1. Introduction
New tools and technology are needed to understand the transport of hazardous agents and track them in our oceans and waterways [
1]. Such technology can be used to determine when people can safely return to their homes and work after a natural disaster and when it is safe for first responders to conduct rescue and recovery efforts in and around contaminated floodwaters. Modeling efforts based on the identification or prediction of hazards [
2,
3] can be used to prioritize the deployment of unmanned assets, positioning unmanned systems at the right time and place to do dull, dirty, and dangerous missions [
4].
Powers et al. [
5] showed the value of coordinated unmanned systems in the air and water to track the plume of a fluorescent dye. A small quadcopter was used to capture images of the plume, and heatmaps were generated from drone images to estimate plume concentrations. An unmanned surface vehicle (USV) equipped with an onboard fluorometer was used to provide real-time measurements of the dye in the plume while the drone was capturing the images. Other studies have found utility in the use of unmanned aerial systems (UASs) to estimate loads of hazardous agents such as harmful algal blooms (HABs) [
6]. Consumer-grade drones, or UASs, have been increasingly used in oceanography over the past two decades, with technological advances allowing light-weight sensors and instruments to be mounted on small UAS [
7]. The U.S. Integrated Ocean Observing System (IOOS) has been incorporating measurements from UAS-mounted sensors for over 15 years [
8]. UASs have also been used in coral ecology [
9], hydrography [
10,
11,
12,
13] and to track pathogens in the air [
14]. Benson et al. [
15] developed a DrOne Water Sampling SystEm (DOWSE) to study microorganisms in freshwater habitats. This system This system consisted of a 3D-printed sampling device tethered to a drone, and was used to collect water samples at the surface of eight different lakes in Austria.
Powers et al. [
5] used an UAS to detect an artificial dye plume (fluorescein) in a freshwater lake. The UAS images were used
a posteriori to construct the heat maps and were compared to concentration measurements collected from a fluorescence sensor mounted on the USV. The heat maps were generated from the images’ red, blue and green (RGB) decomposition, using the fluorescein RGB value of (100, 200, 60) as a reference. The images were scaled as a percentage of this maximum intensity and then scaled based on minimum and maximum dye concentrations from the USV fluorescein sensor. Extending this method to the open ocean remains challenging, however, in part due to the sun reflection induced by the surface wave state, a problem often encountered in the literature [
16,
17]. Zeng et al. [
17] investigated how images acquired with UAS can be used to evaluate water quality and which environmental variables affected the results; they found that surface wave height and sun glint were particularly challenging and a significant factor in the accuracy of regression models. The wave height can also be an obstacle to UAS navigation. Flying at higher altitudes is much safer and more economical than having to lower the drone close to the ocean surface (<10 m). Moreover, open ocean plume studies could find unique applications from swarms of UASs tracking plumes at higher altitudes (>100 m). To our knowledge, the effect of altitude on the accuracy of dye concentration heat maps has not yet been assessed in detail. Another barrier to UAS-based plume studies is the cost associated with photogrammetric processing of the images; though commercial software are available, such as Agisoft PhotoScan used in [
9,
16], their cost is a significant barrier to widespread adoption.
The present study took place as part of a larger project studying Lagrangian dynamics in the coastal ocean. Two plumes of fluorescent rhodamine WT dye were released south of Martha’s Vineyard, Massachusetts, concurrently with surface and subsurface drifters. Rhodamine WT was chosen as a surrogate hazardous agent because of its low environmental impact. Field et al. [
18] studied the ecotoxicity of fluorescent dyes, including Rhodamine WT, and found low levels of concern for concentrations under
mg
L
, a threshold orders of magnitude higher than the concentrations used in this study. Earlier work by Parker [
19] tested the toxicity of Rhodamine WT dye on the larval development of oysters and on juvenile salmon and trout; with concentrations up to
mg
L
for oysters and
mg
L
for fish, no mortalities or abnormalities were observed. Dye plumes are frequently released to conduct dispersion studies in oceanography [
20,
21,
22]. In the past few years, several studies investigated how to estimate concentrations of rhodamine dye using images from a small UAS. Legleiter et al. [
23] stirred dye in experimental tanks to evaluate the potential to map dye patterns via remote sensing in turbid rivers. Baek et al. [
24] released dye in an experimental channel and built artificial neural networks to construct the relationship between UAS images and dye concentrations. Both studies were conducted from land, and ground control points (GCPs) were used for altitude calibration and orientation of the UAS. To our knowledge, these investigations have not yet been extended to a coastal or open ocean environment, where the field conditions present significant challenges. The deep learning approach of Baek et al. [
24], for example, required high volumes of images to build reliable neural networks. The authors were able to record between 13,440 and 23,688 frames per experiment, but these high numbers are much harder to obtain when launching UAS from a research vessel in the open ocean.
