Combining Tracking and Remote Sensing to Identify Critical Year-Round Site, Habitat Use and Migratory Connectivity of a Threatened Waterbird Species
Abstract
:1. Introduction
2. Materials and Methods
3. Results
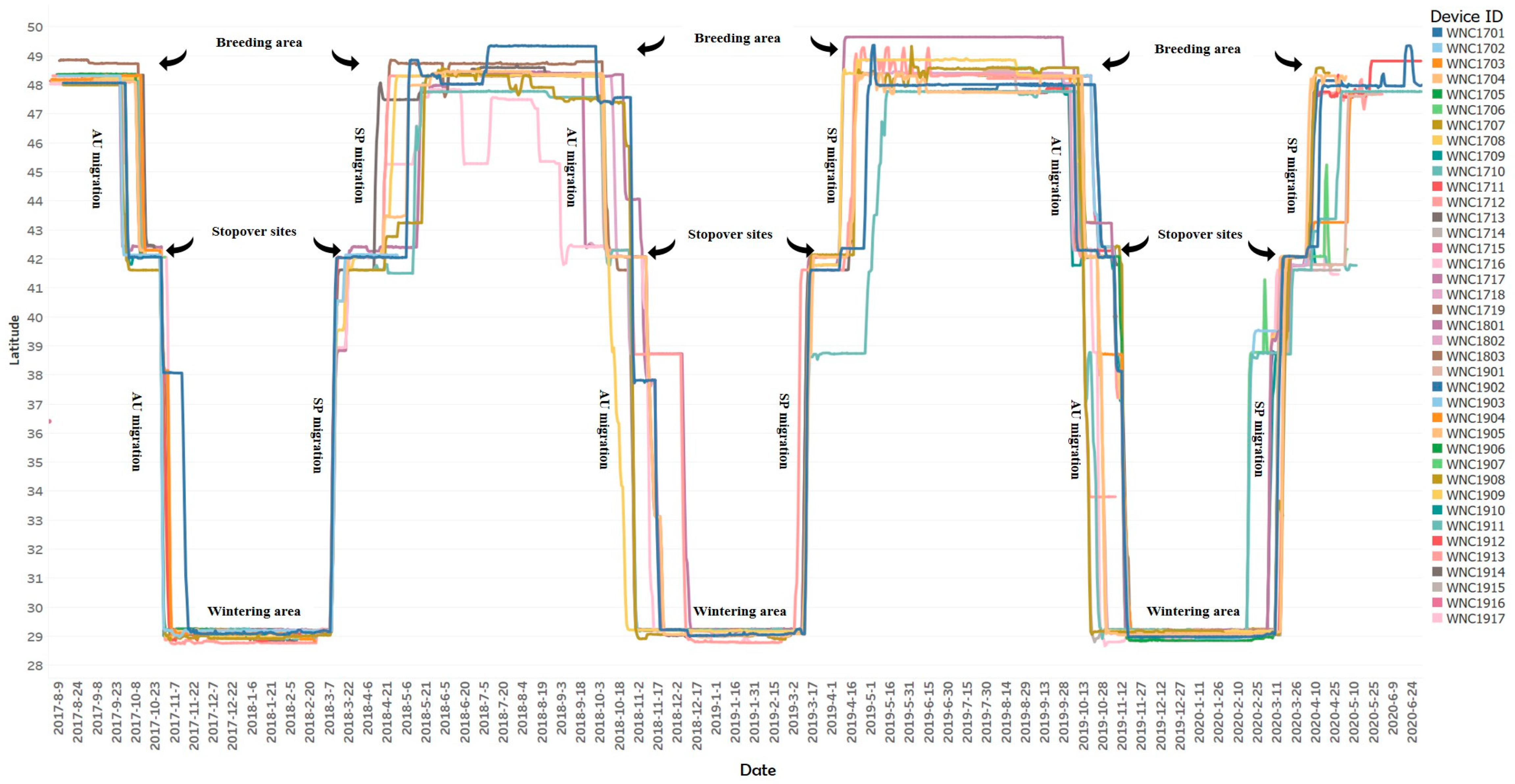
3.1. Migration Patterns
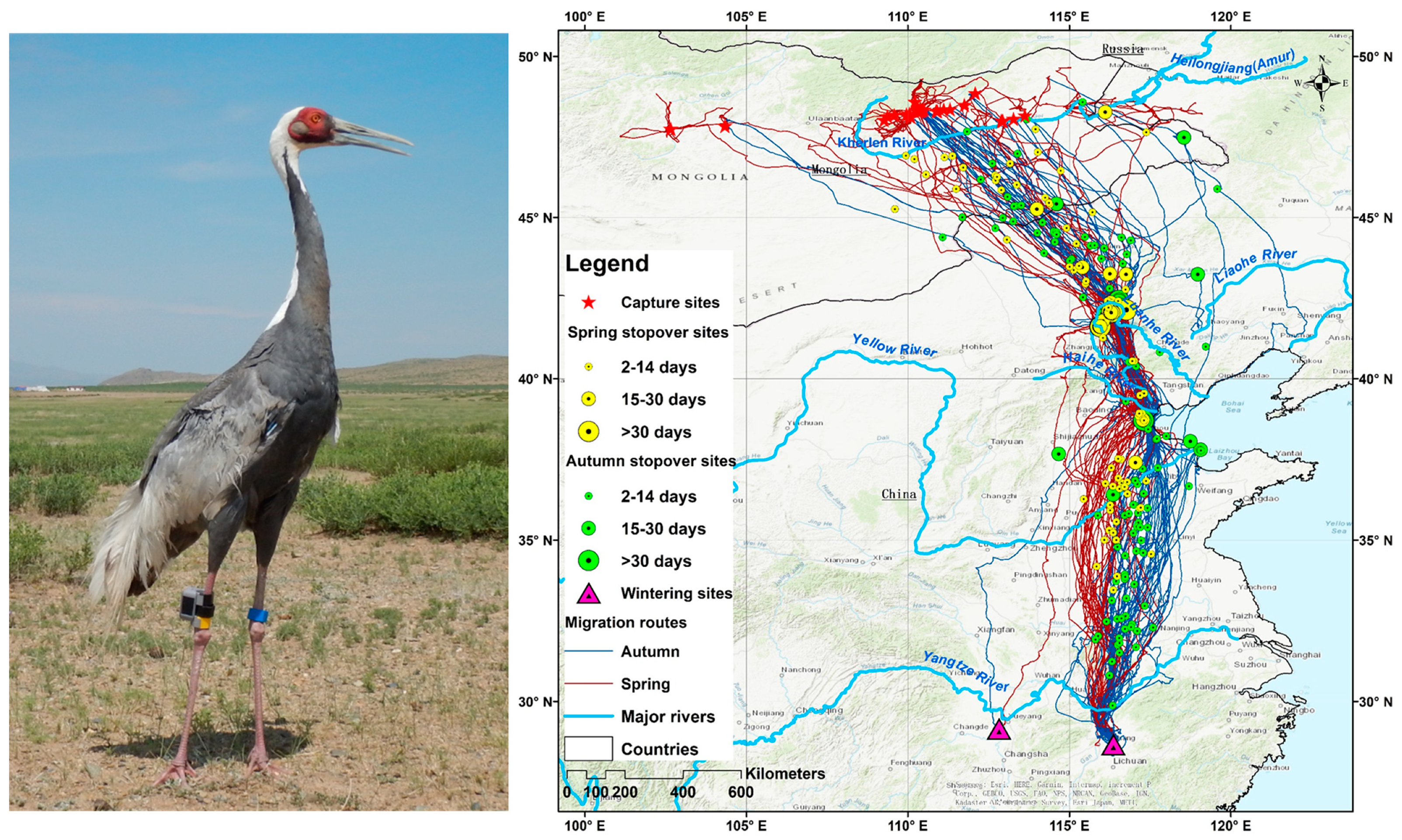
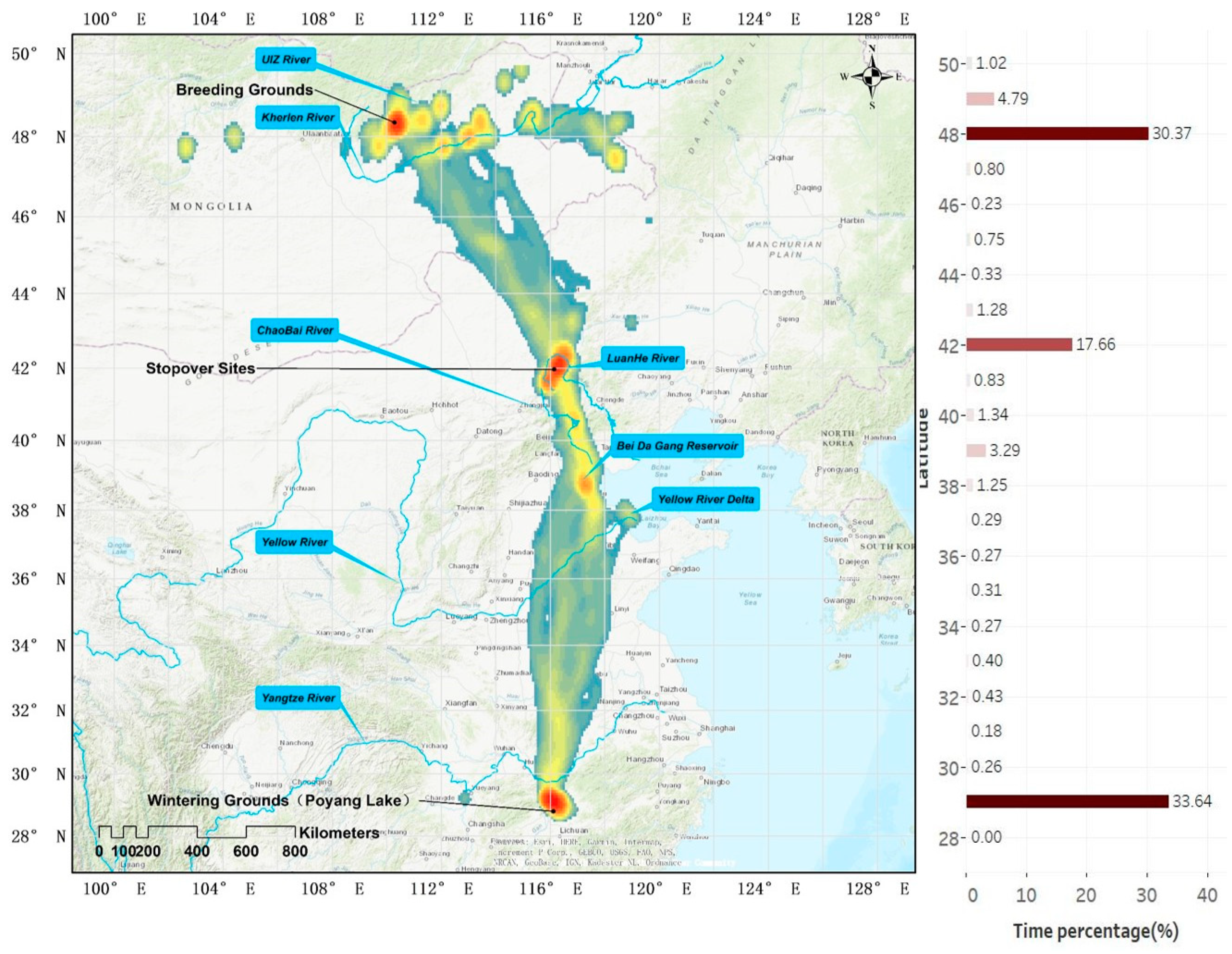
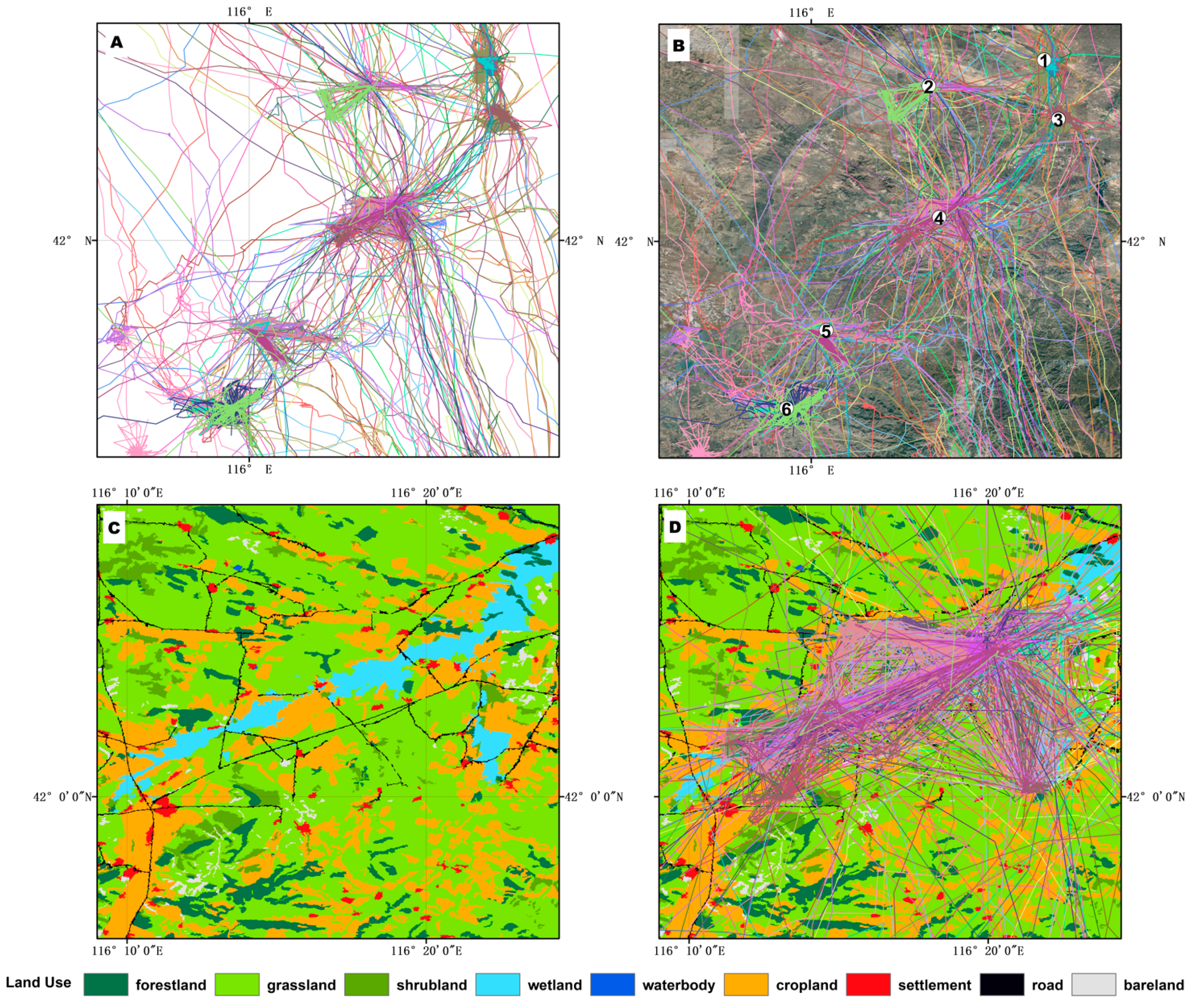
3.2. Migration Routes and Critical Stopover Areas
3.3. Crane Habitat Use
3.4. Protected Area Status of Crane Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.B.; Taylor, R.; Saracco, J.F.; Helton, L.; Stock, S. GPS-tracking reveals non-breeding locations and apparent molt migration of a Black-headed Grosbeak. J. Field Ornithol. 2016, 87, 196–203. [Google Scholar] [CrossRef]
