Distinguishing Original and Non-Original Stands at the Zhanjiang Mangrove National Nature Reserve (P.R. China): Remote Sensing and GIS for Conservation and Ecological Research
Abstract
:1. Introduction
2. Materials and Methods
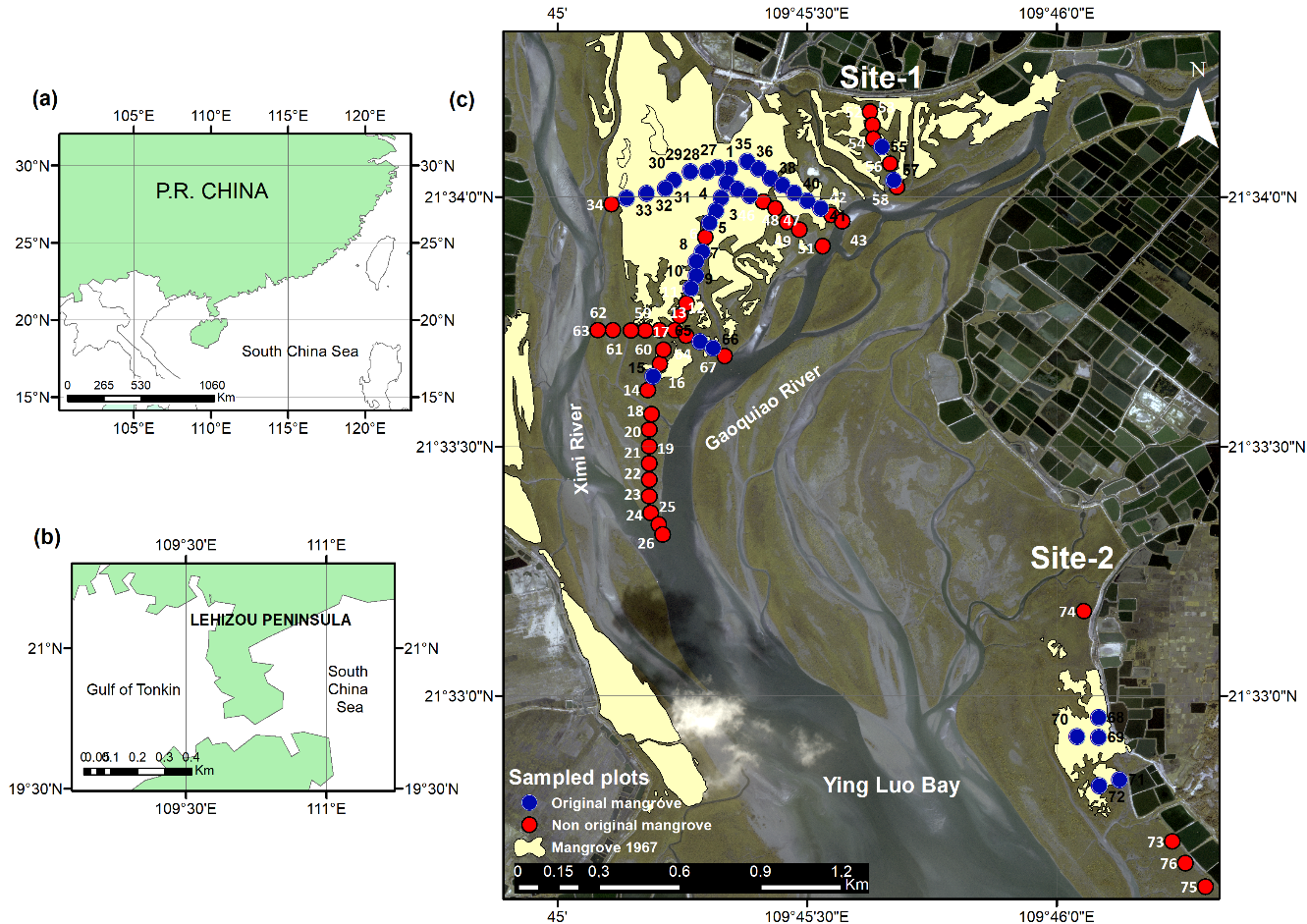
2.1. Study Area
2.2. Forest Stands Identification Methodological Framework
2.3. Remote Sensing Data
2.4. Ground-Truth Data
2.5. Mangrove Community Structural Analysis
3. Results
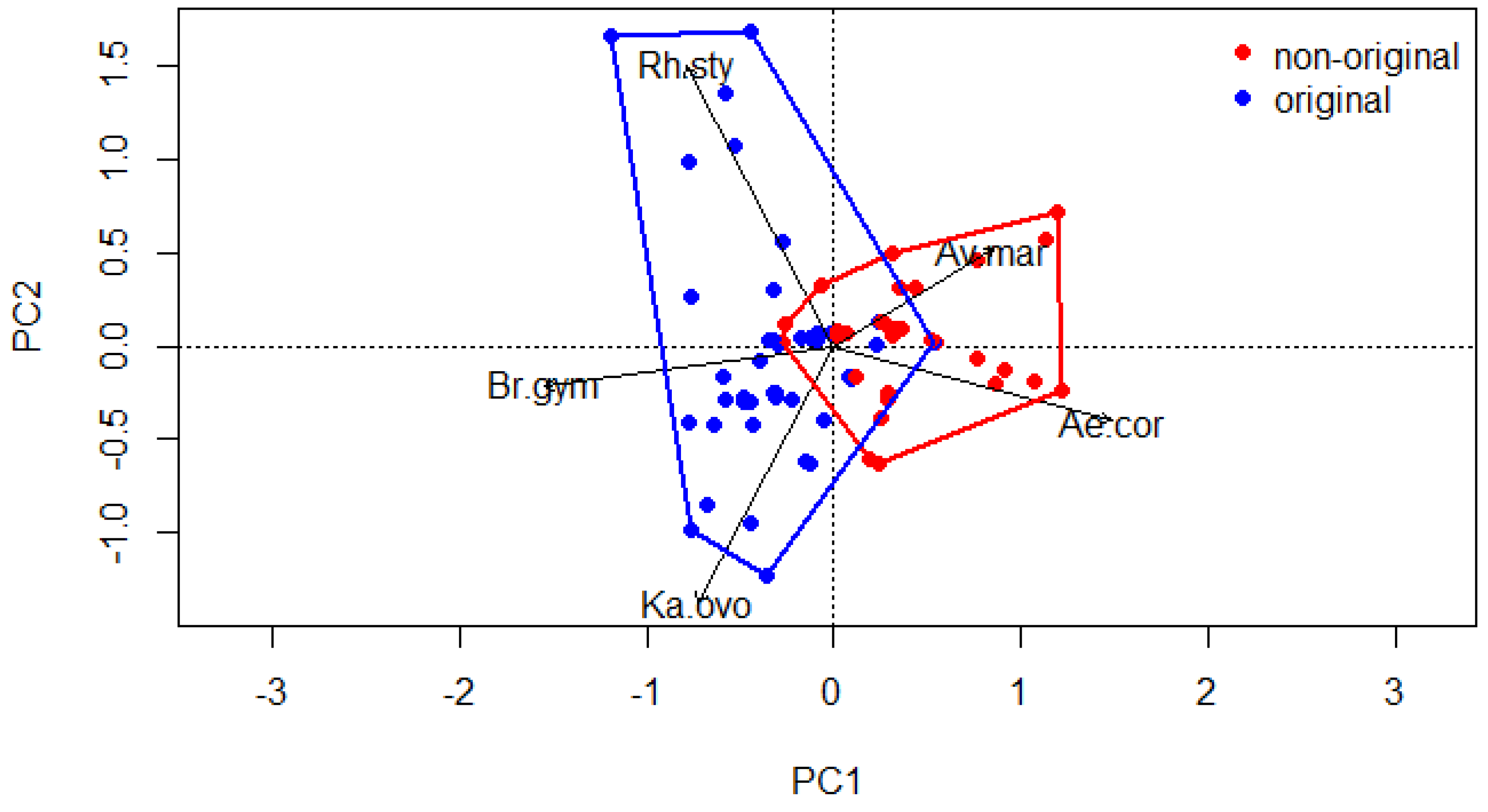
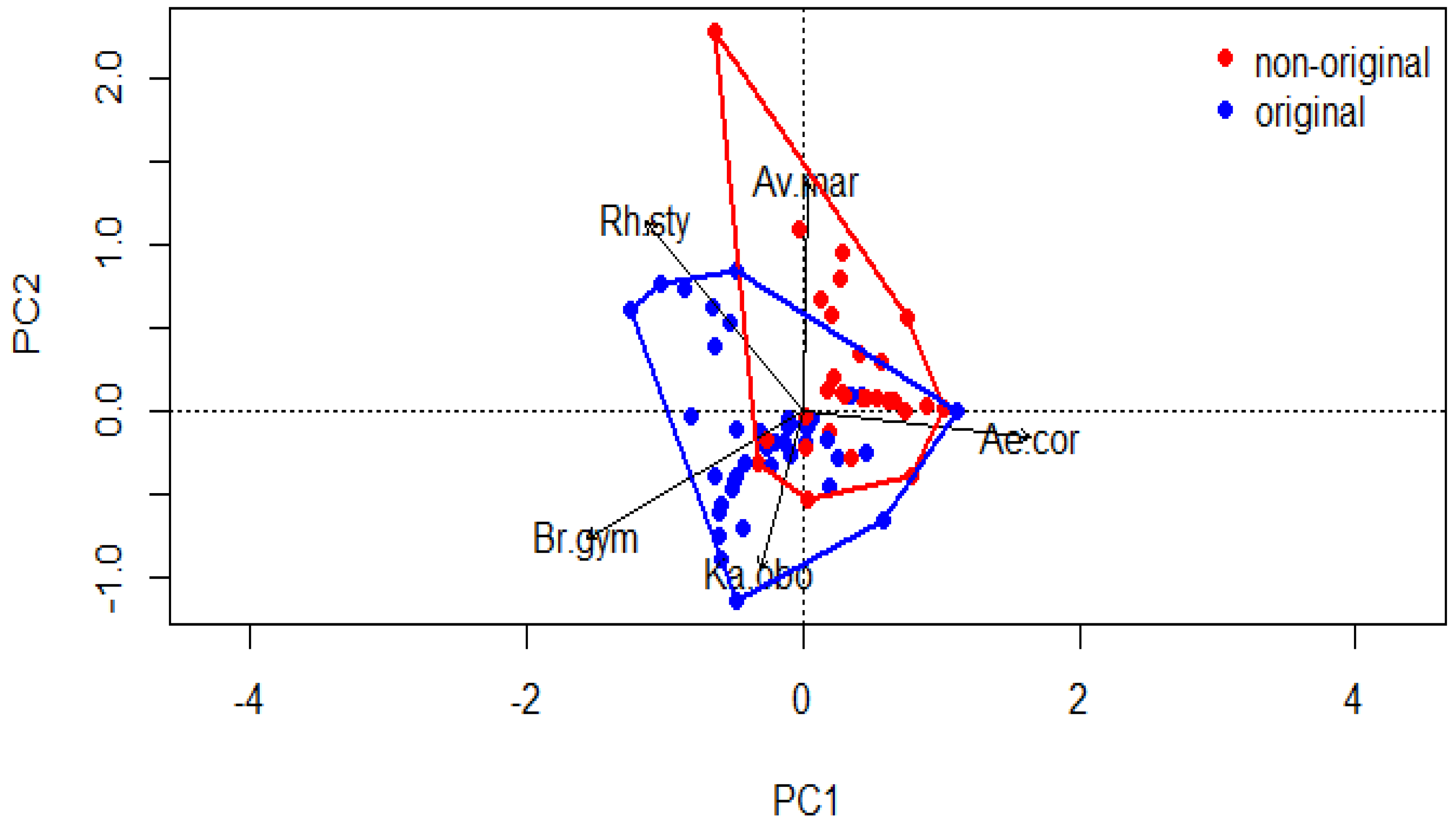
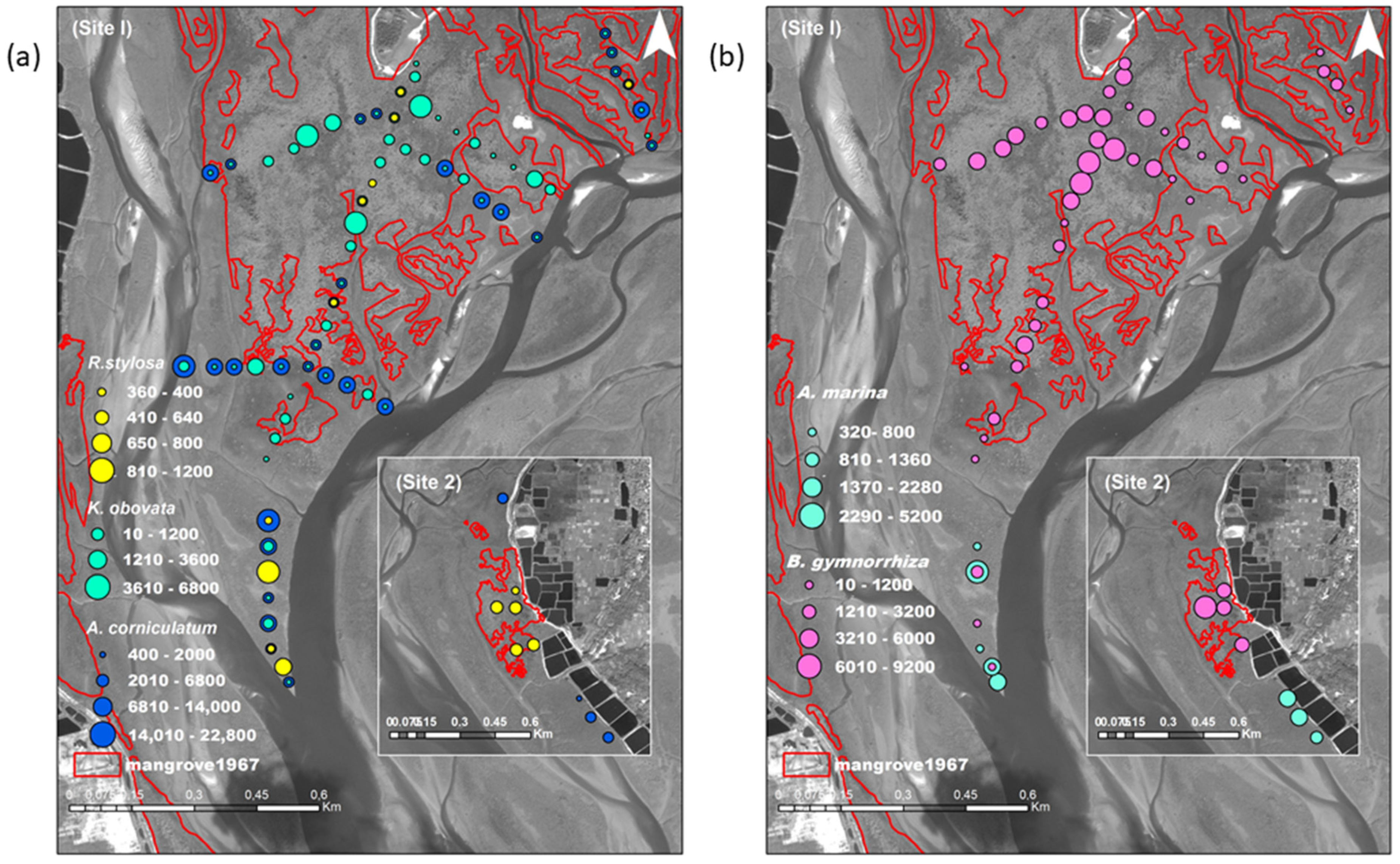
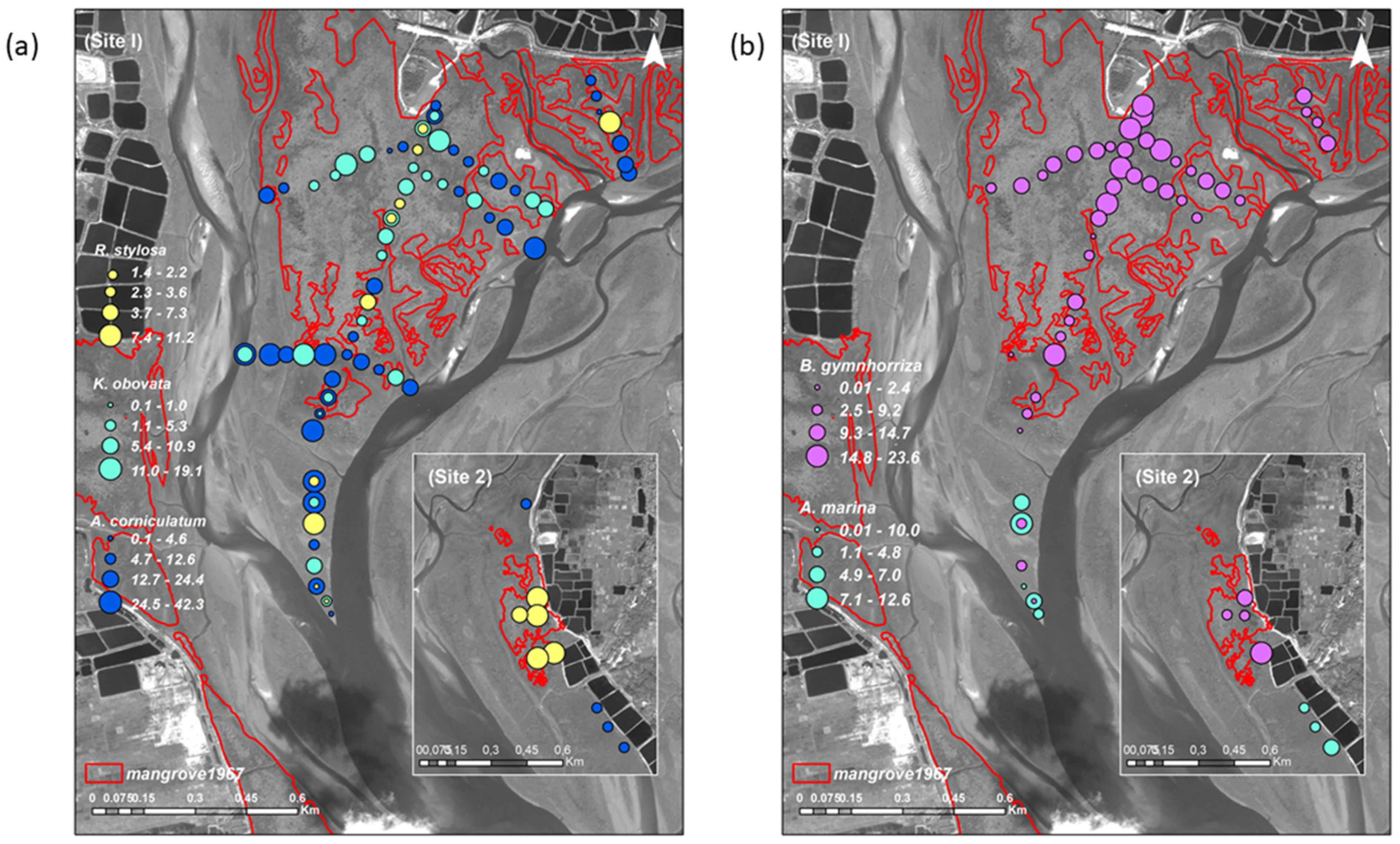
3.1. Mangrove Vegetation Structure and Distinction of Original and Non-Original Stands
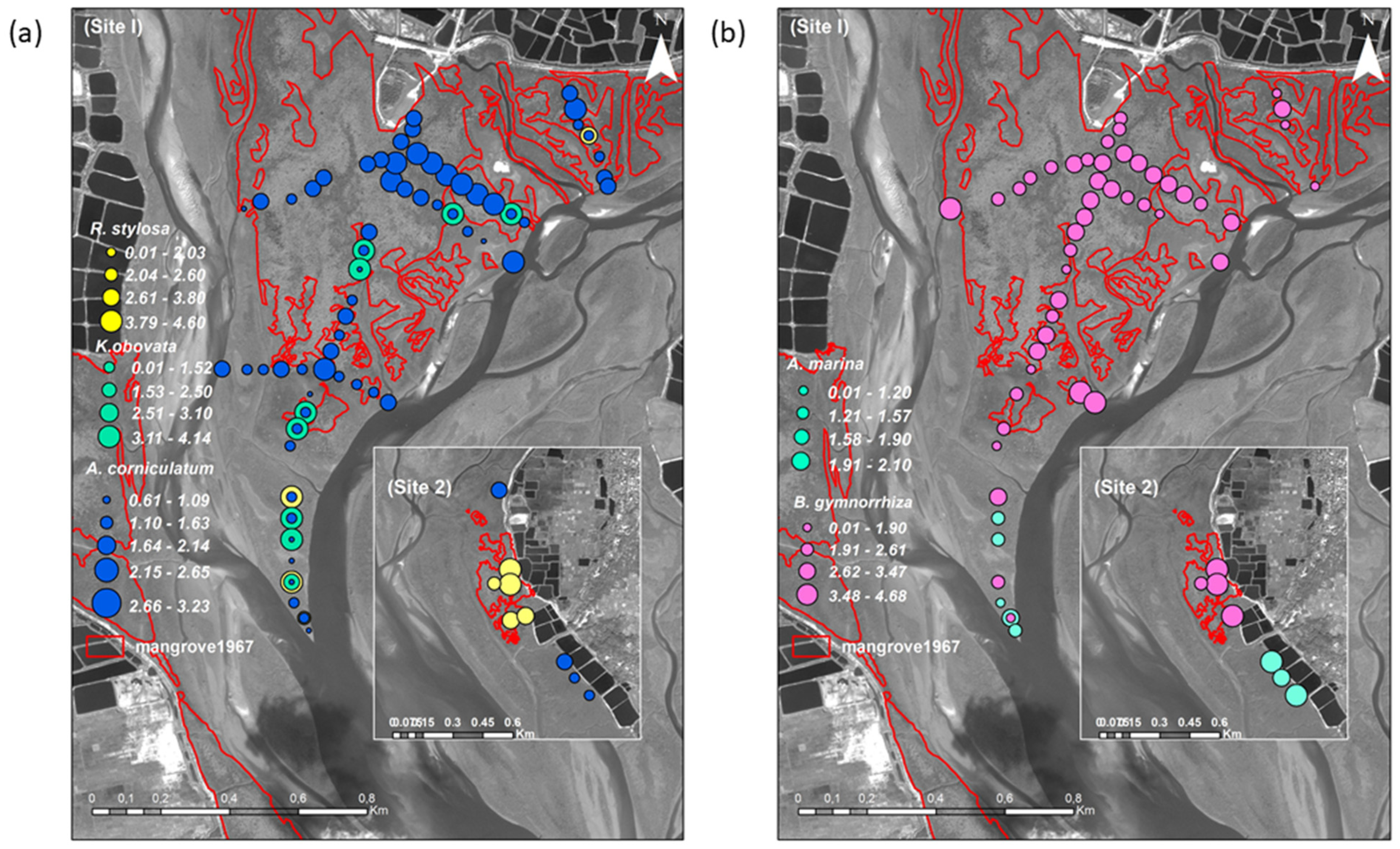
3.2. Differences of Mangrove Spatial Distribution at the Species and Sites Level
4. Discussion
4.1. Distinction of Original and Non-Original Stands
4.2. Characteristics Affecting Mangrove Zonation
4.3. Restoration Activities and Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vegetation Parameter | Original | Intersection | Non-Original |
|---|---|---|---|
| Height | 2, 4, 6, 8, 13, 22, ---25, 28, 31, 32, 33, --36, 37, 41, ---, 46, ---,55, −57, −59, 67, 68, 69, 70, 71, | 1, −3, 7, 10, ---, 13, 14, 15, 16, 17, ---, 20, 21, 26, 34, 35, ---,38, 39, 40, ---, 44, --, 47, --−53, -, 55, ---, 61, --−65, 67 | 9, 11, 12, ---,18, 19, 23, 24, -−27, −29, 30 31, --−42, 43, -,45, ---, 48, 49, 50, 51, 52, --−54, 55, 56,---,60, 62, 63, −64, −66, --−72, -, 73, 74, 75, 76 |
| Density | 1, 2, 3, 4, 5, 6, -, 8, 9, 10, -, 12, ---, 17, ---, 27, 28, 29, 30, 31, ---, 35, 36, 37, 38, 39, ---, 46, ---, 67, 68, 69, 70, 71. | 7, ---, 13, -, 15, 16, 18, ---, 32, 33, 34, ---, 40, ---, 44, 45, -,47, 48, 49, -, 51, ---, 56, -, 58, 59, ---, 64, 65. | 11, --, 14, --,16, -, 18, 19, 20, 21, 22, 23, 24, 25, 26, ---, 36, 37, 39, -, 41, 42, 43, ---, 50, -, 52, 53, 54, 55, -, 57, -----, 60, 61, 62, 63, -−66, ----- 72, 73, 74, 75, 76. |
| Basal area | 1, 2, 3, 4, 5, 6, 7, 8, 12, ---, 22, 23, 25, 28, 36, 46, 58, 59, 67, 68, 69, 70, 71, | 9, 10, 11, −13, 14, 15, 16, 17, 18, 19, 20, 21, -−24, 26, 27, 29, 30, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 47, 48, 49, 51, 52, 53, 54, 56, 62, 63, 64, 65, 66, | 50, 55, 57, 60, 61, 72, 73, 74, 75, 76 |
|
|
|
|
|


References
- Dahdouh-Guebas, F.; Ajonina, G.N.; Amir, A.A.; Andradi-Brown, D.A.; Aziz, I.; Balke, T.; Barbier, E.B.; Cannicci, S.; Cragg, S.M.; Cunha-Lignon, M.; et al. Public Perceptions of Mangrove Forests Matter for Their Conservation. Front. Mar. Sci. 2020, 7, 901. [Google Scholar] [CrossRef]
- Del Valle, A.; Eriksson, M.; Ishizawa, O.A.; Miranda, J.J. Mangroves protect coastal economic activity from hurricanes. Proc. Natl. Acad. Sci. USA 2020, 117, 265–270. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological role and services of tropical mangrove ecosystems: A reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Cohen, R.; Kaino, J.; Okello, J.A.; Bosire, J.O.; Kairo, J.G.; Huxham, M.; Mencuccini, M. Propagating uncertainty to estimates of above-ground biomass for Kenyan mangroves: A scaling procedure from tree to landscape level. For. Ecol. Manag. 2013, 310, 968–982. [Google Scholar] [CrossRef]