In this paper, we describe a unique study using drones to examine the transport of a kilometer-scale dye release in an open ocean environment, where the field conditions presented significant challenges. A drone water-sampling system was used to sample and image rhodamine dye plume released in the Atlantic Ocean in 2018. Images of the dye plume were taken, and rhodamine concentrations were determined onboard the research vessel using a fluorometer immediately after the samples were collected. The specific objectives of this study were to: (1) track the movement of a rhodamine dye plume within three hours following a rhodamine dye release in situ using measurements from unmanned systems, (2) estimate concentrations of rhodamine dye from post-processed drone images taken at the sea surface and at altitude, and without using expensive commercial software, and (3) compare concentrations of rhodamine dye among drone water samples and drone-image estimation methods. Since the images were taken from a UAS, standard homography methods could not be applied because the sea surface was photographed from different angles and altitudes.
2. Materials and Methods
2.1. Rhodamine Dye Release
A kilometer-scale Rhodamine dye release was planned and then carried out on 16 August 2018, south of Martha’s Vineyard in Massachusetts, USA. The specific location was chosen because real-time forecasts predicted the occurrence of a salinity intrusion around this location. The output from the forecasts for the Martha’s Vineyard shelf, along with ocean physics data, including temperature and salinity conditions, as well as the atmospheric and tidal forcing that were assimilated in the models, can be found at
http://mseas.mit.edu/Sea_exercises/NSF_ALPHA/2018/ (accessed on 15 October 2021). Drifter trajectories that were in the vicinity in of the study site during the release indicated an average mean surface velocity of
.
The location of the plume study is shown in
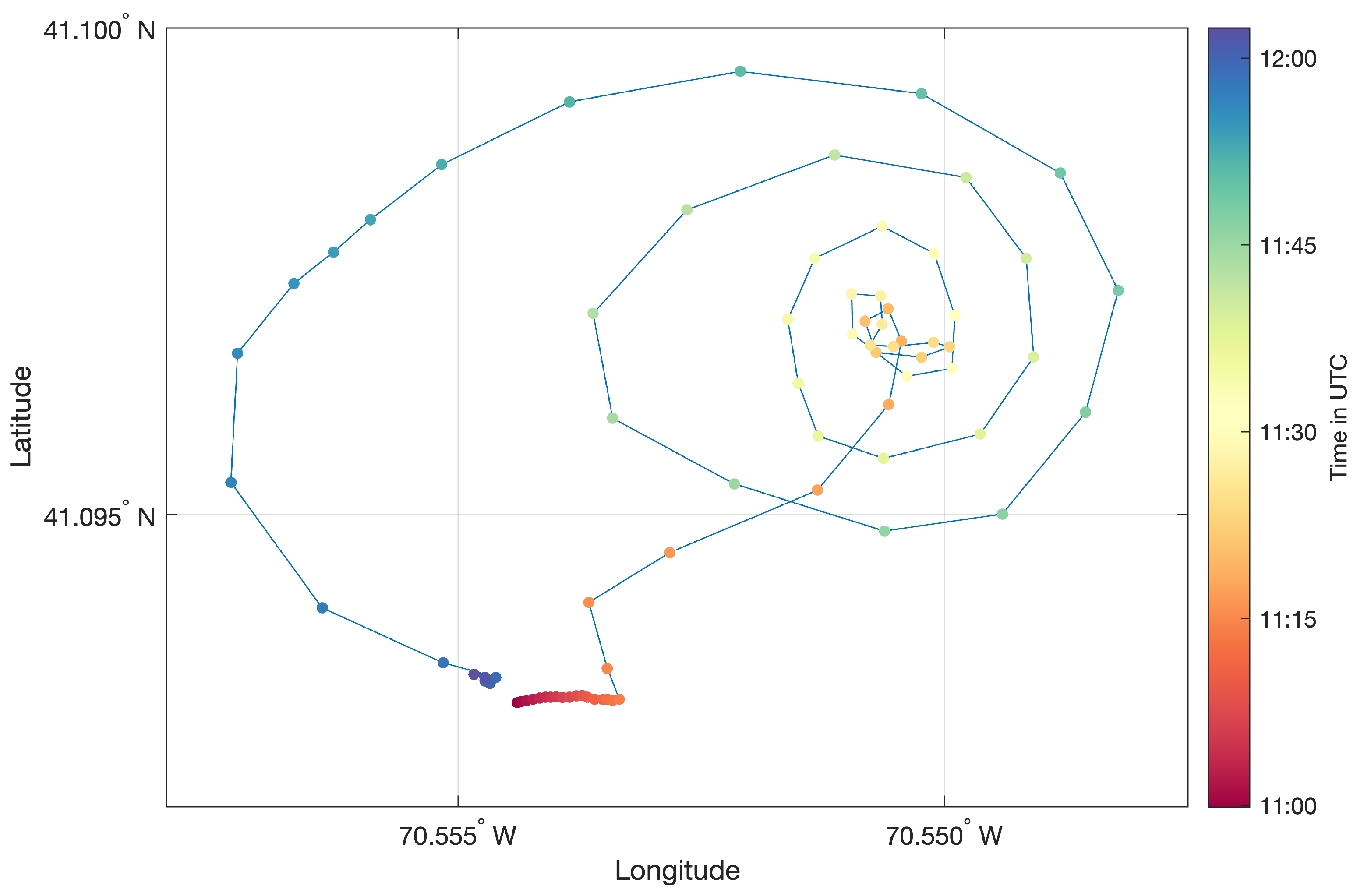
Figure 1. Our research team worked on board R/V Connecticut (R/V UCONN), a 27.43-m steel research vessel, owned and operated by the Department of Marine Sciences, University of Connecticut.
Rhodamine WT and ethanol alcohol were mixed to approximate the near-surface seawater density of
, following the method in [
20]. The dye was released from the back of R/V UCONN in an outward spiral pattern, with 3 spiral loops reaching approximately