- McGowan, J.; Beger, M.; Lewison, R.L.; Harcourt, R.; Campbell, H.; Priest, M.; Dwyer, R.G.; Lin, H.Y.; Lentini, P.; Dudgeon, C.; et al. Integrating research using animal-borne telemetry with the needs of conservation management. J. Appl. Ecol. 2017, 54, 423–429. [Google Scholar] [CrossRef]
- Carter, M.I.D.; Cox, S.L.; Scales, K.L.; Bicknell, A.W.J.; Nicholson, M.D.; Atkins, K.M.; Morgan, G.; Morgan, L.; Grecian, W.J.; Patrick, S.C.; et al. GPS tracking reveals rafting behaviour of Northern Gannets (Morus bassanus): Implications for foraging ecology and conservation. Bird Study 2016, 63, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Alerstam, T.; Backman, J. Ecology of animal migration. Curr. Biol. 2018, 28, R968–R972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, W.; Martinuzzi, S.; Estes, A.B.; Pidgeon, A.M.; Dettki, H.; Ericsson, G.; Radeloff, V.C. Opportunities for the application of advanced remotely-sensed data in ecological studies of terrestrial animal movement. Mov. Ecol. 2015, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, J.; Krause, S.; Arlinghaus, R.; Psorakis, I.; Roberts, S.; Rutz, C. Reality mining of animal social systems. Trends Ecol. Evol. 2013, 28, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.J.; Taylor, L.A.; Benhamou, S.; Bijleveld, A.I.; Clay, T.A.; de Grissac, S.; Demsar, U.; English, H.M.; Franconi, N.; Gomez-Laich, A.; et al. Optimizing the use of biologgers for movement ecology research. J. Anim. Ecol. 2020, 89, 186–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevanda, M.; Horning, N.; Reineking, B.; Heurich, M.; Wegmann, M.; Mueller, J. Adding structure to land cover-using fractional cover to study animal habitat use. Mov. Ecol. 2014, 2, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuenzer, C.; Ottinger, M.; Wegmann, M.; Guo, H.; Wang, C.; Zhang, J.; Dech, S.; Wikelski, M. Earth observation satellite sensors for biodiversity monitoring: Potentials and bottlenecks. Int. J. Remote Sens. 2014, 35, 6599–6647. [Google Scholar] [CrossRef] [Green Version]
- Pimm, S.L.; Alibhai, S.; Bergl, R.; Dehgan, A.; Giri, C.; Jewell, Z.; Joppa, L.; Kays, R.; Loarie, S. Emerging technologies to conserve biodiversity. Trends Ecol. Evol. 2015, 30, 685–696. [Google Scholar] [CrossRef]
- Wetlands International. Waterbird Population Estimates. Wageningen, The Netherland Wetlands International, 2012. Available online: https://www.wetlands.org/publications/waterbird-populations-estimates-fifth-edition/ (accessed on 8 October 2021).