- Osland, M.J.; Feher, L.C.; Spivak, A.C.; Nestlerode, J.A.; Almario, A.E.; Cormier, N.; From, A.S.; Krauss, K.W.; Russell3, M.J.; Alvarez, F.; et al. Rapid peat development beneath created, maturing mangrove forests: Ecosystem changes across 25-year chronosequence. Ecol. Appl. 2020, 30, 1–12. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Kuzyakov, Y.; Lubis, A.A.; Murdiyarso, D.; Hutley, L.B.; Bachri, S.; Friess, D.A.; Martius, C.; Borchard, N. Organic carbon burial and sources in soils of coastal mudflat and mangrove ecosystems. Catena 2020, 187, 104414. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J. Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. For. Ecol. Manag. 2002, 166, 111–129. [Google Scholar] [CrossRef]
- Bell, A.M.; Duke, N.C. Effects of Photosystem II inhibiting herbicides on mangroves—Preliminary toxicology trials. Mar. Pollut. Bull. 2005, 51, 297–307. [Google Scholar] [CrossRef]
- Wolswijk, G.; Satyanarayana, B.; Dung, L.Q.; Siau, Y.F.; Ali, A.N.B.; Saliu, I.S.; Fisol, M.A.B.; Gonnelli, C.; Dahdouh-Guebas, F. Distribution of mercury in sediments, plant and animal tissues in Matang Mangrove Forest Reserve, Malaysia. J. Hazard. Mater. 2020, 387, 121665. [Google Scholar] [CrossRef] [Green Version]
- FAO Mangrove Ecosystem Restoration and Management. Available online: http://www.fao.org/sustainable-forest-management/toolbox/modules/mangroves-restoration-and-management/basic-knowledge/en/#:~:text=Mangrove ecosystems provide habitat for,for birds and other wildlife (accessed on 6 March 2020).
- GNF Mangrove Rehabilitation in Asia—Local Action and Cross-Border Transfer of Knowledge for the Conservation of Climate, Forests and Biodiversity. Available online: https://www.globalnature.org/Mangroves (accessed on 6 March 2020).
- Martínez-Espinosa, C.; Wolfs, P.; Vande Velde, K.; Satyanarayana, B.; Dahdouh-Guebas, F.; Hugé, J. Call for a collaborative management at Matang Mangrove Forest Reserve, Malaysia: An assessment from local stakeholders’ view point. For. Ecol. Manag. 2020, 458, 117741. [Google Scholar] [CrossRef]
- Friess, D.A.; Yando, E.S.; Abuchahla, G.M.O.; Adams, J.B.; Cannicci, S.; Canty, S.W.J.; Cavanaugh, K.C.; Connolly, R.M.; Cormier, N.; Dahdouh-Guebas, F.; et al. Mangroves give cause for conservation optimism, for now. Curr. Biol. 2020, 30, R153–R154. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr. 2016, 25, 729–738. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Mulder, S.; Jayatissa, L.P.; Dahdouh-Guebas, F. Are the mangroves in the Galle-Unawatuna area (Sri Lanka) at risk? A social-ecological approach involving local stakeholders for a better conservation policy. Ocean Coast. Manag. 2013, 71, 225–237. [Google Scholar] [CrossRef] [Green Version]
- Curnick, D.J.; Pettorelli, N.; Amir, A.A.; Balke, T.; Barbier, E.B.; Crooks, S.; Dahdouh-Guebas, F.; Duncan, C.; Endsor, C.; Friess, D.A.; et al. The value of small mangrove patches. Science (80-) 2019, 363, 239. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, A.P.; Riggio, J.; Tait, A.M.; Baillie, J.E.M. Global areas of low human impact (‘Low Impact Areas’) and fragmentation of the natural world. Sci. Rep. 2019, 9, 14179. [Google Scholar] [CrossRef]