in the outermost diameter. The spiral pattern, plotted in
Figure 2, started near 40°05.8′N, 70°33.0′W at 07:25 EST/11:25 UTC on 16 August 2018 and finished at 07:58 EST/11:58 UTC. Due to the advection of dye by oceanic currents, the original spiral got distorted over the course of the dye release. About 130 gallons of dye solution containing roughly 20 gallons of pure Rhodamine WT were injected just below the surface using a weight tied to the end of the dye hose. The dye reservoir tank was continually stirred to keep the dye solution homogeneous. A movie of the experiment can be found at
https://youtu.be/0e0fz0F38eg (accessed on 15 October 2021).
2.2. DOWSE: DrOne Water Sampling SystEm
A DrOne Water Sampling SystEm (DOWSE), consisting of a 3D-printed sampling device tethered to a drone, was used to collect water samples at different locations around the dye plume. The water sampler consisted of four separate 3D-printed pieces (.stl files are available here:
https://github.com/SchmaleLab/Schmale-Lab-3D-Printing-Files-Benson-et-al-Water-2018, accessed on 15 October 2021) assembled with 2.5 mm stainless steel screws. The sampler was tethered to the center of a carbon fiber beam on a 4.5 m orange nylon string tied between two 3D-printed mounts attached to the landing gear. A carabiner was used to quickly attach and remove the water sampler following each collection.
The DOWSE was loaded with a sterile 50 mL conical tube, and the drone was flown manually to each desired sampling location to collect the samples. Once the drone reached the sampling location, it descended to the surface of the water and allowed the DOWSE to rest on the surface for a few seconds. During this time, the DOWSE filled with water. An overhead image was then taken of the sampling device in the water and 10 m above the sampling location, which provided a precise record of the location and sampling time embedded in the EXIF file of each of the images. Following each collection, the sample was returned to the boat and the 50 mL tube (now containing a sample of ocean water with dye) was switched out with a new tube while the drone was still in flight. The drone then flew to another location to collect another sample, until all targeted collections had been completed. The samples were collected over four different flights.