- Fujita, G.; Guan, H.L.; Ueta, M.; Goroshko, O.; Krever, V.; Ozaki, K.; Mita, N.; Higuchi, H. Comparing areas of suitable habitats along travelled and possible shortest routes in migration of White-naped Cranes Grus vipio in East Asia. Ibis 2004, 146, 461–474. [Google Scholar] [CrossRef]
- Higuchi, H.; Ozaki, K.; Fijita, G.; Minton, J.; Ueta, M.; Soma, M.; Mita, N. Satellite tracking of White-naped crane migration and the importance of the Korean demilitarized zone. Conserv. Biol. 1996, 10, 806–812. [Google Scholar] [CrossRef]
- Higuchi, H.; Pierre, J.P.; Krever, V.; Andronov, V.; Fujita, G.; Ozaki, K.; Goroshko, O.; Ueta, M.; Smirensky, S.; Mita, N. Using a remote technology in conservation: Satellite tracking White-naped Cranes in Russia and Asia. Conserv. Biol. 2004, 18, 136–147. [Google Scholar] [CrossRef]
- Harris, J.; Mirande, C. A global overview of cranes: Status, threats and conservation priorities. Chin. Birds 2013, 4, 189–209. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, M.; Buuveibaatar, B.; Fine, A.E.; Jambal, L.; Strindberg, S. Declining breeding populations of White-naped Cranes in Eastern Mongolia, a ten-year update. Bird Conserv. Int. 2016, 26, 490–504. [Google Scholar] [CrossRef]
- Harcourt, R.; Sequeira, A.M.M.; Zhang, X.L.; Roquet, F.; Komatsu, K.; Heupel, M.; McMahon, C.; Whoriskey, F.; Meekan, M.; Carroll, G.; et al. Animal-Borne Telemetry: An Integral Component of the Ocean Observing Toolkit. Front. Mar. Sci. 2019, 6, 326. [Google Scholar] [CrossRef] [Green Version]
- Sinnott, R.W. Virtues of the Haversine. Sky Telesc. 1984, 68, 159. [Google Scholar]
- Hijmans, R.; Williams, E.; Vennes, C. Geosphere: Spherical trigonometry. R Package Version 1.5. Available online: https://cran.r-project.org/web/packages/geosphere/geosphere.pdf (accessed on 8 October 2021).
- van Wijk, R.E.; Kolzsch, A.; Kruckenberg, H.; Ebbinge, B.S.; Muskens, G.; Nolet, B.A. Individually tracked geese follow peaks of temperature acceleration during spring migration. Oikos 2012, 121, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cao, L.; Bysykatova, I.; Xu, Z.G.; Rozenfeld, S.; Jeong, W.; Vangeluwe, D.; Zhao, Y.L.; Xie, T.H.; Yi, K.P.; et al. The Far East taiga forest unrecognized inhospitable terrain for migrating Arctic-nesting waterbirds? Peerj 2018, 6, e4353. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Q.; Fang, L.; Xu, Z.; Wang, X.; He, H.; Cao, L.; Fox, A.D. Spring migration duration exceeds that of autumn migration in Far East Asian Greater White-fronted Geese (Anser albifrons). Avian Res. 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Kolzsch, A.; Muskens, G.; Kruckenberg, H.; Glazov, P.; Weinzierl, R.; Nolet, B.A.; Wikelski, M. Towards a new understanding of migration timing: Slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 2016, 125, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dobra, A. Measuring human activity spaces from GPS data with density ranking and summary curves. Ann. Appl. Stat. 2020, 14, 409–432. [Google Scholar] [CrossRef] [Green Version]
- Barraquand, F.; Benhamou, S. animal movements in heterogeneous landscapes: Identifying profitable places and homogeneous movement bouts. Ecology 2008, 89, 3336–3348. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, M.; Dussault, C.; Côté, S.D. Detecting changes in the annual movements of terrestrial migratory species: Using the first-passage time to document the spring migration of caribou. Mov. Ecol. 2014, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edelhoff, H.; Signer, J.; Balkenhol, N. Path segmentation for beginners: An overview of current methods for detecting changes in animal movement patterns. Mov. Ecol. 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavielle, M. Using penalized contrasts for the change-point problem. Signal Process. 2005, 85, 1501–1510. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Liu, H.; Zhang, M.N.; Li, C.C.; Wang, J.; Huang, H.B.; Clinton, N.; Ji, L.Y.; Li, W.Y.; Bai, Y.Q.; et al. Stable classification with limited sample: Transferring a 30-m resolution sample set collected in 2015 to mapping 10-m resolution global land cover in 2017. Sci. Bull. 2019, 64, 370–373. [Google Scholar] [CrossRef] [Green Version]