- Li, M.S.; Mao, L.J.; Shen, W.J.; Liu, S.Q.; Wei, A.S. Change and fragmentation trends of Zhanjiang mangrove forests in southern China using multi-temporal Landsat imagery (1977–2010). Estuar. Coast. Shelf Sci. 2013, 130, 111–120. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Masqué, P.; Glass, L.; Benson, L.; Kennedy, H.; Duarte, C.M.; Garcia-Orellana, J.; Benitez-Nelson, C.R.; Humphries, M.S.; Ratefinjanahary, I.; et al. Losses of Soil Organic Carbon with Deforestation in Mangroves of Madagascar. Ecosystems 2021, 24, 1–19. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Craig, H.; Jara-Cavieres, A.; Lundy, A.; Preziosi, R.F.; Rowntree, J.K. Multiplex microsatellite PCR panels for the neotropical red mangrove, Rhizophora mangle: Combining efforts towards a cost-effective and modifiable tool to better inform conservation and management. Conserv. Genet. Resour. 2020, 12, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef] [PubMed]
- Sippo, J.Z.; Lovelock, C.E.; Santos, I.R.; Sanders, C.J.; Maher, D.T. Mangrove mortality in a changing climate: An overview. Estuar. Coast. Shelf Sci. 2018, 215, 241–249. [Google Scholar] [CrossRef]
- Adeel, Z.; Pomeroy, R. Assessment and management of mangrove ecosystems in developing countries. Trees Struct. Funct. 2002, 16, 235–238. [Google Scholar] [CrossRef]
- Marchio, D.A.; Savarese, M.; Bovard, B.; Mitsch, W.J. Carbon sequestration and sedimentation in mangrove swamps influenced by hydrogeomorphic conditions and urbanization in Southwest Florida. Forests 2016, 7, 116. [Google Scholar] [CrossRef] [Green Version]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M.F.; Benson, L.; Bukoski, J.J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D.; et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The global mangrove watch—A new 2010 global baseline of mangrove extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef] [Green Version]
- Spalding, M. World Atlas of Mangroves; Routledge: London, UK, 2010. [Google Scholar]
- Goldberg, L.; Lagomasino, D.; Thomas, N.; Fatoyinbo, T. Global declines in human-driven mangrove loss. Glob. Chang. Biol. 2020, 26, 5844–5855. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granado, R.; Pinto Neta, L.; Nunes-Freitas, A.; Voloch, C.; Lira, C. Assessing Genetic Diversity after Mangrove Restoration in Brazil: Why Is It So Important? Diversity 2018, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer-Novelli, Y.; Cintrón-Molero, G.; Reis-Neto, A.S.; Abuchahla, G.M.O.; Neta, L.C.P.; Lira-Medeiros, C.F. The mangroves of Araçá Bay through time: An interdisciplinary approach for conservation of spatial diversity at large scale. Ocean Coast. Manag. 2018, 164, 60–67. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Nehemia, A.; Kochzius, M. Reduced genetic diversity and alteration of gene flow in a fiddler crab due to mangrove degradation. PLoS ONE 2017, 12, e0182987. [Google Scholar] [CrossRef] [Green Version]
- Do, B.T.N.; Koedam, N.; Triest, L. Avicennia marina maintains genetic structure whereas Rhizophora stylosa connects mangroves in a flooded, former inner sea (Vietnam). Estuar. Coast. Shelf Sci. 2019, 222, 195–204. [Google Scholar] [CrossRef]
- Fairuz-Fozi, N.; Triest, L.; Mat Zauki, N.A.; Kaben, A.M.; Nelson, B.R.; Chatterji, A.; Akhir, M.F.; Satyanarayana, B.; Dahdouh-Guebas, F. Mangrove horseshoe crab (Carcinoscorpius rotundicauda Latreille, 1802) populations show genetic break in Strait of Malacca with a connectivity along southern coasts of Peninsular Malaysia. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021. [Google Scholar] [CrossRef]
- Guo, Z.; Li, X.; He, Z.; Yang, Y.; Wang, W.; Zhong, C.; Greenberg, A.J.; Wu, C.I.; Duke, N.C.; Shi, S. Extremely low genetic diversity across mangrove taxa reflects past sea level changes and hints at poor future responses. Glob. Chang. Biol. 2018, 24, 1741–1748. [Google Scholar] [CrossRef]