The drone was a Phantom 4 quadcopter (DJI, Shenzhen, China) powered by a 5870 mAh Lithium-ion polymer battery. This battery provided a flight time of about 20 min. The drone weighed 1.38 kg, and was equipped with a gimbaled high definition camera. Additional photography parameters are described in
Section 2.4.2. Drone specifications as per the manufacturer can be found at
https://www.dji.com/phantom-4/info (accessed on 15 October 2021).
2.3. Determination of Rhodamine Dye Concentrations following Collection with the DOWSE
Small volume surface collections in the Atlantic Ocean were used to determine Rhodamine dye concentrations following the plume release. Individual locations in close proximity to the ship were sampled by filling 50 mL conical tubes (Genesee Scientific #28-108) with ocean water. We utilized the DOWSE sampling system to retrieve the ocean water samples [
15]. Samples were stored on board in the dark.
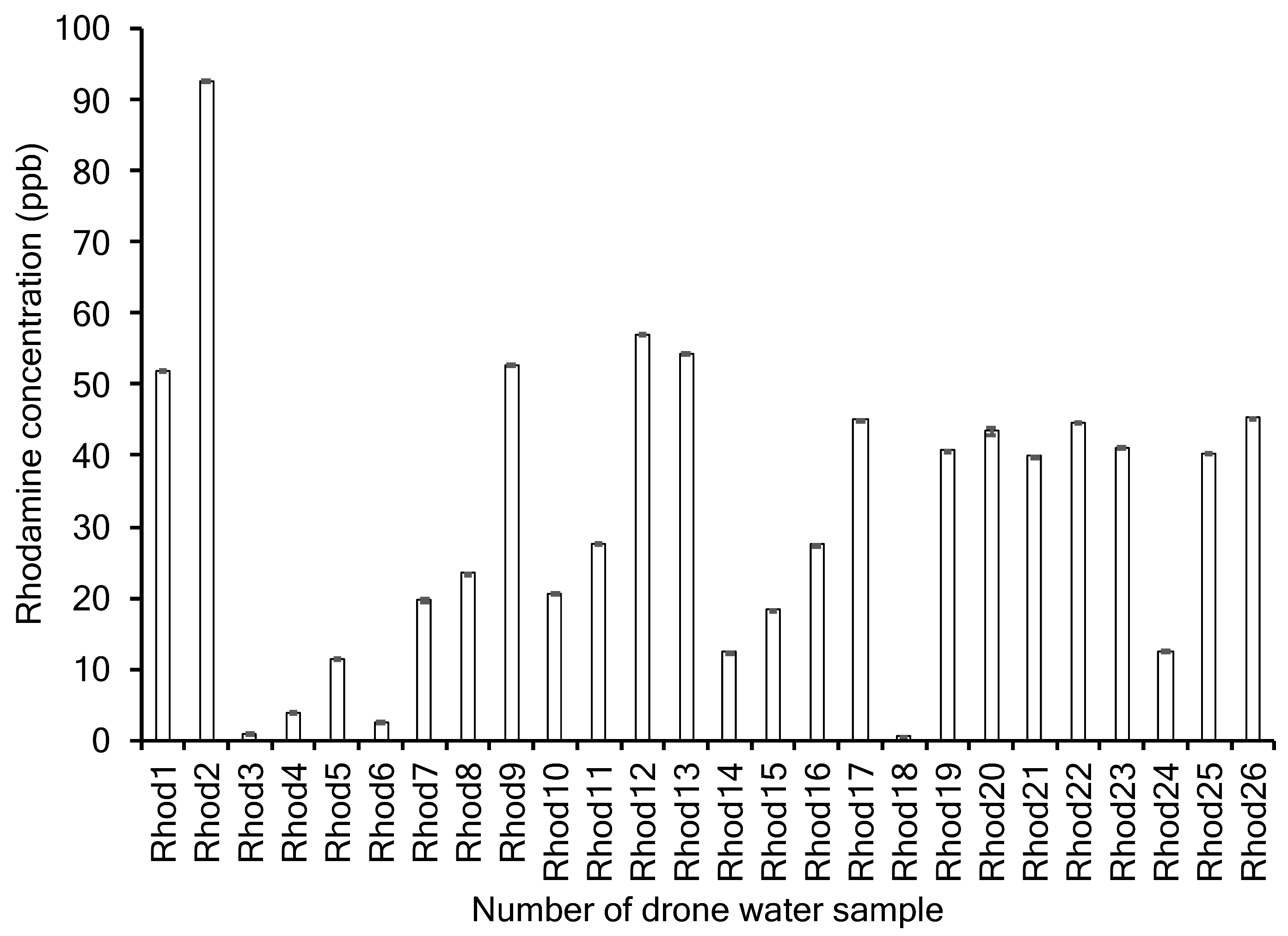
A Turner Designs handheld data bank (serial #2900495) was used on board to determine the Rhodamine concentration of each sample. The Rhodamine sensor (serial #2104382) was calibrated on board using a solid-state calibration unit (P/N 2100-900) set to 20 ppb. Immediately following calibration, the Rhodamine sensor was placed in a 50 mL conical tube with 15 mL of the collected ocean sample. The sensor was held in place 2.4 cm from the bottom of the tube and concentration values were logged in the dark. The concentration of Rhodamine in each drone water sample was recorded in triplicate on the same afternoon.
The dye concentrations at each of the sampling points are shown in
Figure 3.
2.4. Photogrammetry
The DJI Phantom 4 quadcopter’s gimbaled camera recorded images at each dye sampling location, typically capturing an image close to the surface and an image at about 10 m of altitude. Out of 26 sampling locations, 50 images were captured, with locations 5 and 9 not having surface images. The average lag between the surface and the altitude images was and the average difference in the gimbal’s yaw was °. To compare the distribution of dye between the surface images and the altitude images, the sea surface footprint (i.e., the extent of sea surface photographed in each image) had to be calculated from the gimbal’s rotation and altitude.
