The Impact of Phenological Developments on Interferometric and Polarimetric Crop Signatures Derived from Sentinel-1: Examples from the DEMMIN Study Site (Germany)
Abstract
:1. Introduction
2. Materials and Methods
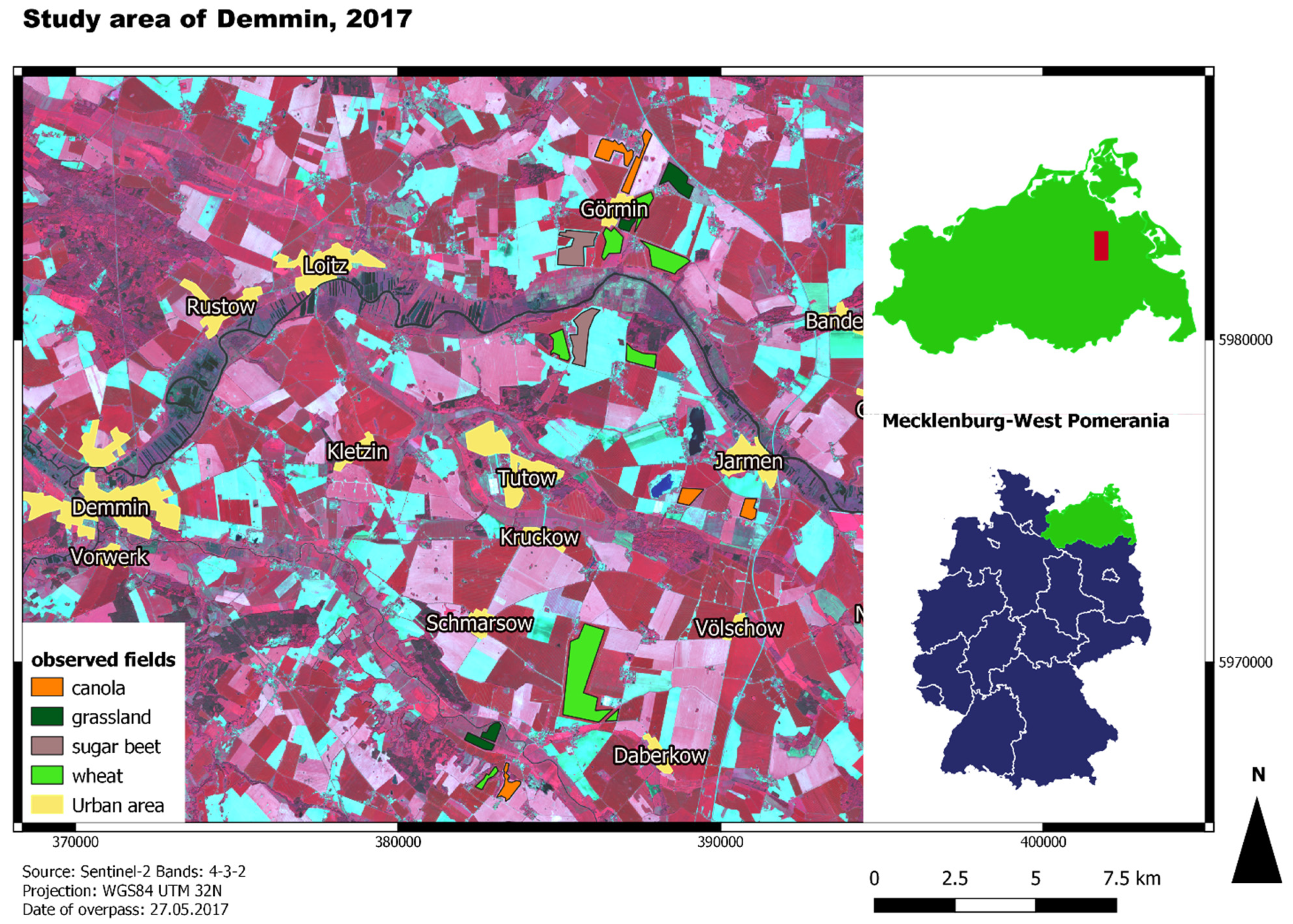
2.1. Study Area and In Situ Data
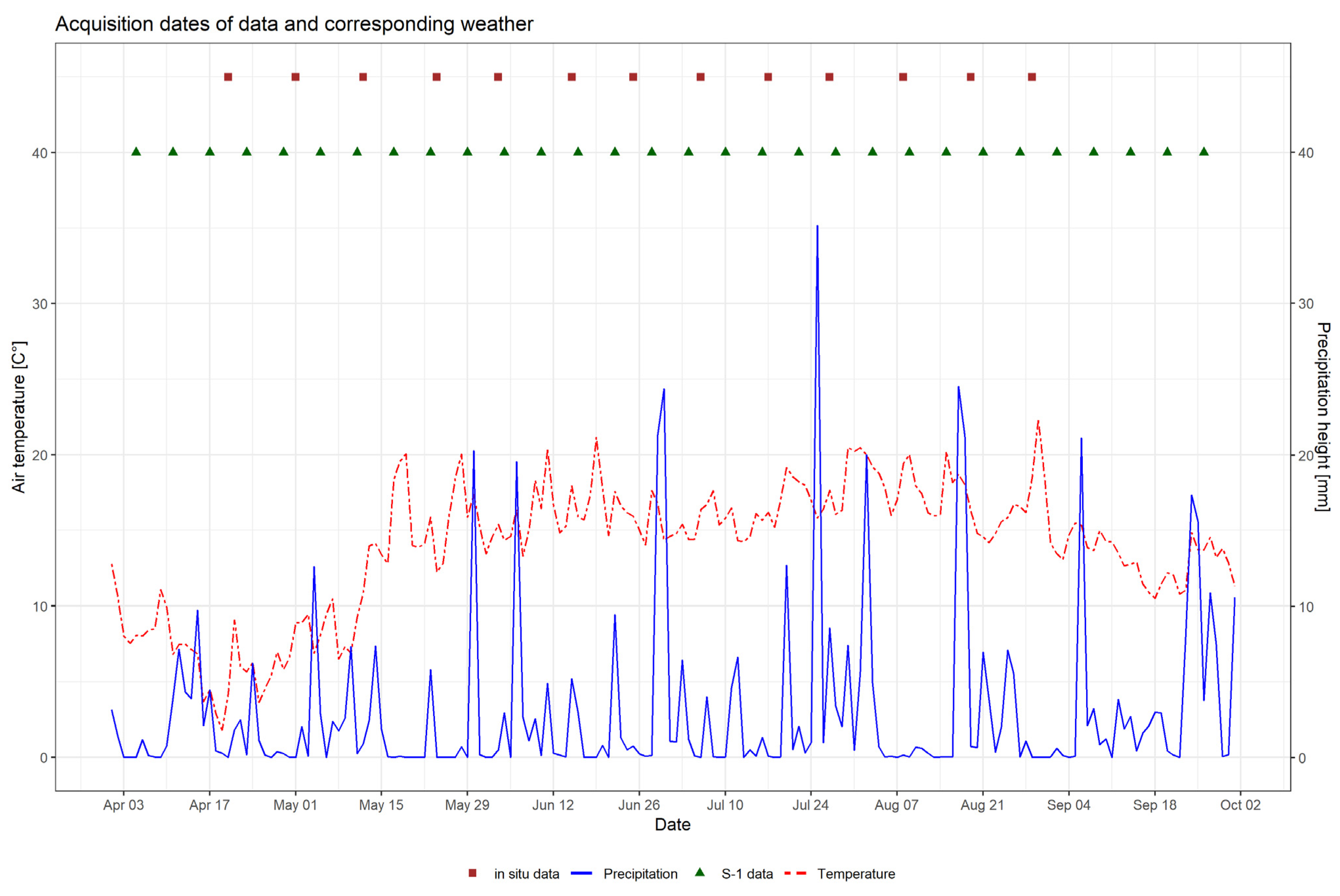
2.2. Sentinel-1 Time Series
2.3. Methods
2.3.1. Elementary Analysis of S-1 Features
2.3.2. Signature Analysis
3. Results
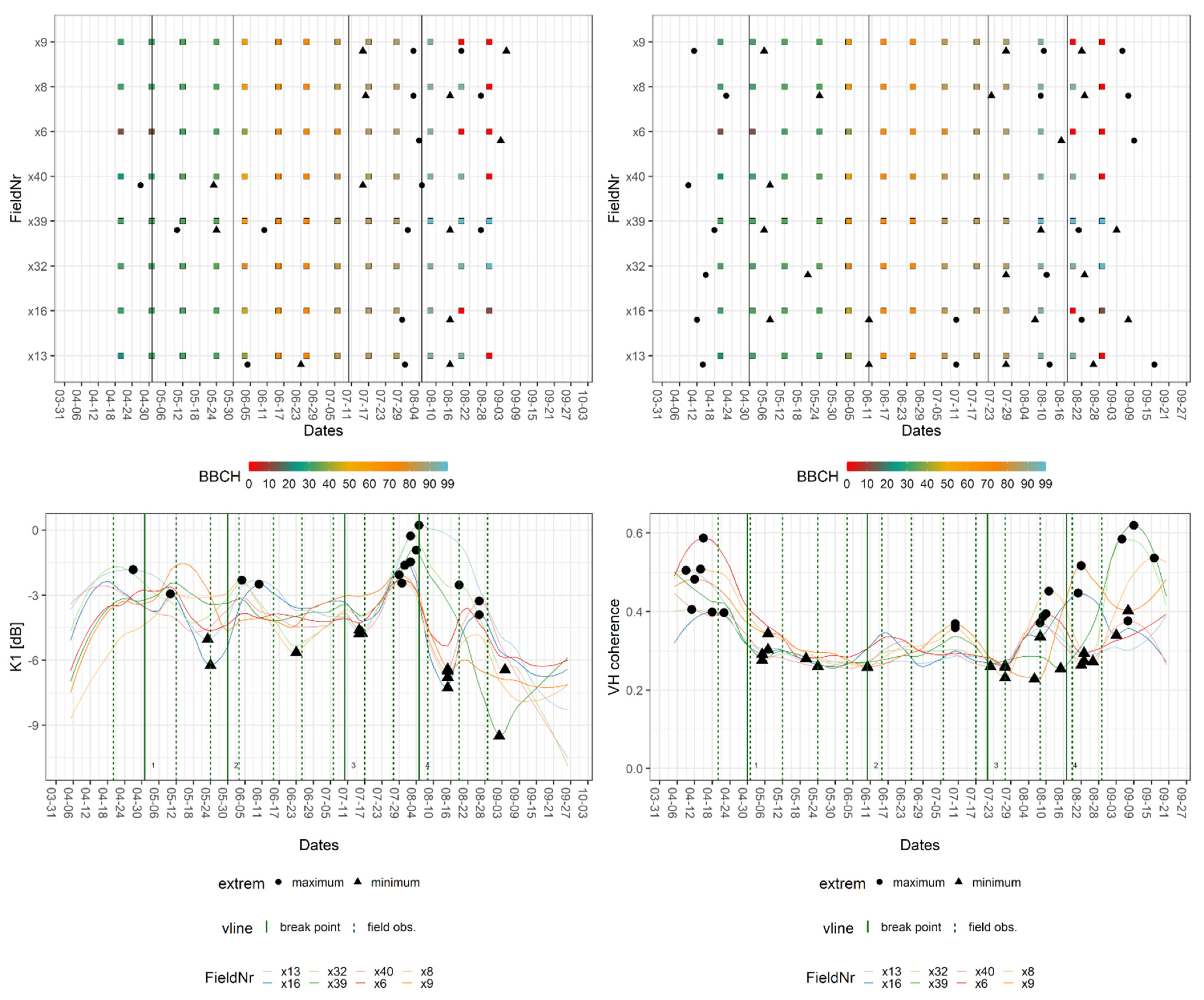
3.1. Elementary Data Analysis
3.2. Signature Analyses
3.2.1. Signatures of Wheat
3.2.2. Signatures of Canola
3.2.3. Signatures of Sugar Beet
4. Discussion
4.1. Elementary Data Analysis
4.2. Realisations of the Signature Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieth, H. (Ed.) Phenology and Seasonality Modeling; Springer: New York, NY, USA, 1974; ISBN 9783642518652. [Google Scholar]
- Gao, F.; Anderson, M.C.; Zhang, X.; Yang, Z.; Alfieri, J.G.; Kustas, W.P.; Mueller, R.; Johnson, D.M.; Prueger, J.H. Toward mapping crop progress at field scales through fusion of Landsat and MODIS imagery. Remote Sens. Environ. 2017, 188, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Stathers, T.; Lamboll, R.; Mvumi, B.M. Postharvest agriculture in changing climates: Its importance to African smallholder farmers. Food Secur. 2013, 5, 361–392. [Google Scholar] [CrossRef]
- Sakamoto, T.; Gitelson, A.A.; Arkebauer, T.J. MODIS-based corn grain yield estimation model incorporating crop phenology information. Remote Sens. Environ. 2013, 131, 215–231. [Google Scholar] [CrossRef]
- Steele-Dunne, S.C.; McNairn, H.; Monsivais-Huertero, A.; Judge, J.; Liu, P.W.; Papathana, K. Radar Remote Sensing of Agriciltural Canopies. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 2249–2273. [Google Scholar] [CrossRef] [Green Version]
- Ulaby, F.; Moore, R. Radar sensing of soil moisture. In Proceedings of the 1973 Antennas and Propagation Society International Symposium, Boulder, CO, USA, 22–24 April 1973; Volume 11, pp. 362–365. [Google Scholar]
