Vertical Variability of Total and Size-Partitioned Phytoplankton Carbon in the South China Sea
Abstract
1. Introduction
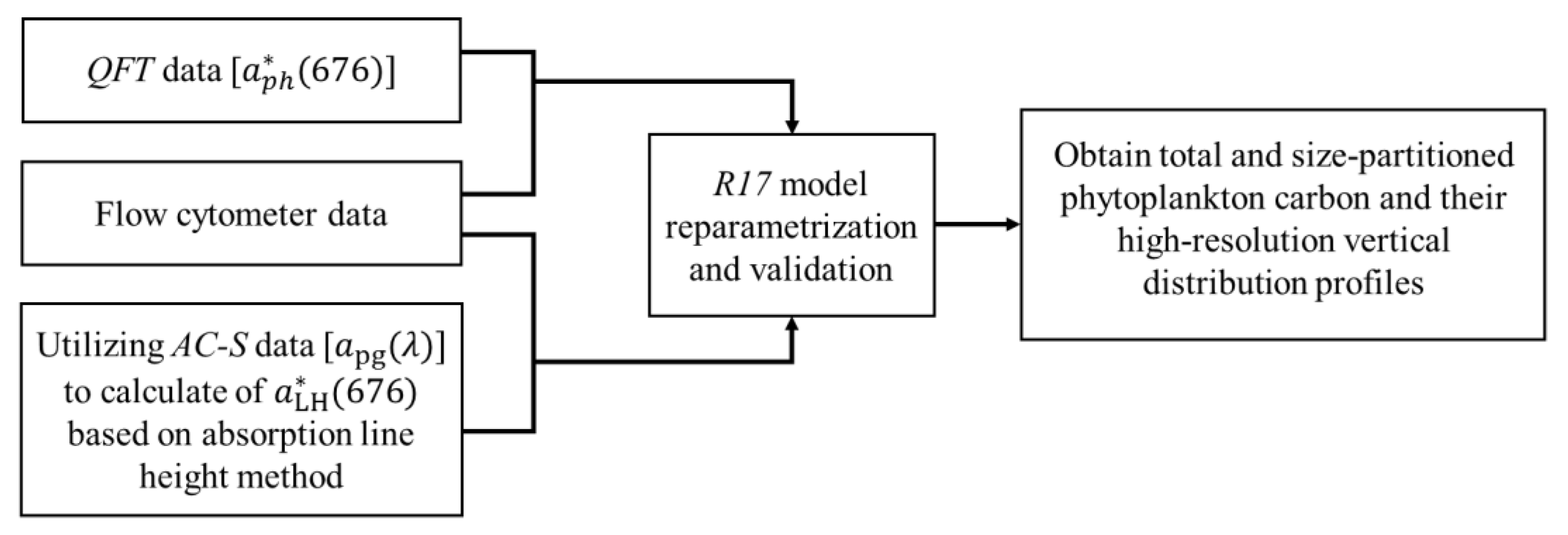
2. Materials and Methods