To calculate the altitude and the sea surface footprint, the parameters used were as follows: the camera was a 1/2.3″ CMOS with a sensor size of 6.17-by-4.55 mm. The lens had a 20 mm f/2.8 focus at ∞ and a focal length of 3.61 mm. The field of view parameters were ° in the horizontal and ° in the vertical. The image dimensions were 4000-by-2500 pixels.
2.4.1. Image Pre-Processing and Altitude Calculations
For each image, the gimbal’s orientation (roll, pitch and yaw) was used to compute the corresponding rotation matrix R and correct the image’s distortion. For all images, there was no roll angle. On average, the pitch angle was °. The latitude and longitude coordinates of each image were converted to Easting and Northing in UTM Zone 19 N.
The drone flight logs recorded the following altitude measurements, all in meters: the OSD altitude, which is the altitude at which the picture was taken; the Home height, which is the reference height used for calibration; and the OSD height, which yields the height of the picture above ground. The difference between OSD altitude and Home height should have given the altitude of the image, but upon inspection, this difference did not explain the OSD height and often resulted in negative altitudes, even for pictures that had clearly been taken above sea level.
The drone’s altitude measurements being prone to errors, the altitude of each image was calculated using a photographed object as a reference: typically, a drifter or the DOWSE sampler floating at the surface. The sampler measured ∼7.75 in or in length and in diameter, and the drifter sails measured ∼. Out of 26 images taken at altitude, only 4 images had captured an object of reference at the surface: images 2 and 5, which captured a drifter, image 16, which captured the vessel’s auxiliary Rigid-hull Inflatable Boat (RIB), and image 22, which captured floating algae. Image 5 did not have a surface equivalent.
The altitude is given by:
The distance on camera, or length of object, is the length of the object of interest in pixels over the focal plane resolution:
The focal plane resolution in pixel per mm was obtained through the following ratio:
The reference object and resulting altitude for each image are listed in
Table S1 of the Supplementary Materials.