- Ngeve, M.N.; Van der Stocken, T.; Menemenlis, D.; Koedam, N.; Triest, L. Hidden founders? Strong bottlenecks and fine-scale genetic structure in mangrove populations of the Cameroon Estuary complex. Hydrobiologia 2017, 803, 189–207. [Google Scholar] [CrossRef]
- Binks, R.M.; Byrne, M.; McMahon, K.; Pitt, G.; Murray, K.; Evans, R.D. Habitat discontinuities form strong barriers to gene flow among mangrove populations, despite the capacity for long-distance dispersal. Divers. Distrib. 2019, 25, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Ragavan, P.; Zhou, R.; Ng, W.L.; Rana, T.S.; Mageswaran, T.; Mohan, P.M.; Saxena, A. Natural hybridization in mangroves—An overview. Bot. J. Linn. Soc. 2017, 185, 208–224. [Google Scholar] [CrossRef]
- Andersen, G.L. How to detect desert trees using corona images: Discovering historical ecological data. J. Arid Environ. 2006, 65, 491–511. [Google Scholar] [CrossRef] [Green Version]
- Otero, V.; Van De Kerchove, R.; Satyanarayana, B.; Mohd-Lokman, H.; Lucas, R.; Dahdouh-Guebas, F. An analysis of the early regeneration of mangrove forests using Landsat time series in the matang mangrove forest reserve, Peninsular Malaysia. Remote Sens. 2019, 11, 774. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Chui, T.F.M. Annual variations in regional mangrove cover in southern China and potential macro- climatic and hydrological indicators. Ecol. Indic. 2020, 110, 105927. [Google Scholar] [CrossRef]
- Nikinmaa, L.; Lindner, M.; Cantarello, E.; Jump, A.S.; Seidl, R.; Winkel, G.; Muys, B. Reviewing the Use of Resilience Concepts in Forest Sciences. Curr. For. Rep. 2020, 6, 61–80. [Google Scholar] [CrossRef]
- Swales, A.; Reeve, G.; Cahoon, D.R.; Lovelock, C.E. Landscape Evolution of a Fluvial Sediment-Rich Avicennia marina Mangrove Forest: Insights from Seasonal and Inter-annual Surface-Elevation Dynamics. Ecosystems 2019, 22, 1232–1255. [Google Scholar] [CrossRef] [Green Version]
- Swales, A.; Lovelock, C.E. Comparison of sediment-plate methods to measure accretion rates in an estuarine mangrove forest (New Zealand). Estuar. Coast. Shelf Sci. 2020, 236, 106642. [Google Scholar] [CrossRef]
- Lucas, R.; Van De Kerchove, R.; Otero, V.; Lagomasino, D.; Fatoyinbo, L.; Omar, H.; Satyanarayana, B.; Dahdouh-Guebas, F. Structural characterisation of mangrove forests achieved through combining multiple sources of remote sensing data. Remote Sens. Environ. 2020, 237, 111543. [Google Scholar] [CrossRef]
- Dangremond, E.M.; Feller, I.C. Precocious reproduction increases at the leading edge of a mangrove range expansion. Ecol. Evol. 2016, 6, 5087–5092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, R.; Ferreira, A.C.; Lacerda, L.D. Systematic planning and ecosystem-based management as strategies to reconcile mangrove conservation with resource use. Front. Mar. Sci. 2017, 4, 353. [Google Scholar] [CrossRef] [Green Version]
- Song, D.X.; Huang, C.; Sexton, J.O.; Channan, S.; Feng, M.; Townshend, J.R. Use of landsat and corona data for mapping forest cover change from the mid-1960s to 2000s: Case studies from the eastern united states and central brazil. ISPRS J. Photogramm. Remote Sens. 2015, 103, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Bosire, J.O.; Dahdouh-Guebas, F.; Walton, M.; Crona, B.I.; Lewis, R.R.; Field, C.; Kairo, J.G.; Koedam, N. Functionality of restored mangroves: A review. Aquat. Bot. 2008, 89, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Salmo, S.G.; Lovelock, C.; Duke, N.C. Vegetation and soil characteristics as indicators of restoration trajectories in restored mangroves. Hydrobiologia 2013, 720, 1–18. [Google Scholar] [CrossRef]