- Lang, X.; Gankhuyag Purev, O.; Oyunchimeg, T.; Dashdorj, K.; Baasansuren, E.; Amarkhuu, G.; Mi, C.; Guo, Y. Luan River upper reaches: The important stopover site of the white-naped crane (Grus vipio) western population. Biodivers. Sci. 2020, 28, 1213–1221. [Google Scholar] [CrossRef]
- Harris, J.; Liying, S.; Higuchi, H.; Ueta, M.; Zhengwang, Z.; Yanyun, Z.; Xijun, N. Migratory stopover and wintering locations in eastern China used by White-naped Cranes Grus vipio and Hooded Cranes, G. monacha, as determined by satellite tracking. Forktail 2000, 16, 93–100. [Google Scholar]
- Sun, C.Z.; Zhen, L.; Wang, C.; Yan, B.Y.; Cao, X.C.; Wu, R.Z. Impacts of ecological restoration and human activities on habitat of overwintering migratory birds in the wetland of Poyang Lake, Jiangxi Province, China. J. Mt. Sci. 2015, 12, 1302–1314. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Cao, L.; de Boer, W.F.; Fox, A.D. Wintering Swan Geese maximize energy intake through substrate foraging depth when feeding on buried Vallisneria natans tubers. Avian Res. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.; Li, H.; Wang, X.; Fang, L.; Li, X.; Cao, L.; Fox, A.D. Size matters: Wintering ducks stay longer and use fewer habitats on largest Chinese lakes. Avian Res 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Dong, B.; Zhu, M.; Huang, H.; Cui, Y.-h.; Gao, X.; Liu, L.-P. Habitat selection of wintering cranes (Gruidae) in typical lake wetland in the lower reaches of the Yangtze River, China. Environ. Sci. Pollut. Res. 2019, 26, 8266–8279. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, L.E.I.; Barter, M.; Fox, A.D.; Zhao, M.; Meng, F.; Shi, H.; Jiang, Y.; Zhu, W. Changing distribution and abundance of Swan Goose Anser cygnoides in the Yangtze River floodplain: The likely loss of a very important wintering site. Bird Conserv. Int. 2010, 21, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.J.; Shankman, D.; Huber, C.; Yesou, H.; Huang, Q.; Jiang, J.H. Sand mining and increasing Poyang Lake’s discharge ability: A reassessment of causes for lake decline in China. J. Hydrol. 2014, 519, 1698–1706. [Google Scholar] [CrossRef]
- Guo, H.; Hu, Q.; Zhang, Q.; Feng, S. Effects of the Three Gorges Dam on Yangtze River flow and river interaction with Poyang Lake, China: 2003–2008. J. Hydrol. 2012, 416, 19–27. [Google Scholar] [CrossRef]
- Mei, X.; Dai, Z.; Du, J.; Chen, J. Linkage between Three Gorges Dam impacts and the dramatic recessions in China’s largest freshwater lake, Poyang Lake. Sci. Rep. 2015, 5, 18197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Li, L.; Wang, Y.G.; Werner, A.D.; Xin, P.; Jiang, T.; Barry, D.A. Has the Three-Gorges Dam made the Poyang Lake wetlands wetter and drier? Geophys. Res. Lett. 2012, 39, L20402. [Google Scholar] [CrossRef] [Green Version]
- Jiao, L. CHINA Scientists line up against Dam that would alter protected wetlands. Science 2009, 326, 508–509. [Google Scholar] [CrossRef] [PubMed]
- An, A.; Cao, L.; Jia, Q.; Wang, X.; Zhu, Q.; Zhang, J.; Ye, X.; Gao, D. Changing abundance and distribution of the wintering swan goose Anser cygnoides in the Middle and Lower Yangtze River floodplain: An investigation combining a field survey with satellite telemetry. Sustainability 2019, 11, 1398. [Google Scholar] [CrossRef] [Green Version]