2.4.2. Sea Surface Footprint
The sea surface footprint was calculated from the gimbal’s rotation
, the UTM coordinates in Zone 19 N of each image and the camera specifications. From the
, the rays delimiting the boundaries of what is captured by the camera can be calculated as follows:
The rays were then rotated according to and their intersections with the sea surface were calculated, assuming an altitude of 0 m for the surface. The extent of sea surface coverage resulted from the difference of these intersection points.
2.4.3. Dye Heat Maps
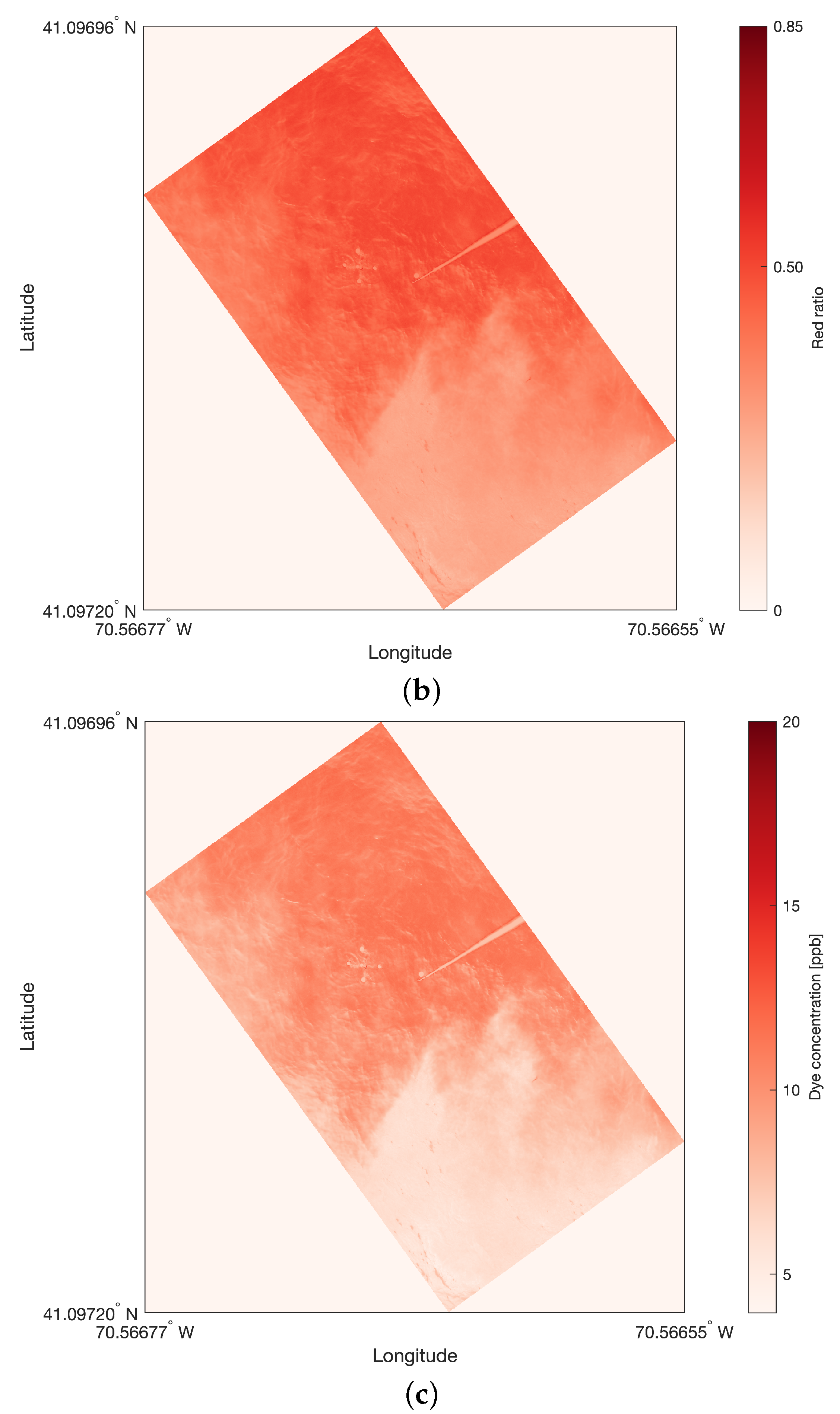
All images were imported in MATLAB and decomposed into their red, green and blue (RGB) components, ranging from 0 to 255. The percentage of the red component was calculated for the pre-processed image, which was rotated and slightly distorted from the original due to the gimbal’s rotation. An example is provided for Rhod 5, the image at sample location #5, shown in
Figure 4a. The resulting heat map of red ratio is shown in
Figure 4b. While the wave crests were still discernible in the heat map, the signature of the sun rays, which were still visible in the RGB decomposition, have been erased in the heat map. The limits of the dye plume are discernible from the difference of red ratio. The buoys on the drifters, which were white and have no rhodamine, had lower ratios of red, around
.