- Lee, R.Y.; Porubsky, W.P.; Feller, I.C.; McKee, K.L.; Joye, S.B. Porewater biogeochemistry and soil metabolism in dwarf red mangrove habitats (Twin Cays, Belize). Biogeochemistry 2008, 87, 181–198. [Google Scholar] [CrossRef]
- Pupin, B.; Nahas, E. Microbial populations and activities of mangrove, restinga and Atlantic forest soils from Cardoso Island, Brazil. J. Appl. Microbiol. 2014, 116, 851–864. [Google Scholar] [CrossRef]
- Ren, H.; Jian, S.; Lu, H.; Zhang, Q.; Shen, W.; Han, W.; Yin, Z.; Guo, Q. Restoration of mangrove plantations and colonisation by native species in Leizhou bay, South China. Ecol. Res. 2008, 23, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, B.; Mohamad, K.A.; Idris, I.F.; Husain, M.L.; Dahdouh-Guebas, F. Assessment of mangrove vegetation based on remote sensing and ground-truth measurements at Tumpat, Kelantan Delta, East Coast of Peninsular Malaysia. Int. J. Remote Sens. 2011, 32, 1635–1650. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Castellanos-Galindo, G.A.; Millones-Mayer, M.; Chen, M. Remote sensing of mangrove forests: Current techniques and existing databases. Coast. Res. Libr. 2018, 25, 497–520. [Google Scholar] [CrossRef]
- Kuenzer, C.; Bluemel, A.; Gebhardt, S.; Quoc, T.V.; Dech, S. Remote sensing of mangrove ecosystems: A review. Remote Sens. 2011, 3, 878–928. [Google Scholar] [CrossRef] [Green Version]
- Ruwaimana, M.; Satyanarayana, B.; Otero, V.; Muslim, A.M.; Muhammad Syafiq, A.; Ibrahim, S.; Raymaekers, D.; Koedam, N.; Dahdouh-Guebas, F. The advantages of using drones over space-borne imagery in the mapping of mangrove forests. PLoS ONE 2018, 13, e0200288. [Google Scholar] [CrossRef] [Green Version]
- Grosse, G.; Schirrmeister, L.; Kunitsky, V.V.; Hubberten, H.W. The use of CORONA images in remote sensing of periglacial geomorphology: An illustration from the NE Siberian coast. Permafr. Periglac. Process. 2005, 16, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Nita, M.D.; Munteanu, C.; Gutman, G.; Abrudan, I.V.; Radeloff, V.C. Widespread forest cutting in the aftermath of World War II captured by broad-scale historical Corona spy satellite photography. Remote Sens. Environ. 2018, 204, 322–332. [Google Scholar] [CrossRef]
- Ghosh, A.; Schmidt, S.; Fickert, T.; Nüsser, M. The Indian Sundarban mangrove forests: History, utilization, conservation strategies and local perception. Diversity 2015, 7, 149–169. [Google Scholar] [CrossRef]
- Leempoel, K.; Satyaranayana, B.; Bourgeois, C.; Zhang, J.; Chen, M.; Wang, J.; Bogaert, J.; Dahdouh-Guebas, F. Dynamics in mangroves assessed by high-resolution and multi-temporal satellite data: A case study in Zhanjiang Mangrove National Nature Reserve (ZMNNR), P. R. China. Biogeosciences 2013, 10, 5681–5689. [Google Scholar] [CrossRef]
- Gao, X.M.; Han, W.D.; Liu, S.Q. The mangrove and its conservation in Leizhou Peninsula, China. J. For. Res. 2009, 20, 174–178. [Google Scholar] [CrossRef]
- Ramsar Convention Bureau. List of Wetlands of International Importance; Ramsar Convention Bureau: Gland, Switzerland, 2002. [Google Scholar]
- World Weather Online Zhanjiang Monthly Climate Averages. Available online: https://www.worldweatheronline.com/zhanjiang-weather-averages/guangdong/cn.aspx (accessed on 21 September 2020).