- Ulaby, F. Radar response to vegetation. IEEE Trans. Antennas Propag. 1975, 23, 36–45. [Google Scholar] [CrossRef]
- Weise, K.; Davidson, M.W.J. Dualband—TerraSAR simulation/campaign results for L-band configuration. In Proceedings of the IGARSS 2004 IEEE International Geoscience and Remote Sensing Symposium, Anchorage, AK, USA, 20–24 September 2004; Volume 7, pp. 4556–4559. [Google Scholar]
- Su, Z.; Timmermans, W.J.; van der Tol, C.; Dost, R.; Bianchi, R.; Gómez, J.A.; House, A.; Hajnsek, I.; Menenti, M.; Magliulo, V.; et al. EAGLE 2006—Multi-purpose, multi-angle and multi-sensor in-situ and airborne campaigns over grassland and forest. Hydrol. Earth Syst. Sci. 2009, 13, 833–845. [Google Scholar] [CrossRef]
- Wegmüller, U.; Werner, C. Farmland monitoring with SAR interferometry. Geosci. Remote Sens. Symp. 1995, 1, 544–546. [Google Scholar]
- Baghdadi, N.; Boyer, N.; Todoroff, P.; El Hajj, M.; Bégué, A. Potential of SAR sensors TerraSAR-X, ASAR/ENVISAT and PALSAR/ALOS for monitoring sugarcane crops on Reunion Island. Remote Sens. Environ. 2009, 113, 1724–1738. [Google Scholar] [CrossRef]
- Mascolo, L.; Lopez-Sanchez, J.M.; Vicente-Guijalba, F.; Mazzarella, G.; Nunziata, F.; Migliaccio, M. Retrieval of phenological stages of onion fields during the first year of growth by means of C-band polarimetric SAR measurements. Int. J. Remote Sens. 2015, 36, 3077–3096. [Google Scholar] [CrossRef] [Green Version]
- Cloude, S.R.; Pottier, E. A review of target decomposition theorems in radar polarimetry. IEEE Trans. Geosci. Remote Sens. 1996, 34, 498–518. [Google Scholar] [CrossRef]
- Canisius, F.; Shang, J.; Liu, J.; Huang, X.; Ma, B.; Jiao, X.; Geng, X.; Kovacs, J.M.; Walters, D. Tracking crop phenological development using multi-temporal polarimetric Radarsat-2 data. Remote Sens. Environ. 2018, 210, 508–518. [Google Scholar] [CrossRef]
