sensor onboard the Terra satellite will be referred hereafter as MODIS
T. The few references the MODIS instrument on the Aqua satellite will be denoted as MODIS-Aqua.
MODIS
T has an exact orbital repeat time of 16 days on a 705 km high orbit in descending node. The MERIS sensor has a repeat time of 35 days, which allows global coverage in ~3 days at an orbit height of 800 km. The OLCI sensor has a repeat time of 27 days at an orbital height of 815 km. OLCI has a spatial resolution of 300 m, as did MERIS when data was collected in the Full Resolution (FR) mode. FR collection was not routine over the USA and Canada until 2008 when the Canadian Center for Remote Sensing began acquiring direct broadcast data. The MERIS data set was routinely resampled onboard to a 1200-m spatial resolution in the Reduced Resolution (RR) mode. The RR data was more reliably archived and will be used here, as the FR data was not reliably archived over much of North America. While the algorithm has been applied successfully to all three sensors (OLCI, MODIS, and MERIS) in a variety of water bodies, the validation of the algorithm is not the focus of this manuscript. For more detailed results of the algorithmic performance with these sensors, the reader is directed to Wynne et al. [
19] for a detailed look into MODIS, Wynne et al. [
18] for validation of MERIS, and Mishra et al. [
16] for application to OLCI.
1.3. Study Area
The Laurentian Great Lakes lie between the border of Canada and the United States and feature three catchments that are routinely affected by cyanobacterial blooms: western Lake Erie, Saginaw Bay, and Green Bay [
20] (
Figure 2). These are large water bodies that have been routinely experienced cyanobacterial blooms over the last 20 years and will be the regions of interest for this study. These lakes are used by millions of people to supply drinking water, and cyanobacterial blooms can be detrimental to human health. For example, in 2014, the metropolitan area of Toledo, OH (USA), issued a “Do Not Drink” order on its municipal water supply due to contamination from the biotoxin, microcystin, caused by cyanobacterial blooms [
21]. Green Bay and Lake Erie are separated by over 500 km and do not necessarily appear in the same swath on MERIS (1150 km) or OLCI (1270 km). The CI algorithm has been routinely run on all three basins to monitor and detect cyanobacterial blooms [
22]. Western Lake Erie has the largest blooms of the three catchments, and these blooms exhibit a large degree of interannual variability in size and severity (interannual variability is >20 fold) [
19]. Saginaw Bay has intermediate-sized blooms with a small degree of interannual variability [
19]. Green Bay exhibits the smallest blooms of the three basins, with a high degree of interannual variability in its cyanobacterial biomass [
20]. These three regions will be used to make the three CI intercalibration factors (MERIS-to-MODIS
T, MODIS
T-to-OLCI, and MERIS-to-OLCI). Using three distinct basins in three different lakes (
Figure 2) will yield a more robust dataset as opposed to using only one single basin. All three basins are shallow, relatively warm, eutrophic environments that have the preponderance of their nutrients delivered by a single river. The Fox River supplies 60% of nutrients into Green Bay [
23], the Saginaw River supplies approximately 90% of the nutrients into Saginaw Bay [
24], and the Maumee River, along with the smaller Cuyahoga and Sandusky Rivers, supplies approximately half of the nutrients into western Lake Erie [
25] (
Figure 2).
4. Discussion
The need to merge cyanobacterial index (CI) data from multiple sensors for environmental analyses will become increasingly common [
33]. This will be necessary to determine the efficacy of management strategies to control cyanobacterial bloom intensity, such as nutrient reductions on decadal time scales. If agricultural best management practices are amended, the need to synoptically differentiate any impacts through the changing of these practices must be taken into account [
34]. The timing, severity, location, and size of cyanobacterial blooms can be used to test the efficacy of these changes. Further, if there are differences in the physical manifestation of the bloom (i.e., timing, severity, location, and size), this could be a harbinger for an ecological shift within a given ecosystem. This study examined both the traditional pixel-to-pixel technique and a new image-to-image integrated technique for intercalibrating the CI algorithm from the MODIS
T, MERIS, and OLCI sensors. Being able to reliably compare the CI values between sensors will allow the production of a >20 year CI time-series in numerous geographically dispersed water bodies. These time-series will prove useful in retrospectively evaluating previous management decisions and in documenting temporal changes in cyanobacterial bloom intensity.