- Wang, G.; Guan, D.; Xiao, L.; Peart, M.R.; Zhang, H.; Singh, M. Changes in mangrove community structures affecting sediment carbon content in Yingluo Bay of South China. Mar. Pollut. Bull. 2019, 149, 110581. [Google Scholar] [CrossRef]
- Laben, C.A.; Brower, B. Process for Enhancing the Spatial Resolution of Multispectral Imagery Using Pan-Sharpening of Multispectral Imagery Using Pan-Sharpening. U.S. Patent No. 6,011,875, 1 January 2000. [Google Scholar]
- ESRI Fundamentals of Panchromatic Sharpening. Available online: https://desktop.arcgis.com/en/arcmap/10.3/manage-data/raster-and-images/fundamentals-of-panchromatic-sharpening.htm (accessed on 1 June 2021).
- U.S. EPA. Methods for Evaluating Wetland Condition: Using Algae To Assess Environ- mental Conditions in Wetlands; U.S. EPA: Washington, DC, USA, 2002; Volume 11, pp. 246–259.
- Tomlinson, P.B. The Botany of Mangroves, 1st ed.; Cambridge University Press: Cambridge, UK, 1986; ISBN 0521255678. [Google Scholar]
- Brokaw, N.; Thompson, J. The H for DBH. For. Ecol. Manag. 2000, 129, 89–91. [Google Scholar] [CrossRef]
- Cintrón, G.; Schaeffer-Novelli, Y. Methods for studying mangrove structure. In The Mangrove Ecosystem: Research Methods; UNESCO: Paris, France, 1984; pp. 91–113. ISBN 9231021818. [Google Scholar]
- Ellison, A.M.; Mukherjee, B.B.; Karim, A. Testing patterns of zonation in mangroves: Scale dependence and environmental correlates in the Sundarbans of Bangladesh. J. Ecol. 2000, 88, 813–824. [Google Scholar] [CrossRef]
- Piotrowska, M.; Stepien, P.P.; Bartnik, E.; Zakrzewska, E. Basic and neutral amino acid transport in Aspergillus nidulans. J. Gen. Microbiol. 1976, 92, 89–96. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.r-project.org/ (accessed on 1 February 2020).
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Triest, L. Molecular ecology and biogeography of mangrove trees towards conceptual insights on gene flow and barriers: A review. Aquat. Bot. 2008, 89, 138–154. [Google Scholar] [CrossRef]
- Bosire, J.O.; Dahdouh-Guebas, F.; Kairo, J.G.; Cannicci, S.; Koedam, N. Spatial variations in macrobenthic fauna recolonisation in a tropical mangrove bay. Biodivers. Conserv. 2004, 13, 1059–1074. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira Côrtes, L.H.; Zappes, C.A.; Di Beneditto, A.P.M. The crab harvest in a mangrove forest in south-eastern Brazil: Insights about its maintenance in the long-term. Perspect. Ecol. Conserv. 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Hugé, J.; Abuchahla, G.M.O.; Cannicci, S.; Jayatissa, L.P.; Kairo, J.G.; Kodikara Arachchilage, S.; Koedam, N.; Mafaziya Nijamdeen, T.W.G.F.; Mukherjee, N.; et al. Reconciling nature, people and policy in the mangrove social-ecological system through the adaptive cycle heuristic. Estuar. Coast. Shelf Sci. 2021, 248, 106942. [Google Scholar] [CrossRef]
- Mukherjee, N.; Sutherland, W.J.; Dicks, L.; Hugé, J.; Koedam, N.; Dahdouh-Guebas, F. Ecosystem service valuations of mangrove ecosystems to inform decision making and future valuation exercises. PLoS ONE 2014, 9, e107706. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.F.; Cortez, L.S.R.; Longhi, S.J. Native forest management in subtropical South America: Long-term effects of logging and multiple-use on forest structure and diversity. Biodivers. Conserv. 2012, 21, 1953–1969. [Google Scholar] [CrossRef]
- Floyd Sabins, F.; Ellis, J.M. Remote Sensing: Principles, Interpretation, and Applications, Fourth Edition; Waveland: Long Grove, IL, USA, 2020. [Google Scholar]
- Alfaro, A.C. Benthic macro-invertebrate community composition within a mangrove/seagrass estuary in northern New Zealand. Estuar. Coast. Shelf Sci. 2006, 66, 97–110. [Google Scholar] [CrossRef]
- Jayakody, J.; Amarasinghe, M.; Pahalawattaarachchi, V.; De Silva, K. Vegetation structure and potential gross primary productivity of mangroves at Kadolkele in Meegamuwa (Negombo) estuary, Sri Lanka. Sri Lanka J. Aquat. Sci. 2010, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.E.; Virginia, R.A.; Wall, D.H.; Parsons, A.N.; Powers, L.E.; Burkins, M.B. Variation in biogeochemistry and soil biodiversity across spatial scales in a polar desert ecosystem. Ecology 2004, 85, 3105–3118. [Google Scholar] [CrossRef]
- Lira-Medeiros, C.F.; Parisod, C.; Fernandes, R.A.; Mata, C.S.; Cardoso, M.A.; Ferreira, P.C.G. Epigenetic variation in mangrove plants occurring in contrasting natural environment. PLoS ONE 2010, 5, e10326. [Google Scholar] [CrossRef]
- Dittmann, S.; Thiessen, E.; Hartung, E. Applicability of different non-invasive methods for tree mass estimation: A review. For. Ecol. Manag. 2017, 398, 208–215. [Google Scholar] [CrossRef]
- Jenerowicz, A.; Siok, K.; Schismak, A.; Ewiak, I. Improvement of interpretability of archival aerial photographs using remote sensing tools. SPIE Proc. 2018, 10789, 82. [Google Scholar]
- Chen, Q.; Zhao, Q.; Chen, P.; Lu, H.; Jian, S. Eco-exergy based self-organization of the macrobenthic faunal assemblage during mangrove succession in Zhanjiang, China. Ecol. Indic. 2018, 95, 887–894. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Koedam, N. Coastal vegetation and the Asian tsunami. Science (80-) 2006, 311, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, W.; Wu, Q.; Fang, B.; Lin, P. The growth of Kandelia candel seedlings in mangrove habitats of the Zhangjiang estuary in Fujian, China. Acta Ecol. Sin. 2006, 26, 1648–1655. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Verneirt, M.; Cannicci, S.; Kairo, J.G.; Tack, J.F.; Koedam, N. An exploratory study on grapsid crab zonation in Kenyan mangroves. Wetl. Ecol. Manag. 2002, 10, 179–187. [Google Scholar] [CrossRef]
- Van der Stocken, T.; Wee, A.K.S.; De Ryck, D.J.R.; Vanschoenwinkel, B.; Friess, D.A.; Dahdouh-Guebas, F.; Simard, M.; Koedam, N.; Webb, E.L. A general framework for propagule dispersal in mangroves. Biol. Rev. 2019, 94, 1547–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromard, F.; Vega, C.; Proisy, C. Half a century of dynamic coastal change affecting mangrove shorelines of French Guiana. A case study based on remote sensing data analyses and field surveys. Mar. Geol. 2004, 208, 265–280. [Google Scholar] [CrossRef]