- Ndikumana, E.; Ho Tong Minh, D.; Baghdadi, N.; Courault, D.; Hossard, L. Deep Recurrent Neural Network for Agricultural Classification using multitemporal SAR Sentinel-1 for Camargue, France. Remote Sens. 2018, 10, 1217. [Google Scholar] [CrossRef] [Green Version]
- Schlund, M.; Erasmi, S. Sentinel-1 time series data for monitoring the phenology of winter wheat. Remote Sens. Environ. 2020, 246, 111814. [Google Scholar] [CrossRef]
- Torres, R.; Snoeij, P.; Geudtner, D.; Bibby, D.; Davidson, M.; Attema, E.; Potin, P.; Rommen, B.; Floury, N.; Brown, M.; et al. GMES Sentinel-1 mission. Remote Sens. Environ. 2012, 120, 9–24. [Google Scholar] [CrossRef]
- Ruml, M.; Vulic, T. Importance of phenological observations and predictions in agriculture. J. Agric. Sci. Belgrade 2005, 50, 217–225. [Google Scholar] [CrossRef]
- Wali, E.; Tasumi, M.; Moriyama, M. Combination of linear regression lines to understand the response of sentinel-1 dual polarization SAR data with crop phenology-case study in Miyazaki, Japan. Remote Sens. 2020, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, J. Mapping winter wheat planting area and monitoring its phenology using Sentinel-1 backscatter time series. Remote Sens. 2019, 11, 449. [Google Scholar] [CrossRef] [Green Version]
- D’Andrimont, R.; Taymans, M.; Lemoine, G.; Ceglar, A.; Yordanov, M.; van der Velde, M. Detecting flowering phenology in oil seed rape parcels with Sentinel-1 and -2 time series. Remote Sens. Environ. 2020, 239, 111660. [Google Scholar] [CrossRef]
- Khabbazan, S.; Vermunt, P.; Steele-Dunne, S.; Arntz, L.R.; Marinetti, C.; van der Valk, D.; Iannini, L.; Molijn, R.; Westerdijk, K.; van der Sande, C. Crop monitoring using Sentinel-1 data: A case study from The Netherlands. Remote Sens. 2019, 11, 1887. [Google Scholar] [CrossRef] [Green Version]
- Meroni, M.; d’Andrimont, R.; Vrieling, A.; Fasbender, D.; Lemoine, G.; Rembold, F.; Seguini, L.; Verhegghen, A. Comparing land surface phenology of major European crops as derived from SAR and multispectral data of Sentinel-1 and -2. Remote Sens. Environ. 2021, 253. [Google Scholar] [CrossRef]