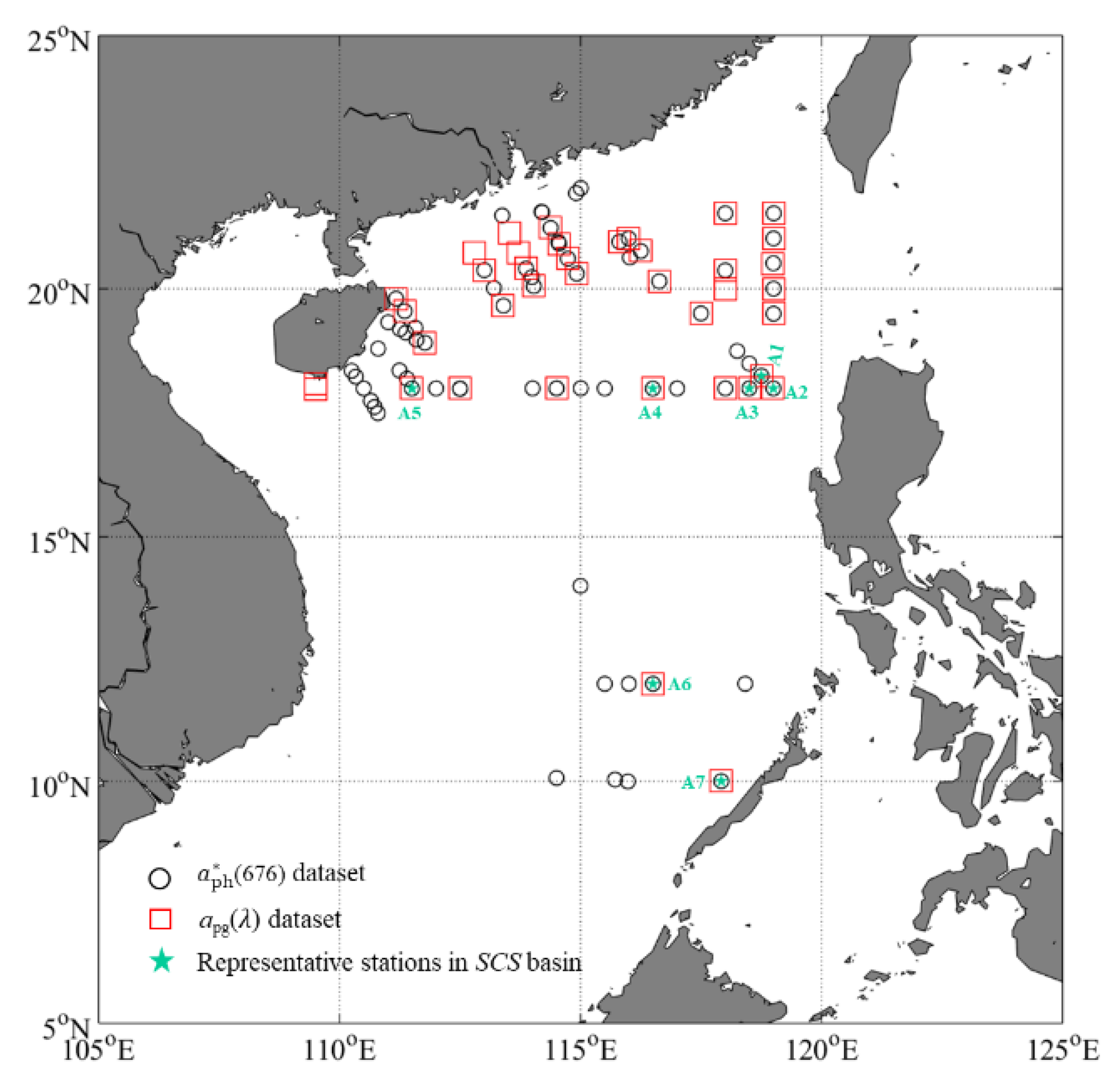
2.1. Study Area and Field Sampling
2.2. Field in Situ Data
2.2.1. Measurement of aph() and Calculation of ()
2.2.2. Measurement of apg()
2.2.3. Pigment Concentration