- Putz, F.E.; Chan, H.T. Tree growth, dynamics, and productivity in a mature mangrove forest in Malaysia. For. Ecol. Manag. 1986, 17, 211–230. [Google Scholar] [CrossRef]
- Smith, T.J. Forest structure. In Tropical Mangrove Ecosystems; Robertson, A.I., Alongi, D.M., Eds.; Wiley & Sons: Hoboken, NJ, USA, 1992; pp. 101–136. [Google Scholar]
- Dahdouh-Guebas, F.; De Bondt, R.; Abeysinghe, P.D.; Kairo, J.G.; Cannicci, S.; Triest, L.; Koedam, N. Comparative study of the disjunct zonation pattern of the grey mangrove Avicennia marina (Forsk.) Vierh. in Gazi Bay (Kenya). Bull. Mar. Sci. 2004, 74, 237–252. [Google Scholar]
- Gardner, T.A.; Hernández, M.I.M.; Barlow, J.; Peres, C.A. Understanding the biodiversity consequences of habitat change: The value of secondary and plantation forests for neotropical dung beetles. J. Appl. Ecol. 2008, 45, 883–893. [Google Scholar] [CrossRef]
- Okello, J.A.; Robert, E.M.R.; Beeckman, H.; Kairo, J.G.; Dahdouh-Guebas, F.; Koedam, N. Effects of experimental sedimentation on the phenological dynamics and leaf traits of replanted mangroves at Gazi bay, Kenya. Ecol. Evol. 2014, 4, 3187–3200. [Google Scholar] [CrossRef]
- He, B.; Lai, T.; Fan, H.; Wang, W.; Zheng, H. Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the Beibu Gulf. Estuar. Coast. Shelf Sci. 2007, 74, 254–262. [Google Scholar] [CrossRef]
- Osborne, D.J.; Berjak, P. The making of mangroves: The remarkable pioneering role played by seeds of Avicennia marina. Endeavour 1997, 21, 143–147. [Google Scholar] [CrossRef]
- Muoghalu, J.I. Tree species population dynamics in a secondary forest at Ile-Ife, Nigeria after a ground fire. Afr. J. Ecol. 2007, 45, 62–71. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Koedam, N.; De Smet, K.; Di Nitto, D.; Bauwens, M.; Jayatissa, L.P.; Cannicci, S.; Dahdouh-Guebas, F. Long-term mangrove forest development in Sri Lanka: Early predictions evaluated against outcomes using VHR remote sensing and VHR ground-truth data. Mar. Ecol. Prog. Ser. 2011, 443, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- López-Portillo, J.; Lewis, R.R.; Saenger, P.; Rovai, A.; Koedam, N.; Dahdouh-Guebas, F.; Agraz-Hernández, C.; Rivera-Monroy, V.H. Mangrove forest restoration and rehabilitation. In Mangrove Ecosystems: A Global Biogeographic Perspective: Structure, Function, and Services; Springer International Publishing: Cham, Switzerland, 2017; pp. 301–345. ISBN 9783319622064. [Google Scholar]
- Ellison, A.M. Mangrove restoration: Do we know enough? Restor. Ecol. 2000, 8, 219–229. [Google Scholar] [CrossRef]
- Kairo, J.G.; Dahdouh-Guebas, F.; Bosire, J.; Koedam, N. Restoration and management of mangrove systems—A lesson for and from the East African region. S. Afr. J. Bot. 2001, 67, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Vannucci, M. Indo-West Pacific Mangroves. In Mangrove Ecosystems; Springer: Berlin/Heidelberg, Germany, 2002; pp. 123–215. [Google Scholar]
- Islam, S.N.; Yahya, U.A.A. Bin Impacts of coastal land use changes on mangrove wetlands at sungai mangsalut basin in Brunei Darussalam. In Coastal Research Library; Springer: New York, NY, USA, 2017; Volume 21, pp. 133–157. [Google Scholar]
- Feagin, R.A.; Mukherjee, N.; Shanker, K.; Baird, A.H.; Cinner, J.; Kerr, A.M.; Koedam, N.; Sridhar, A.; Arthur, R.; Jayatissa, L.P.; et al. Shelter from the storm? Use and misuse of coastal vegetation bioshields for managing natural disasters. Conserv. Lett. 2010, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kodikara, K.A.S.; Mukherjee, N.; Jayatissa, L.P.; Dahdouh-Guebas, F.; Koedam, N. Have mangrove restoration projects worked? An in-depth study in Sri Lanka. Restor. Ecol. 2017, 25, 705–716. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Hettiarachchi, S.; Lo Seen, D.; Batelaan, O.; Sooriyarachchi, S.; Jayatissa, L.P.; Koedam, N. Transitions in ancient inland freshwater resource management in Sri Lanka affect biota and human populations in and around coastal lagoons. Curr. Biol. 2005, 15, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; Di Nitto, D.; Bosire, J.O.; Lo Seen, D.; Koedam, N. How effective were mangroves as a defence against the recent tsunami? Curr. Biol. 2005, 15, R443–R447. [Google Scholar] [CrossRef] [Green Version]
- Gourlay, I.D. The Definition of Seasonal Growth Zones in Some African Acacia Species—A Review. IAWA J. 2014, 16, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Robert, E.M.R.; Schmitz, N.; Okello, J.A.; Boeren, I.; Beeckman, H.; Koedam, N. Mangrove growth rings: Fact or fiction? Trees Struct. Funct. 2011, 25, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, N.; Verheyden, A.; Kairo, J.G.; Beeckman, H.; Koedam, N. Successive cambia development in Avicennia marina (Forssk.) Vierh. is not climatically driven in the seasonal climate at Gazi Bay, Kenya. Dendrochronologia 2007, 25, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Melville, F.; Burchett, M.; Pulkownik, A. Genetic variation among age-classes of the mangrove Avicennia marina in clean and contaminated sediments. Mar. Pollut. Bull. 2004, 49, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y. Effects of plant density and age on the mating system of Kandelia candel Druce (Rhizophoraceae), a viviparous mangrove species. Hydrobiologia 2000, 432, 189–193. [Google Scholar] [CrossRef]
- Ge, J.P.; Cai, B.; Ping, W.; Song, G.; Ling, H.; Lin, P. Mating system and population genetic structure of Bruguiera gymnorrhiza (Rhizophoraceae), a viviparous mangrove species in China. J. Exp. Mar. Bio. Ecol. 2005, 326, 48–55. [Google Scholar] [CrossRef]


| Hypothetical Ecological Study Objectives | Field | Example Reference(s) |
|---|---|---|
| to execute sampling for conservation genetics | Conservation genetics | Binks et al., 2019; Ragavan et al., 2017 |
| to estimate tree age | Silviculture | Lucas et al., 2020 |
| to identify late successional stands (e.g., capable of mass seeding) | Reproductive botany | Dangremond and Feller, 2016 |