- Shang, J.; Liu, J.; Poncos, V.; Geng, X.; Qian, B.; Chen, Q.; Dong, T.; Macdonald, D.; Martin, T.; Kovacs, J.; et al. Detection of crop seeding and harvest through analysis of time-series Sentinel-1 interferometric SAR data. Remote Sens. 2020, 12, 1551. [Google Scholar] [CrossRef]
- Schmitt, A.; Wendleder, A.; Hinz, S. The Kennaugh element framework for multi-scale, multi-polarized, multi-temporal and multi-frequency SAR image preparation. ISPRS J. Photogramm. Remote Sens. 2015, 102, 122–139. [Google Scholar] [CrossRef] [Green Version]
- Verbesselt, J.; Zeileis, A.; Herold, M. Near real-time disturbance detection using satellite image time series. Remote Sens. Environ. 2012, 123, 98–108. [Google Scholar] [CrossRef]
- Verbesselt, J.; Hyndman, R.; Zeileis, A.; Culvenor, D. Phenological change detection while accounting for abrupt and gradual trends in satellite image time series. Remote Sens. Environ. 2010, 114, 2970–2980. [Google Scholar] [CrossRef] [Green Version]
- Meier, U. Growth Stages of Mono-and Dicotyledonous Plants. BBCH Monograph, 2nd ed.; Meier, U., Ed.; Federal Biological Research Centre for Agriculture and Forestry: Berlin/Braunschweig, Germany, 2001. [Google Scholar]
- Bastidas, A.M.; Setiyono, T.D.; Dobermann, A.; Cassman, K.G.; Elmore, R.W.; Graef, G.L.; Specht, J.E. Soybean sowing date: The vegetative, reproductive, and agronomic impacts. Crop Sci. 2008, 48, 727–740. [Google Scholar] [CrossRef] [Green Version]
- Araki, T. Transition from vegetative to reproductive phase. Curr. Opin. Plant Biol. 2001, 4, 63–68. [Google Scholar] [CrossRef]
- Conradt, S.; Finger, R.; Spörri, M. Climate Risk Management Flexible weather index-based insurance design. Clim. Risk Manag. 2015, 10, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Borg, E.; Lippert, K.; Zabel, E.; Löpmeier, F.-J.; Fichtelmann, B.; Jahncke, D.; Maass, H. DEMMIN Teststandort zur Kalibrierung und Validierung von Fernerkundungsmissionen; DLR Eigenverlag: Neustrelitz, Germany, 2009. [Google Scholar]