Measurement and Determination of Dominant Phytoplankton Species
2.2.4. Picophytoplankton Carbon Biomass and POC Measurements
2.3. Derived Data
2.3.1. Physical and Biogeochemical Layer Depths (Layers) of the Water Column
2.3.2. Definition of Trophic Categories
2.3.3. Algorithm Performance Metrics
2.4. Phytoplankton Carbon Estimation
2.4.1. Phytoplankton Carbon Estimation Algorithm
2.4.2. Modification of the Algorithm
2.4.3. Application of the Algorithm to apg()
3. Results
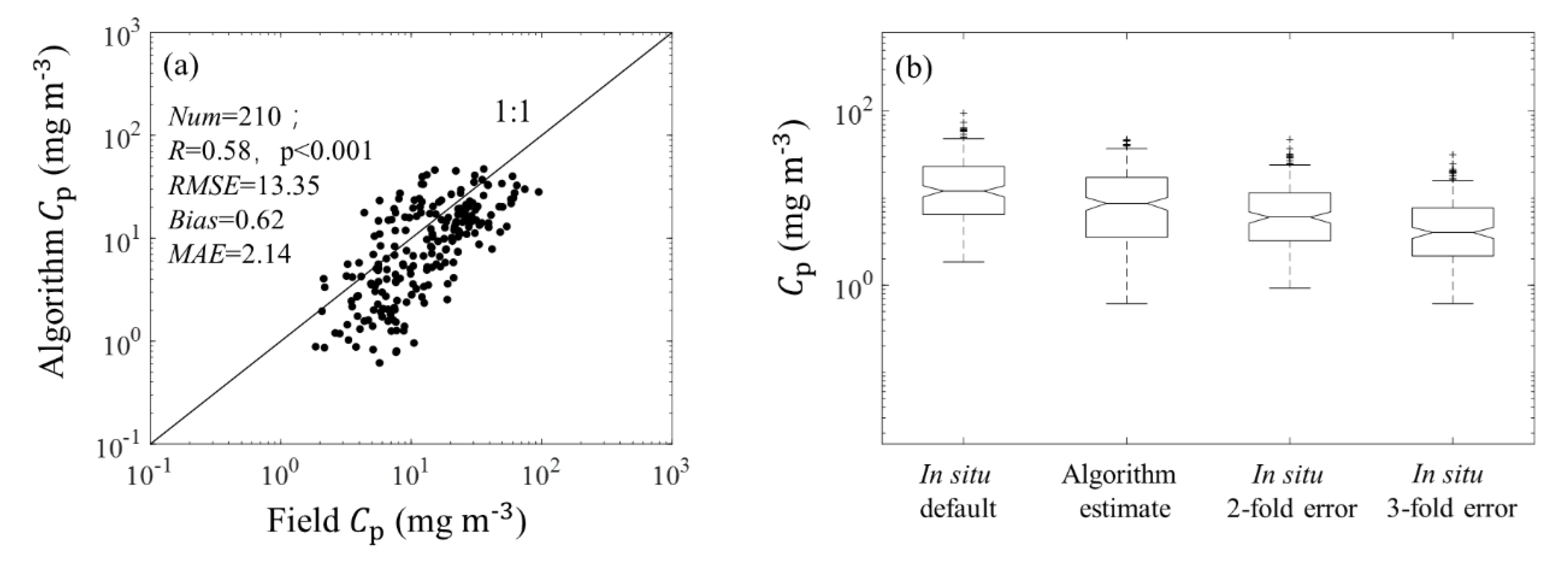
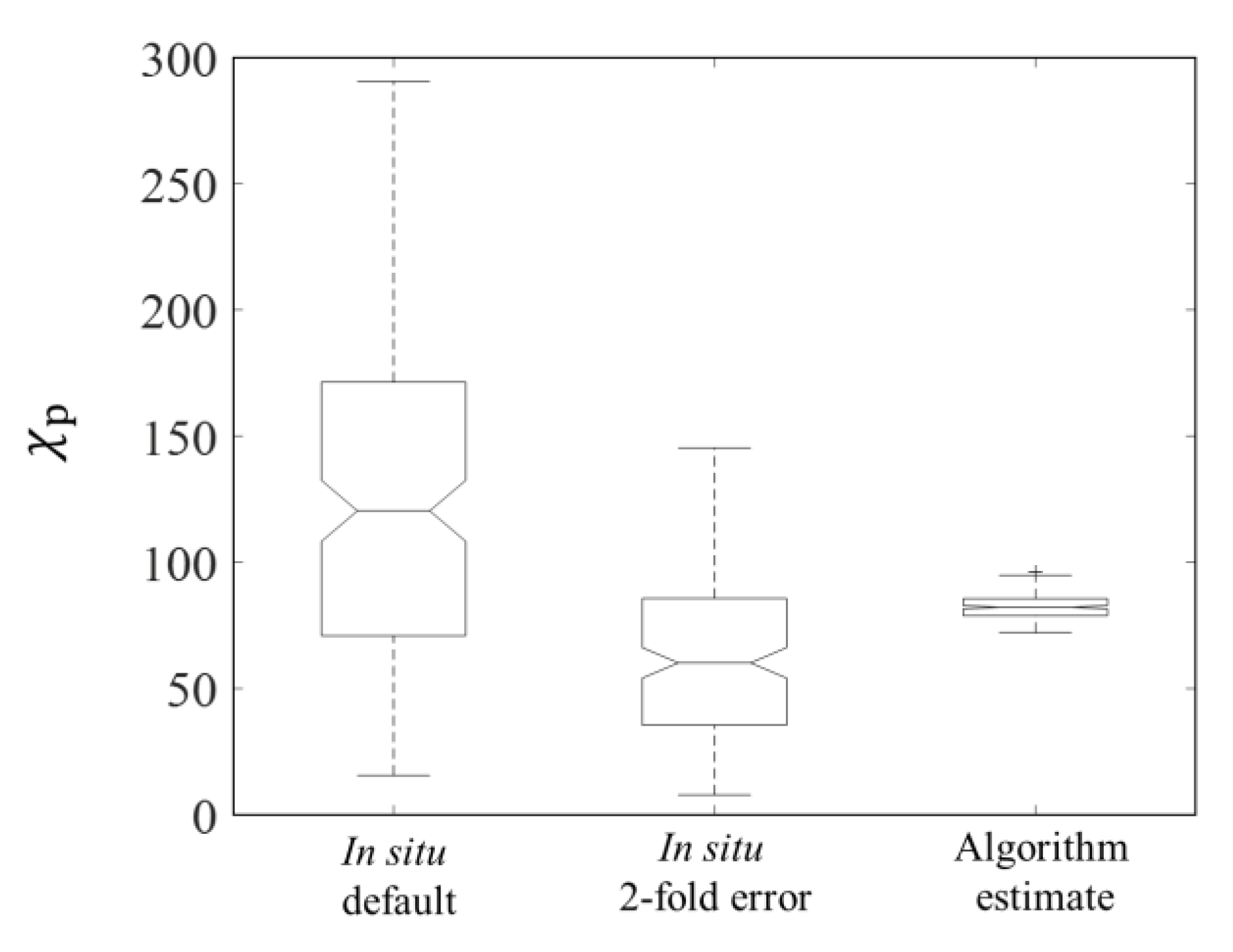
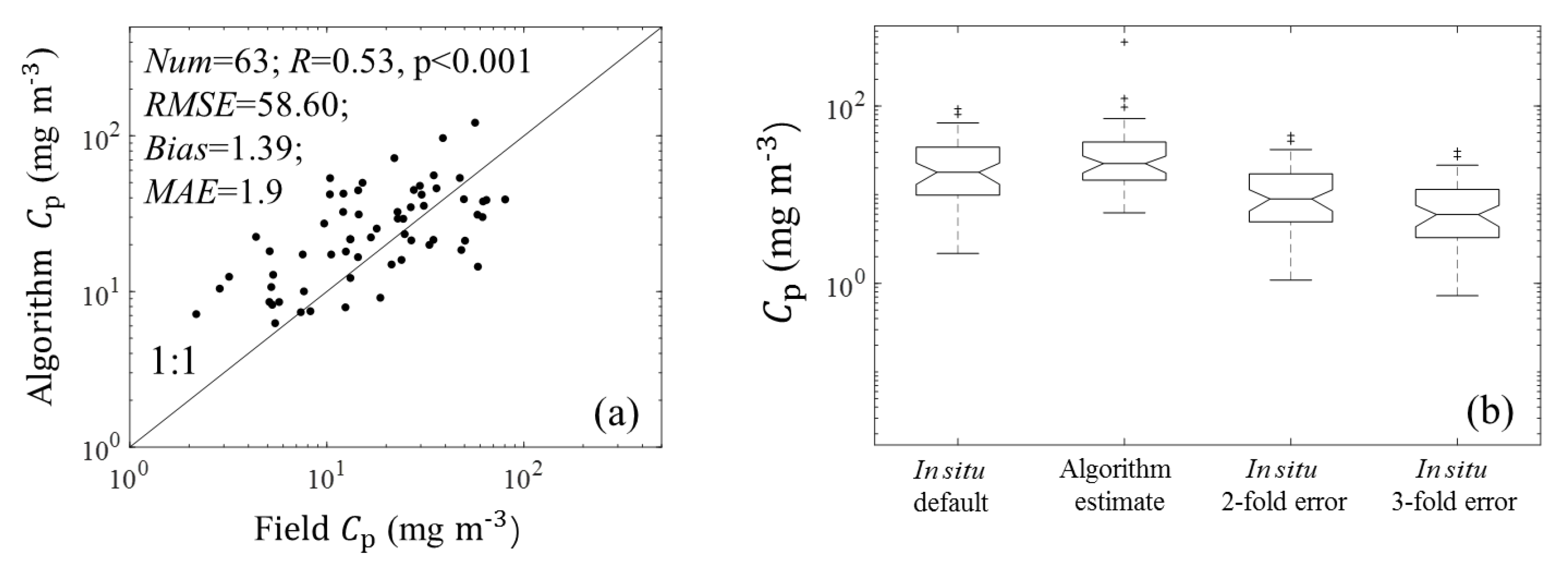