To construct the heat maps of the dye distribution, the assumptions were as follows: first, the rhodamine’s signature on the images corresponds to a high ratio of red; second, the ambient seawater’s signature on the images corresponds to a ratio of red approaching zero due to the ocean light absorption.
2.4.4. Dye Calibration
The constructed heat maps were then calibrated using the mean rhodamine concentrations in ppb, which have been sampled
in situ, as explained in
Section 2.3. The assumptions were as follows. First, the
in situ samples,
, were taken at the centers of the images: for calibration, the red ratio
taken at the center of the image corresponds to the
in situ concentration in ppb. Second, the ambient seawater has no red component, hence 0 ppb corresponds to a red null ratio. The second assumption was verified against images that captured patches of blue ocean: the red ratios were 0 or close to 0. An example is shown in
Figure 4c for the picture in
Figure 4a. The
in situ rhodamine sample had a concentration of 11 ppb, the center of the image had a red ratio of 0.4806 and the lowest ratio in the picture was 0.2827. The patches of blue ocean are even more contrasted than in the ratio ratio heat maps. The limits of the dye plume, the drifter and the DOWSE are more easily discernible.
4. Discussion
Rhodamine dye (a surrogate hazardous agent) was released into the Atlantic ocean in August, 2018, and a series of experiments were conducted to track the dye near the water surface within three hours following the release. Our observed range of dye concentrations (1 ppb to 93 ppb) was similar to dye concentrations commonly used in recent scientific studies; Powers et al. [
5] released fluorescein into a freshwater lake and estimated concentrations using a quadcopter, and Rypina et al. [
20] released a mixture of rhodamine and fluorescein into the ocean and concentrations were measured with a shipboard flowthrough fluorometer.
The red ratio, or the percentage of the red component within the RGB decomposition, was calculated as a proxy to construct heat maps of dye within each picture. Though the red ratio filtered out the signature of the sun rays, there were a number of limitations to this approach as a predictor of dye concentrations. First, the red ratio was unable to reduce the signature of the wave action; consequently wave crests are still discernible in the heat maps. Future dye transport studies could be conducted in calm bays or inlets to eliminate wave crest caveats (e.g., Powers et al. [
5]). Second, glint (momentary flashes of light from the sun’s reflection on the water surface), was observed at the edges of some of the raw images, and the red ratio was unable to account for these ‘glinted areas’. Future missions might consider the use of a proper polarizing camera filter, as such filters have been shown to limit this nuisance in images of the water surface (e.g., Shaw and Vollmer [
27]). Third, the red ratio does not incorporate a working knowledge of the vertical dye plume and/or any potential stratification beneath the sea surface (e.g., Lee et al. [
28] who looked into models of vertical profiles of Chlorophyll from remote sensing of ocean color). Future missions could incorporate sub-surface sampling of the dye using different types of drone-based water sampling devices (e.g., Castendyk et al. [
29]) coupled with multiple Secchi disk observations (e.g., Lee et al. [
30]) at the sampling locations, but these additional observations were beyond the scope of resources and capabilities available to the present study. Consequently, dye estimates based on red ratios derived from our drone images may in fact include some dye beneath the water surface. Fourth, shadows from clouds observed in some of the images (e.g., Li et al. [
31], Zhang et al. [
32]) and those from the drone and the sampling tether line were not taken into account in this analysis. Measuring the self-shadow effect of marine instruments is an active area of research (see Lin et al. [
33] and references therein) and these effects could also have contributed to small errors in dye estimates using the red ratio approach.