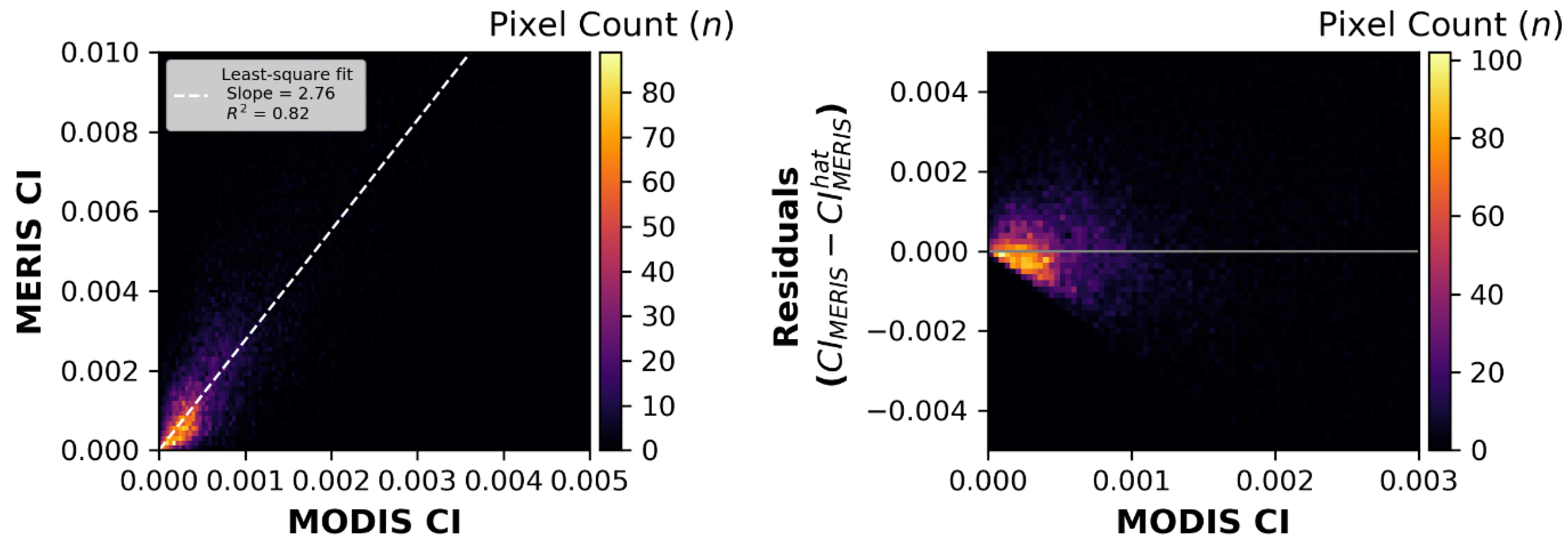
In this study, the image-to-image integrated technique exhibited much lower error and reduced bias than the pixel-to-pixel technique. The residual CI distributions produced by the pixel technique showed skewed bias in the residuals (
Figure 4B and
Figure 7B), which did not occur with the integrated technique (
Figure 5 and
Figure 8). Additionally, as the MODIS
T CI increased, the more negative the MERIS or OLCI residuals became when using the pixel technique (
Figure 4B and
Figure 7B). The integrated technique did not exhibit such biases over the entire range of CI values, with the higher values converging on the 1:1 line (
Figure 5 and
Figure 8). Pixel-to-pixel comparisons would be expected to have this problem because any shifts in the distribution of the variable being measured between overflight times or alignment issues can introduce noise confounding intercalibration (
Figure 4B and
Figure 7B for the pixel technique; 5 and 8 for the pixel technique for integrated technique). The integrated technique, in contrast, provides a more robust analysis option for evaluating the influence of individual image pairs on the calibration (e.g.,
Figure 6A–C and
Figure 9A–C). In a pixel-to-pixel technique, the statistical influence of the pixels from individual scenes cannot be readily evaluated and would require more complex analysis. Any skewing of pixels from CI image pairs at the low or high end (those pairs with maximum statistical leverage) would bias the results of the comparison. Skewing could occur with even a one or two-pixel offset in the actual (or apparent) location of an intense bloom between two images. This problem is mostly removed by the integration technique, as the offset pixels are contained within the integrated region of the bloom.