| to outline core conservation areas to monitor gain/loss of pristine forest | Environmental planning | Borges et al., 2017; Song et al., 2015 |
| to sample species indicative of floristic or faunistic recruitment | Restoration ecology | Bosire et al., 2008; Salmo et al., 2013 |
| to monitor soil biogeochemical processes in interior stands | Biogeochemistry | Lee et al., 2008 |
| to compare microbial activity in outer and interior stands | Environmental microbiology | Pupin and Nahas, 2014 |
| to detect/validate older mangrove presence using spectral analysis in up-to-date high-resolution images | Earth observation science | Andersen, 2006; Otero et al., 2019; Song et al., 2015 |
| to monitor hydrological process in interior stands | Forest hydrology | Luo and Chui, 2020 |
| to test forest resilience in interior stands after disturbance | Forestry science | Nikinmaa et al., 2020 |
| to compare sediment and geological formations in interior stands | Sedimentology | Swales et al., 2019; Swales and Lovelock, 2020 |
| Species | Site 1 | Site 2 | ||
|---|---|---|---|---|
| Original | Non-Original | Original | Non-Original | |
| Density (stems ha−1) | ||||
| Aegiceras corniculatum | 6480 (400–2800) | 7093.3 (3200–19,600) | – | 3750 (2000–5500) |
| Avicennia marina | – | 1800 (400–5200) | – | 1320 (320–2280) |
| Bruguiera gymnorrhiza | 3186.7 (400–9200) | 2114.3 (400–5600) | 290 (80–720) | – |
| Kandelia obovata | 1580 (400–6800) | 711.1 (400–1200) | – | – |
| Rhizophora stylosa | 400 (340–460) | 700 (390–1200) | 5360 (340–640) | – |
| Height (m) | ||||
| Aegiceras corniculatum | 2.27 (0.96–3.23) | 1.63 (0.61–3.08) | – | 2.00 (1.80–2.30) |
| Avicennia marina | – | 1.08 (0.30–1.57) | – | 1.93 (1.80–2.10) |
| Bruguiera gymnorrhiza | 2.69 (1.57–3.85) | 2.03 (0.98–3.04) | 3.87 (2.50–4.68) | – |
| Kandelia obovata | 2.74 (1.50–4.14) | 2.16 (1.38–3.10) | – | – |
| Rhizophora stylosa | 1.87 (1.87–1.87) | 2.21 (1.70–2.88) | 3.82 (2.50–4.60) | – |
| Basal area (m2 ha−1) | ||||
| Aegiceras corniculatum | 10.8 (1.4–42.3) | 17.9 (3.6–38.6) | – | 8.8 (6.3–10.8) |
| Avicennia marina | – | 5.6 (1.0–12.6) | – | 4.6 (3.2–5.9) |
| Bruguiera gymnorrhiza | 11.2 (2.0–23.6) | 7.6 (1.7–16.5) | 10.0 (4.7–13.6) | – |
| Kandelia obovata | 6.4 (1.0–19.1) | 5.5 (1.7–8.2) | – | – |
| Rhizophora stylosa | 5.1 (3.3–9.3) | 4.2 (1.4–9.9) | 9.5 (6.4–1.2) | – |
| IV (rank numbers) | ||||
| Aegiceras corniculatum | 2 (76.56) | 1 (224.79) | – | 1 (146.51) |
| Avicennia marina | – | 4 (10.08) | – | 2 (53.49) |
| Bruguiera gymnorrhiza | 1 (170.78) | 2 (41.90) | 2 (67.07) | – |
| Kandelia obovata | 3 (46.39) | 3 (13.48) | – | – |
| Rhizophora stylosa | 4 (5.14) | 5 (7.89) | 1 (232.93) | – |
| CI | 100.59 | 55.65 | 5.59 | 9.73 |
| Kruskal–Wallis Test (H) | Wilcoxon Sum Rank Test with Bonferroni Correction (T) | |||
|---|---|---|---|---|
| Height | X2 | p-Value | Weight | p-Value |
| Aegiceras corniculatum | 2.3164 | 0.08 | 835.5 | 0.1294 |
| Avicennia marina | 103.27 | 0.001 * | 504 | 0.001 * |
| Bruguiera gymnorrhiza | 2.5732 | 0.1087 | 1192 | 0.001 * |
| Kandelia obovata | 10.811 | 0.001 * | 915.5 | 0.001 * |
| Rhizophora stylosa | 70.927 | 0.001* | 697 | 0.9516 |
| Density | ||||
| Aegiceras corniculatum | 61.603 | 0.001 * | 238 | 0.001 * |
| Avicennia marina | 82.045 | 0.001 * | 504 | 0.001 * |
| Bruguiera gymnorrhiza | f = 45.6 | 0.001 * | t = −7.017 | 0.001 * |
| Kandelia obovata | 34.780 | 0.001 * | 874.5 | 0.02 |
| Rhizophora stylosa | 64.031 | 0.001 * | 793 | 0.1285 |
| Basal area | ||||
| Aegiceras corniculatum | f = 45.6 | 0.001 * | t = −6.050 | 0.001 * |
| Avicennia marina | 123.71 | 0.001 * | 504 | 0.001 * |
| Bruguiera gymnorrhiza | 26.005 | 0.001 * | 1159.5 | 0.001 * |
| Kandelia obovata | 108.15 | 0.001 * | 875.5 | 0.0267 |
| Rhizophora stylosa | 117.96 | 0.001 * | 804.5 | 0.0898 |
| Tree Height | PC1 | PC2 | PC3 |
|---|---|---|---|
| Aegiceras corniculatum | −1.536 | 0.652 | −0.193 |
| Avicennia marina | 0.758 | 0.979 | 1.361 |
| Bruguiera gymnorrhiza | −0.791 | −1.506 | 0.206 |
| Kandelia obovata | −1.171 | −0.537 | 1.228 |
| Rhizophora stylosa | 1.314 | −1.187 | 0.201 |
| Variation explained (%) | 35 | 28 | 18 |
| Cumulative proportion (%) | 35 | 63 | 81 |
| Density | |||
| Aegiceras corniculatum | 1.484 | −0.399 | 0.676 |
| Avicennia marina | 0.855 | 0.516 | −1.608 |
| Bruguiera gymnorrhiza | −1.549 | −0.214 | 0.094 |
| Kandelia obovata | −0.731 | −1.371 | −0.763 |
| Rhizophora stylosa | −0.793 | 1.493 | 0.049 |
| Variation explained (%) | 39 | 27 | 18 |
| Cumulative proportion (%) | 39 | 66 | 77 |
| Basal area | |||
| Aegiceras corniculatum | 1.643 | −0.154 | −0.146 |
| Avicennia marina | 0.037 | 1.391 | −1.194 |
| Bruguiera gymnorrhiza | −1.565 | −0.762 | 0.037 |
| Kandelia obovata | −0.312 | −0.959 | −1.582 |
| Rhizophora stylosa | −1.139 | 1.133 | 0.133 |
| Variation explained (%) | 35 | 25 | 20 |
| Cumulative proportion (%) | 35 | 60 | 80 |
| Technique | Advantage | Limitation |
|---|---|---|
| GIS |
|
|
| Remote sensing |
|
|
| Kruskal–Wallis-PCA-ordination |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durango-Cordero, J.; Satyanarayana, B.; Chan, J.C.-W.; Bogaert, J.; Dahdouh-Guebas, F. Distinguishing Original and Non-Original Stands at the Zhanjiang Mangrove National Nature Reserve (P.R. China): Remote Sensing and GIS for Conservation and Ecological Research. Remote Sens. 2021, 13, 2781. https://doi.org/10.3390/rs13142781
Durango-Cordero J, Satyanarayana B, Chan JC-W, Bogaert J, Dahdouh-Guebas F. Distinguishing Original and Non-Original Stands at the Zhanjiang Mangrove National Nature Reserve (P.R. China): Remote Sensing and GIS for Conservation and Ecological Research. Remote Sensing. 2021; 13(14):2781. https://doi.org/10.3390/rs13142781
Chicago/Turabian StyleDurango-Cordero, Juan, Behara Satyanarayana, Jonathan Cheung-Wai Chan, Jan Bogaert, and Farid Dahdouh-Guebas. 2021. "Distinguishing Original and Non-Original Stands at the Zhanjiang Mangrove National Nature Reserve (P.R. China): Remote Sensing and GIS for Conservation and Ecological Research" Remote Sensing 13, no. 14: 2781. https://doi.org/10.3390/rs13142781