- Spengler, D.; Itzerott, S.; Ahmadian, N.; Borg, E.; Hüttich, C.; Maass, H.; Missling, K.-D.; Schmullius, C.; Truckenbrodt, S.; Conrad, C. The German JECAM site DEMMIN: Status and future perspectives. In Proceedings of the Annual JECAM Meeting, Taichung City, Taiwan, 17–20 September 2018. [Google Scholar]
- Team, S. Sentinel-1 User Handbook; ESA Communications: Noordwijk, The Netherlands, 2013. [Google Scholar]
- Ullmann, T.; Serfas, K.; Büdel, C.; Padashi, M.; Baumhauer, R. Data Processing, Feature Extraction, and Time-Series Analysis of Sentinel-1 Synthetic Aperture Radar (SAR) Imagery: Examples from Damghan and Bajestan Playa (Iran). Z. für Geomorphol. Suppl. Issues 2019, 62, 9–39. [Google Scholar] [CrossRef]
- Ullmann, T.; Sauerbrey, J.; Hoffmeister, D.; May, S.M.; Baumhauer, R.; Bubenzer, O. Assessing spatiotemporal variations of sentinel-1 InSAR coherence at different time scales over the atacama desert (Chile) between 2015 and 2018. Remote Sens. 2019, 11, 2960. [Google Scholar] [CrossRef] [Green Version]
- Small, D. Flattening Gamma: Radiometric Terrain Correction for SAR Imagery. IEEE Trans. Geosci. Remote Sens. 2011, 49, 3081–3093. [Google Scholar] [CrossRef]
- Richards, J.A. Remote Sensing with Imaging Radar; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780387765679. [Google Scholar]
- Zhang, Z.; Wang, C.; Zhang, H.; Tang, Y.; Liu, X. Analysis of Permafrost Region Coherence Variation in the Qinghai–Tibet Plateau with a High-Resolution TerraSAR-X Image. Remote Sens. 2018, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Pichierri, M.; Hajnsek, I.; Zwieback, S.; Rabus, B. On the potential of Polarimetric SAR Interferometry to characterize the biomass, moisture and structure of agricultural crops at L-, C- and X-Bands. Remote Sens. Environ. 2018, 204, 596–616. [Google Scholar] [CrossRef]