- Aharon-Rotman, Y.; McEvoy, J.; Zheng, Z.J.; Yu, H.; Wang, X.; Si, Y.L.; Xu, Z.G.; Yuan, Z.; Jeong, W.; Cao, L.; et al. Water level affects availability of optimal feeding habitats for threatened migratory waterbirds. Ecol. Evol. 2017, 7, 10440–10450. [Google Scholar] [CrossRef]
- Amano, T.; Szekely, T.; Koyama, K.; Amano, H.; Sutherland, W.J. A framework for monitoring the status of populations: An example from wader populations in the East Asian-Australasian flyway. Biol. Conserv. 2010, 143, 2238–2247. [Google Scholar] [CrossRef]
- Martin, G.R.; Shaw, J.M. Bird collisions with power lines: Failing to see the way ahead? Biol. Conserv. 2010, 143, 2695–2702. [Google Scholar] [CrossRef]
- Stehn, T.V.; Wassenich, T. Whooping crane collisions with power lines: An issue paper. Proc. North Am. Crane Workshop 2008, 10, 25–36. [Google Scholar]
- Tacha, T.; Martin, D.; Endicott, C. Mortality of Sandhill Cranes associated with utility highlines in Texas. In Proceedings of the 1978 North American Crane Workshop, Rockport, TX, USA, 6–8 December 1979. [Google Scholar]
- Swaddle, J.P.; Moseley, D.L.; Hinders, M.K.; Smith, E.P. A sonic net excludes birds from an airfield: Implications for reducing bird strike and crop losses. Ecol. Appl. 2016, 26, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Gasteren, H.; Krijgsveld, K.L.; Klauke, N.; Leshem, Y.; Metz, I.C.; Skakuj, M.; Sorbi, S.; Schekler, I.; Shamoun-Baranes, J. Aeroecology meets aviation safety: Early warning systems in Europe and the Middle East prevent collisions between birds and aircraft. Ecography 2019, 42, 899–911. [Google Scholar] [CrossRef]
- Blackwell, B.F.; Seamans, T.W.; Fernandez-Juricic, E.; Devault, T.L.; Outward, R.J. Avian responses to aircraft in an airport environment. J. Wildl. Manag. 2019, 83, 893–901. [Google Scholar] [CrossRef]
- Shergalin, J.E.; Keskpaik, J.; Kuznetsov, G.A. The Common Crane with its migration conditions and as a hazard to aircraft in Estonia. In Crane Research and Protection in Europe; Prange, H., Ed.; Martin-Luther Universität: Halle-Wittenberg, Germany, 1995; pp. 165–169. [Google Scholar]
- Navarrete, L.; Griffis-Kyle, K.L. Sandhill Crane collisions with wind turbines in Texas. In Proceedings of the Twelfth North American Crane Workshop, Grand Island, NE, USA, 13–16 March 2011; 2016; pp. 65–67. [Google Scholar]
- BirdLife International. Species factsheet: Grus vipio. 2021. Available online: http://www.birdlife.org (accessed on 30 August 2021).
- Nilsson, C.; Klaassen, R.H.G.; Alerstam, T. Differences in speed and duration of bird migration between spring and autumn. Am. Nat. 2013, 181, 837–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Christie, M.; Coleman, J.; Hassell, C.; Gosbell, K.; Lisovski, S.; Minton, C.; Klaassen, M. Time versus energy minimization migration strategy varies with body size and season in long-distance migratory shorebirds. Mov. Ecol. 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Benhamou, S. How to reliably estimate the tortuosity of an animal’s path: Straightness, sinuosity, or fractal dimension? J. Theor. Biol. 2004, 229, 209–220. [Google Scholar] [CrossRef]
- Batschelet, E. Circular Statistics in Biology. Technometrics 1981, 24, 336. [Google Scholar]
| Parameter | Definitions | Reference |
|---|---|---|
| Departure date | The date of the first position when a bird left wintering/moulting/breeding sites and was judged by the methods described in the text to have acquired flight status. | [21] |
| Arrival date | The date when a bird was determined to have arrived at wintering or breeding sites, based on the methods described in the text to qualify as “non-flight” status after a period of flight. | [21] |
| Migration duration | The time a bird took to travel (including stopovers) between the last summering site and first wintering site (autumn migration) or between the last wintering and the first summering site (spring migration). | [22] |