Despite these limitations, heat maps of rhodamine concentrations (ppb) constructed by calibrating the red ratios with the
in situ concentrations revealed linear fits (
) ranging from 0.511 to 0.670. The linear fit was constructed using two points of reference for dye concentration, assuming that the mean red ratio in the images or their subset corresponded to the measured concentrations in ppb and that a red ratio of 0 corresponded to 0 ppb. A major uncertainty in this approach is the assumption that each image’s center corresponded to the sample of water collected by DOWSE: the images having been taken after the sample collection, we were unable to measure the red ratio of the exact parcel of water sampled. It is assumed that for a parcel of water small enough, i.e., the volume of water around the collected sample, the dye distribution was homogeneous. The
values increased from 0.566 to 0.598 to 0.670 as the saturated images were cropped to 5 × 5-in and to 2 × 2-in squares, which is in agreement with the assumption that the pixels near the center of the images correspond to the parcel of water that was sampled. The red ratios calculated from aerial photographs thus appear to be good indicators of the rhodamine concentration at the surface. The limitations of this assumption are however reflected in the correlation plots in
Figure 5: for the images that had captured some blue ocean, the red ratios were more scattered, most likely because the dye distribution was much more heterogeneous. The assumption that a zero red ratio corresponded to 0 ppb, however, was verified from the blue ocean (no dye) pictures.
Quantitative comparisons of the dye spatial coverage were possible at three locations that had altitude references for both images. At location #2, the images were taken at an altitude difference of 11.1 m and the average percent difference between dye concentrations was 15.15% (see
Figure 7c). At location #22, the altitude difference was 10.3 m and the average percent difference was 21.59%. At location #16, the altitude difference was 16.5 m yet the heat maps differed much less: the average percent difference was 8.55%. The relatively low percent differences are consistent with Zeng et al. [
17], who found “negligible” differences in water spectra from images taken at a 100 m altitude compared to the spectra from images taken at a 20 m altitude. It is important to remember that the estimates of dye concentrations and the comparison between surface and altitude estimates were calculated to determine the loss of accuracy with altitude. The initial plan in the experimental design was to capture the presence of a surface drifter in each image and to sample dye adjacent to the drifter. The field conditions presented some challenges, however, and the fast currents led to a rapid dye dispersion. Consequently, only a few sets of images of higher altitude had objects of reference. In future studies, an object of reference will be dropped at each sample location to help with the altitude calibration.
The ability to obtain quantitative results was limited in part by the lack of accurate altitude measurements. A high-accuracy altimeter on the drone’s gimbal would have enabled the calculations of photometric footprints for all images and allowed for a more systematic comparison. Removing the DOWSE sampler and other objects captured within the images would also yield greater results, but doing so manually is very time-intensive and the sample size was way too small for an automatic approach using machine learning detection algorithms. As more studies are conducted with DOWSE and more images are obtained, the removal of such objects can be automated. The main source of error in the resulting heat maps would then come from the wave action: indeed, the RGB filtering was not sufficient to remove the wave crests. Note that this study was conducted on a very sunny day and it is unclear how much the weather, beyond the waves, would affect the results. It would be of interest to investigate the role of different environmental factors, similarly to Zeng et al. [
17]. The present study was designed to monitor dye around a single dye release within a period of about three hours. Consequently, it is not possible to extend the results to a range of atmospheric and hydrological conditions, but there is an opportunity for future studies to target multiple dye releases under a range of wave regime, cloud coverage or water quality.
Coordinated manned and unmanned systems may be used to detect, track and assist in mitigating hazardous agents in the future. In this study, the surface and altitude drone images were sufficient to detect the dye plume. The concentration estimates from the altitude images were roughly similar to the surface estimates, meaning that if high accuracy is not needed when tracking a plume, drone flights can be reduced by staying at an altitude of about 10 m. This would save survey time, battery life, and would reduce the risk of potential equipment failure due to waves splashing on the drone. As a final remark, this study focused on surface dynamics and did not consider three-dimensional effects nor vertical transport. Many hazardous agents stay at the surface due to buoyancy constraints: with a density of
[
34], the Deepwater Horizon oil was buoyant and many oil particles rose to the surface, for instance. A surface study is thus still relevant to help track such hazards.