The calibration coefficient between the MODIS
T and MERIS for the pixel and integrated techniques differed by 5% (
Table 4), while the coefficient between the pixel and integrated technique for the MODIS
T–OLCI matchup differed by 1%. However, as noted above, the pixel technique for MODIS
T to OLCI had a severe bias, which can lead to questions on the accuracy of the calibration. The integrated technique did not have this problem. OLCI appeared to miss the lowest CI values (
Figure 7) that MODIS
T and MERIS detect. MERIS has had much more time for calibration, and it is now at the fourth reprocessing. As the OLCI calibration is developed and improves, it is likely to see shifts in the calibration, potentially reducing the difference. Most calibration is conducted on a band-by-band basis. Subtle inter-band differences within the uncertainties of the band calibration can affect a shape algorithm such as the CI [
35]. A spectral shape method can detect these residual differences, providing a means of improving the inter-band calibration [
35]. The integrated technique can provide a more reliable method for intercalibrating other biophysical variables across sensors as well. A similar approach has been used to calibrate MODIS [
36,
37]. Specifically, data sets were integrated over space and time to compare satellites for such issues as trends and calibrating for cross-scan polarization.
We carried out sensitivity analysis using bootstrap simulation to find out how stable the slope parameters were with respect to the effect from the points of leverage or influence. We repeated the simulation for 1000 runs for MERIS–MODIS analysis. OLCI–MODIS sensitivity analysis was repeated 171 times based on the number of unique permutations possible with a sample size of 17. At every step of the iteration, 39 out of 42 of the MERIS–MODIS samples and 17 out of 19 samples were randomly selected without replacement. By doing so, 5% and 10% of MERIS–MODIS and OLCI–MODIS pairs were left out to evaluate their influence on the regression slope. The median slope from the sensitivity analysis was identical to the reported slopes in
Figure 5 and
Figure 7 (
Table 7). The variability of the MERIS–OLCI slope is expected to be within 2.87–2.99, which is −1.7% and +2.4% of the median value of 2.92. Similarly, the OLCI–MODIS slope is likely to be within 2.61–2.81 (−3.7% or +3.5% around the median of 2.71). While more samples may lead to more robust statistics, special attention was given to image quality, and only the very best image pairs were selected for analysis.
This study shows the CI intercalibration is consistent between the three basins in the Laurentian Great Lakes and that the integration technique is robust. The intercalibration coefficients presented here were consistent with no outliers compared to those derived with the pixel-to-pixel technique used in Wynne et al. [
10]. We should note that the CI algorithm does not require an atmospheric correction [
26]. It was based on the Maximum Chlorophyll Index (MCI) presented by Gower et al. [
38], who used top-of-atmosphere reflectance. The calibration coefficient between MODIS and MERIS derived here was much higher than in Wynne et al. [
10], primarily due to an issue with the measurement units. The original MERIS reflectance data sets were expressed as L2 files from the original ESA processing as having units of per steradian (sr
−1) (although presented as dimensionless). This translates as having the initial L2 products being off by a factor of π sr. By multiplying the 1.33 correction factor derived by Wynne et al. [
10] by π, the new factor becomes 4.17, which is considerably closer to the correction factor of 2.94 derived here. The remaining ~30 % difference (between 4.17 and 2.94) could be explained by several reasons. ESA has recalibrated and reprocessed the MERIS data several times since the analysis of Wynne [
10]. This study also used rigorous quality assurance for both the image pairs selected and the pixels used for analysis. Further, Wynne et al. [
10] used only MODIS Aqua imagery, which is collected about 3 h later than MERIS and OLCI. By employing MODIS Terra imagery in this study, which has comparable overpass times to MERIS and OLCI, it was possible to reduce any impacts of wind events or biomass changes in surface waters unaccounted for in the original calibration study. This, in turn, reduced misfit, thereby improving the calibrations.
While this study focuses on intercalibrating the CI algorithm among three large satellite missions in the Great Lakes, the techniques presented here could be adapted to other areas and sensors. The integrated technique could be applied to any remotely sensed image products from any number of sensors and algorithms. The integrated technique is not designed to better approximate an in situ measurement but instead to determine how well an algorithm from one sensor approximates the same algorithm on a different sensor. As a result, the integrated technique could also be applied to small satellites, such as CubeSats, which generally have poor absolute radiometric calibration, but show linearity. Two excellent review articles highlight water quality parameters that could be tested using the integrated technique for sensor intercalibration [
39,
40].