- Scheuchl, B.; Ullmann, T.; Koudogbo, F. Change Detection Using High Resolution TerraSAR-X Data Preliminary Results. In Proceedings of the ISPRS Hannover Workshop 2009, Hannover, Germany, 2–5 June 2009. [Google Scholar]
- Zebker, H.A.; Villasenor, J. Decorrelation in interferometric radar echoes. IEEE Trans. Geosci. Remote Sens. 1992, 30, 950–959. [Google Scholar] [CrossRef] [Green Version]
- Esch, S. Determination of Soil Moisture and Vegetation Parameters from Spaceborne C-Band SAR on Agricultural Areas. Ph.D. Thesis, Universität zu Köln, Cologne, Germany, 2018. [Google Scholar]
- Lee, J.-S.; Pottier, E. Polarimetric Radar Imaging: From Basics to Applications; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9781420054989. [Google Scholar]
- Harfenmeister, K.; Itzerott, S.; Weltzien, C.; Spengler, D. Agricultural Monitoring Using Polarimetric Decomposition Parameters of Sentinel-1 Data. Remote Sens. 2021, 13, 575. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Su, S.; Wang, C. Retrieving canopy height and density of paddy rice from Radarsat-2 images with a canopy scattering model. Int. J. Appl. Earth Obs. Geoinf. 2014, 28, 170–180. [Google Scholar] [CrossRef]
- Cleveland, W.S. Robust Locally Weighted Regression and Smoothing Scatterplots Robust Locally Weighted Regression and Smoothing Scatterplots. J. Am. Stat. Assoc. 1979, 74, 829–836. [Google Scholar] [CrossRef]
- Cai, Z.; Jönsson, P.; Jin, H.; Eklundh, L. Performance of smoothing methods for reconstructing NDVI time-series and estimating vegetation phenology from MODIS data. Remote Sens. 2017, 9, 1271. [Google Scholar] [CrossRef] [Green Version]
- Nagendra, H.; Rocchini, D.; Ghate, R.; Sharma, B.; Pareeth, S. Assessing plant diversity in a dry tropical forest: Comparing the utility of landsat and ikonos satellite images. Remote Sens. 2010, 2, 478–496. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 10 June 2021).