| Migration distance | The cumulative travel distance between all adjacent GPS locations which were defined as “flight” status during migration, the distance between two adjacent GPS locations. | [22,23] |
| Migration speed | The migration speed is defined as the total distance (in kilometres) divided by the total duration of migration in days. | [24] |
| Travel speed | The daily travel speed is the migration distance divided by total travel days. | [24] |
| Travel duration | Travel days is the total duration of migration (days) minus stopover days. | [24] |
| Stopover duration | The sum of all days spent at all stopover sites during each migration season. | [24] |
| Migration bout length | Calculated as the migration distance divided by the number of flight legs during each migration season. | [25] |
| Number of Stopovers | Sites where birds rested and fed during migration for more than 2 days. | [23] |
| Straight index | When the goal is located at a finite distance D from the starting point, and the animal reaches it after having travelled some variable path length L; the orientation efficiency is often empirically measured as the ratio D/L; referred to as the straightness index. | [26,27] |
| Parameter | Mean Value ± SD for Autumn Migration | Mean Value ± SD for Spring Migration | Df | t | p-Value |
|---|---|---|---|---|---|
| Migration duration (Days) | 34.7 ± 14.2 | 48.8 ± 15.4 | 75.6 | 3.7 | <0.001 |
| Migration distance (km) | 2555.9 ± 187.9 | 2673.2 ± 342.3 | 50.2 | 1.9 | 0.062 |
| Migration speed (km/Days) | 86.4 ± 40.9 | 60.9 ± 22.2 | 39.3 | −3.0 | 0.004 |
| Travel speed (km/Days) | 310.6 ± 100.0 | 296.2 ± 88.1 | 47.7 | −0.7 | 0.478 |
| Travel duration (Days) | 9.1 ± 3.7 | 9.9 ± 4.0 | 46.0 | 1.0 | 0.339 |
| Stopover duration (Days) | 25.5 ± 13.4 | 38.8 ± 14.8 | 77.7 | 3.6 | <0.001 |
| Migration bout length (km) | 1013.3 ± 224.2 | 1045.0 ± 343.3 | 72.6 | 1.6 | 0.107 |
| Number of stopovers | 1.6 ± 0.6 | 1.8 ± 0.9 | 73.2 | −0.2 | 0.837 |
| Straightness index | 0.9 ± 0.1 | 0.8 ± 0.1 | 80.0 | −2.1 | 0.037 |
| Habitat Types | Breeding | Staging Stopover | Wintering | Total |
|---|---|---|---|---|
| Cropland | 0.65% | 22.63% | 32.30% | 20.72% |
| Forest | 0.04% | 0.77% | 0.03% | 0.24% |
| Grassland | 89.29% | 68.62% | 0.25% | 44.29% |
| Wetland | 4.65% | 1.84% | 17.76% | 9.65% |
| Water | 3.56% | 5.52% | 49.64% | 24.41% |
| Impervious surface | 0.19% | 0.44% | 0.02% | 0.19% |
| Bare substrate | 1.55% | 0.16% | 0.00% | 0.48% |
| Snow/ice | 0.07% | 0.00% | 0.00% | 0.02% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batbayar, N.; Yi, K.; Zhang, J.; Natsagdorj, T.; Damba, I.; Cao, L.; Fox, A.D. Combining Tracking and Remote Sensing to Identify Critical Year-Round Site, Habitat Use and Migratory Connectivity of a Threatened Waterbird Species. Remote Sens. 2021, 13, 4049. https://doi.org/10.3390/rs13204049
Batbayar N, Yi K, Zhang J, Natsagdorj T, Damba I, Cao L, Fox AD. Combining Tracking and Remote Sensing to Identify Critical Year-Round Site, Habitat Use and Migratory Connectivity of a Threatened Waterbird Species. Remote Sensing. 2021; 13(20):4049. https://doi.org/10.3390/rs13204049
Chicago/Turabian StyleBatbayar, Nyambayar, Kunpeng Yi, Junjian Zhang, Tseveenmyadag Natsagdorj, Iderbat Damba, Lei Cao, and Anthony David Fox. 2021. "Combining Tracking and Remote Sensing to Identify Critical Year-Round Site, Habitat Use and Migratory Connectivity of a Threatened Waterbird Species" Remote Sensing 13, no. 20: 4049. https://doi.org/10.3390/rs13204049
APA StyleBatbayar, N., Yi, K., Zhang, J., Natsagdorj, T., Damba, I., Cao, L., & Fox, A. D. (2021). Combining Tracking and Remote Sensing to Identify Critical Year-Round Site, Habitat Use and Migratory Connectivity of a Threatened Waterbird Species. Remote Sensing, 13(20), 4049. https://doi.org/10.3390/rs13204049