- Bai, J.; Perron, P. Computation and analysis of multiple structural change models. J. Appl. Econom. 2003, 18, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Zeileis, A.; Kleiber, C.; Krämer, W.; Hornik, K. Testing and dating of structural changes in practice. Comput. Stat. Data Anal. 2003, 44, 109–123. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Huang, N. Ensemble Empirical Mode Decomposition: A Noise-Assisted Data Analysis Method. Adv. Data Sci. Adapt. Anal. 2009, 1, 1–41. [Google Scholar] [CrossRef]
- Veloso, A.; Mermoz, S.; Bouvet, A.; Le Toan, T.; Planells, M.; Dejoux, J.F.; Ceschia, E. Understanding the temporal behavior of crops using Sentinel-1 and Sentinel-2-like data for agricultural applications. Remote Sens. Environ. 2017, 199, 415–426. [Google Scholar] [CrossRef]
- Vreugdenhil, M.; Wagner, W.; Bauer-Marschallinger, B.; Pfeil, I.; Teubner, I.; Rüdiger, C.; Strauss, P. Sensitivity of Sentinel-1 backscatter to vegetation dynamics: An Austrian case study. Remote Sens. 2018, 10, 1396. [Google Scholar] [CrossRef] [Green Version]
- Mattia, F.; Le Toan, T.; Picard, G.; Posa, F.I.; Alessio, A.D.; Notarnicola, C.; Gatti, A.M.; Rinaldi, M.; Satalino, G. Multitemporal C-Band Radar Measurements on Wheat Fields. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1551–1560. [Google Scholar] [CrossRef]
- Möller, M.; Gerstmann, H.; Gao, F.; Christian, T.; Förster, M. Catena Coupling of phenological information and simulated vegetation index time series: Limitations and potentials for the assessment and monitoring of soil erosion risk. Catena 2017, 150, 192–205. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Lilley, J.M.; Brill, R.D.; Ware, A.H.; Walela, C.K. Field Crops Research The critical period for yield and quality determination in canola (Brassica napus L.). Field Crop. Res. 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Zhang, H.; Flottmann, S. Source-sink manipulations indicate seed yield in canola is limited by source availability. Eur. J. Agron. 2018, 96, 70–76. [Google Scholar] [CrossRef]
- McNairn, H.; Jiao, X.; Pacheco, A.; Sinha, A.; Tan, W.; Li, Y. Estimating canola phenology using synthetic aperture radar. Remote Sens. Environ. 2018, 219, 196–205. [Google Scholar] [CrossRef]
- Younes, N.; Joyce, K.E.; Maier, S.W. All models of satellite-derived phenology are wrong, but some are useful: A case study from northern Australia. Int. J. Appl. Earth Obs. Geoinf. 2021, 97, 102285. [Google Scholar] [CrossRef]





| Wheat | |||||
| DWD label | 12 | 15 | 18 | 21 | 24 |
| BBCH stage | - | Mainshoot | Heading | Ripening | Harvest |
| Dargun: 12615 | - | 10 April 2017 | 4 June 2017 | 23 July 2017 | 6 August 2017 |
| Gülzow: 12563 | - | 20 April 2017 | 3 June 2017 | - | 4 August 2017 |
| Tützpatz: 12508 | - | 2 May 2017 | 31 May 2017 | 12 July 2017 | 4 August 2017 |
| Canola | |||||
| DWD label | 7 | 10 | 12 | 17 | 24 |
| BBCH stage | End of Flowering | Preparing Field | Germation | Bud development | Harvest |
| Dargun: 12615 | 29 May 2017 | 17 August 2017 | 25 August 2017 | 30 March 2017 | 4 August 2017 |
| Gülzow: 12563 | 30 May 2017 | 23 August 2017 | 30 August 2017 | 20 March 2017 | 24 July 2017 |
| Tützpatz: 12508 | 29 May 2017 | 19 August 2017 | 2 September 2017 | 4 April 2017 | 26 July 2017 |
| Feature | Abbreviation | Unit | About |
|---|---|---|---|
| VV coherence | VV Coh | / | InSAR coherence in VV of two consecutive acquisitions * |
| VH coherence | VH Coh | / | InSAR coherence in VH of two consecutive acquisitions * |
| Alpha | ALP | Degree [°] | Relation of VV and VH simulating the dominant scattering mechanism |
| Entropy | ENT | / | Degree of depolarisation |
| Kennaugh element K0 | K0 | dB | Combined backscatter (VV + VH) |
| Kennaugh element K1 | K1 | dB | Difference in backscatter (VV − VH) |
| Kennaugh element K5 | K5 | dB | Real part of inter-channel correlation between VV and VH |
| Kennaugh element K6 | K6 | dB | Imaginary part of inter-channel-correlation between VV and VH |
| VV backscatter | VV | dB | Gamma nought VV intensity in dB |
| VH backscatter | VH | dB | Gamma nought VH intensity in dB |
| Phenological Stages | Median BBCH Value at Breakpoint | Breakpoint Producing SAR Features |
|---|---|---|
| Stem elongation | 31 | VH coh, ALP, ENT, K0, K1, K5, K6 VH int, VV int |
| Stem elongation | 33 | VV coh, VV int |
| Inflorescence/Heading | 57 | K0, K1, K5, K6, VH int |
| Flowering | 69 | VH coh, ALP |
| Fruit development | 71 | VV int |
| Ripening | 83 | K0, K1, K5, K6, VH int |
| Ripening | 85 | VH coh, VV coh, ALP |
| Senescence | 93 | VH coh, K0, K1, K5, K6 |
| Senescence | 99 | VV coh, ALP, ENT |
| Phenological Stage | Median BBCH Value | SAR Feature Producing Minimum | SAR Feature Producing Maximum |
|---|---|---|---|
| Est. Tillering | - | VH coh | |
| Stem elongation | 31 | VH coh | VH int, VV int |
| Fruit development | 70 | - | VV int, K0 |
| Fruit development | 71 | VH int | - |
| Fruit development | 73 | VV int, K0 | - |
| Ripening | 85 | - | VV int |
| Ripening | 86 | VV coh | VH int, K0 |
| Senescence | 93 | VH int, VV int, K0 | VH int |
| Phenological Stages | Median BBCH Value at Breakpoint | Breakpoint Producing SAR Features |
|---|---|---|
| Flowering | 60 | ALP, ENT, K1, K5, VV int |
| Flowering | 65 | K6, VH int |
| Flowering | 67 | VH coh, VV coh, K1, VV int |
| Fruit development | 74 | ALP, ENT |
| Est. harvest | - | VH coh, VV coh, ALP |
| Harvested | - | K0, K1, K5, VV int, VH int |
| Harvested, secondary Vegetation | - | VH coh, VV coh, ALP, ENT, K0, VH int, VV int |
| Phenological Stage | Median BBCH Value | SAR Feature Producing Minimum | SAR Feature Producing Maximum |
|---|---|---|---|
| Flowering: | 60 | - | K0, VH int |
| Flowering: | 67 | K0, VH int | - |
| Fruit development: | 72 | VH int | K1 |
| Est. harvest | - | - | K1, ALP |
| Harvest | - | VH coh, VV coh, K0, VH int, VV int | - |
| Secondary vegetation | VH coh, K0, VH int, VV int | - | - |
| Phenological Stages | Median BBCH Value at Breakpoint | Breakpoint Producing SAR Features |
|---|---|---|
| Leaf development | 10 | VH coh, VV coh, K6 |
| Leaf development | 12 | ALP, ENT, K0, K1, VH int, VV int |
| Leaf development | 19 | VH coh, VV coh, ALP, ENT, K6 |
| Rosette development | 33 | K0, K1, K5, VV int, VH int |
| Rosette development | 39 | VV coh, ALP, ENT, K0, K1, K5, K6, VV int, VH int |
| Phenological Stage | Median BBCH Value | SAR Feature Producing Minimum | SAR Feature Producing Maximum |
|---|---|---|---|
| Leaf development | 10 | - | VH coh, VV coh |
| Leaf development | 12 | - | K0, VH int, VV int |
| Leaf development | 17 | K0, VH int, VV int | - |
| Leaf development | 19 | VH coh, VV coh | ALP, ENT |
| Rosette development | 33 | - | K0, K1, VH int, VV int |
| Rosette development | 39 | K0, VH int | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Löw, J.; Ullmann, T.; Conrad, C. The Impact of Phenological Developments on Interferometric and Polarimetric Crop Signatures Derived from Sentinel-1: Examples from the DEMMIN Study Site (Germany). Remote Sens. 2021, 13, 2951. https://doi.org/10.3390/rs13152951
Löw J, Ullmann T, Conrad C. The Impact of Phenological Developments on Interferometric and Polarimetric Crop Signatures Derived from Sentinel-1: Examples from the DEMMIN Study Site (Germany). Remote Sensing. 2021; 13(15):2951. https://doi.org/10.3390/rs13152951
Chicago/Turabian StyleLöw, Johannes, Tobias Ullmann, and Christopher Conrad. 2021. "The Impact of Phenological Developments on Interferometric and Polarimetric Crop Signatures Derived from Sentinel-1: Examples from the DEMMIN Study Site (Germany)" Remote Sensing 13, no. 15: 2951. https://doi.org/10.3390/rs13152951
APA StyleLöw, J., Ullmann, T., & Conrad, C. (2021). The Impact of Phenological Developments on Interferometric and Polarimetric Crop Signatures Derived from Sentinel-1: Examples from the DEMMIN Study Site (Germany). Remote Sensing, 13(15), 2951. https://doi.org/10.3390/rs13152951