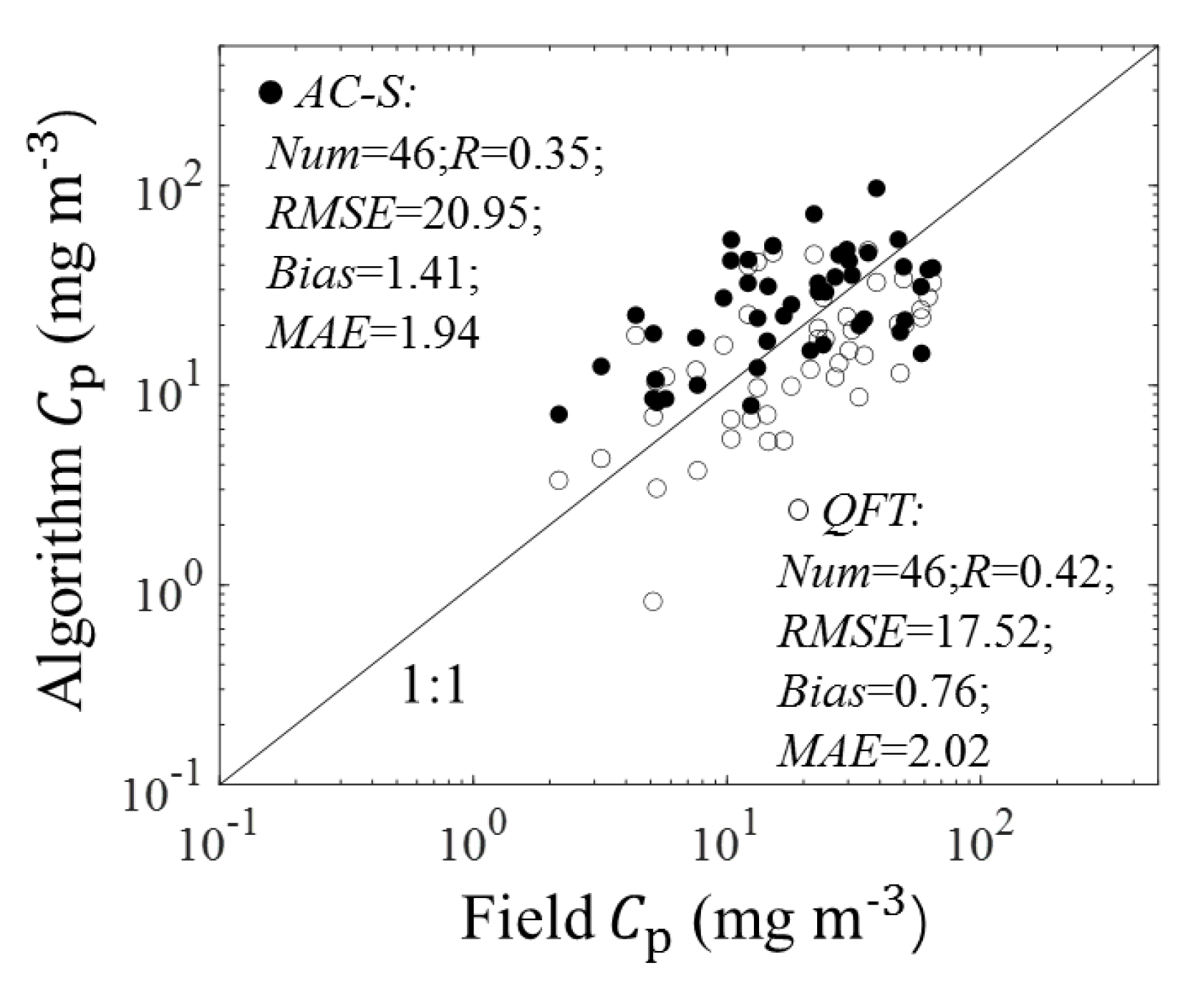
3.1. Algorithm Validation
3.1.1. Validation of Estimations from (676)
3.1.2. Validation of Estimations from (676)
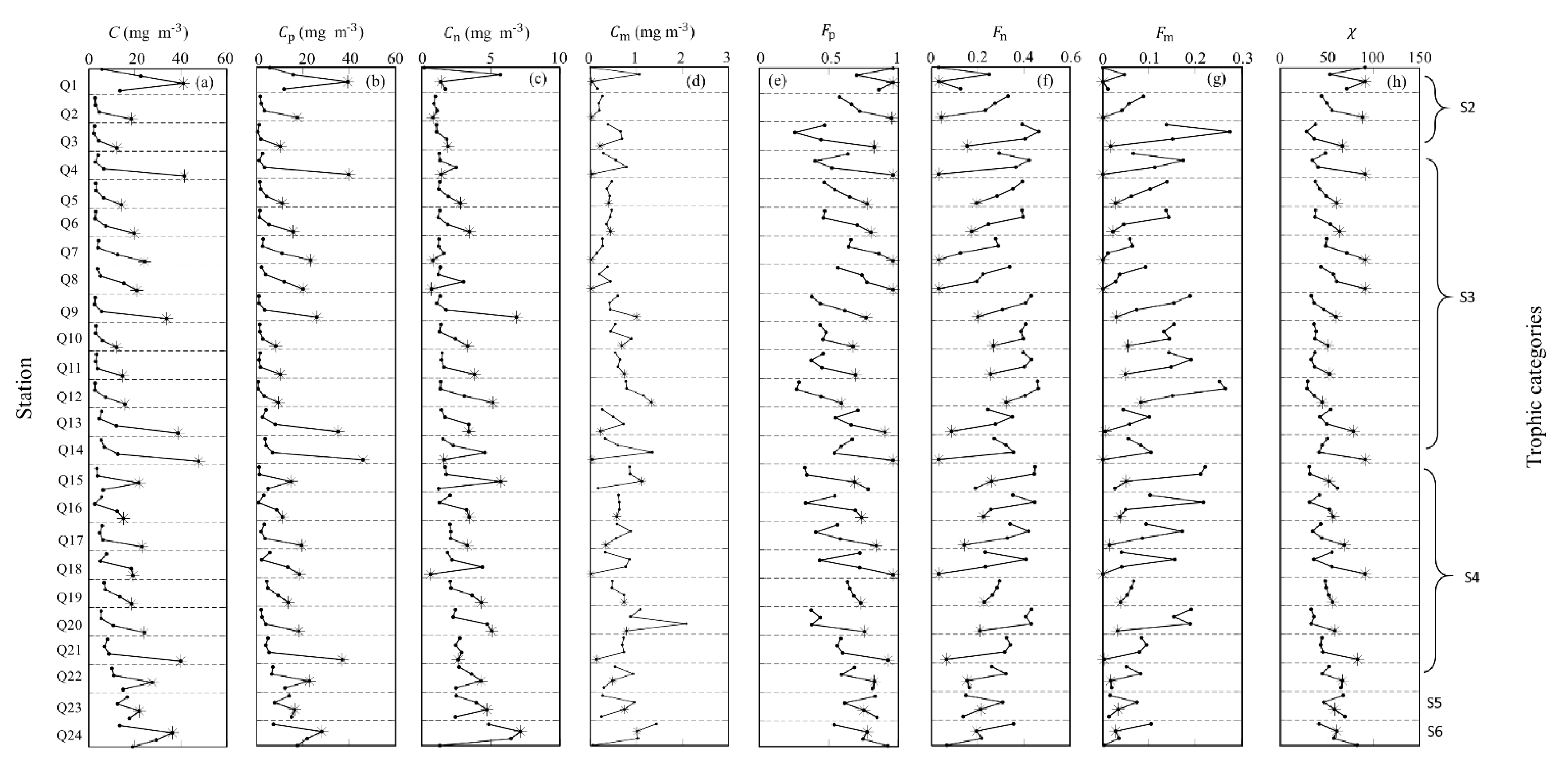
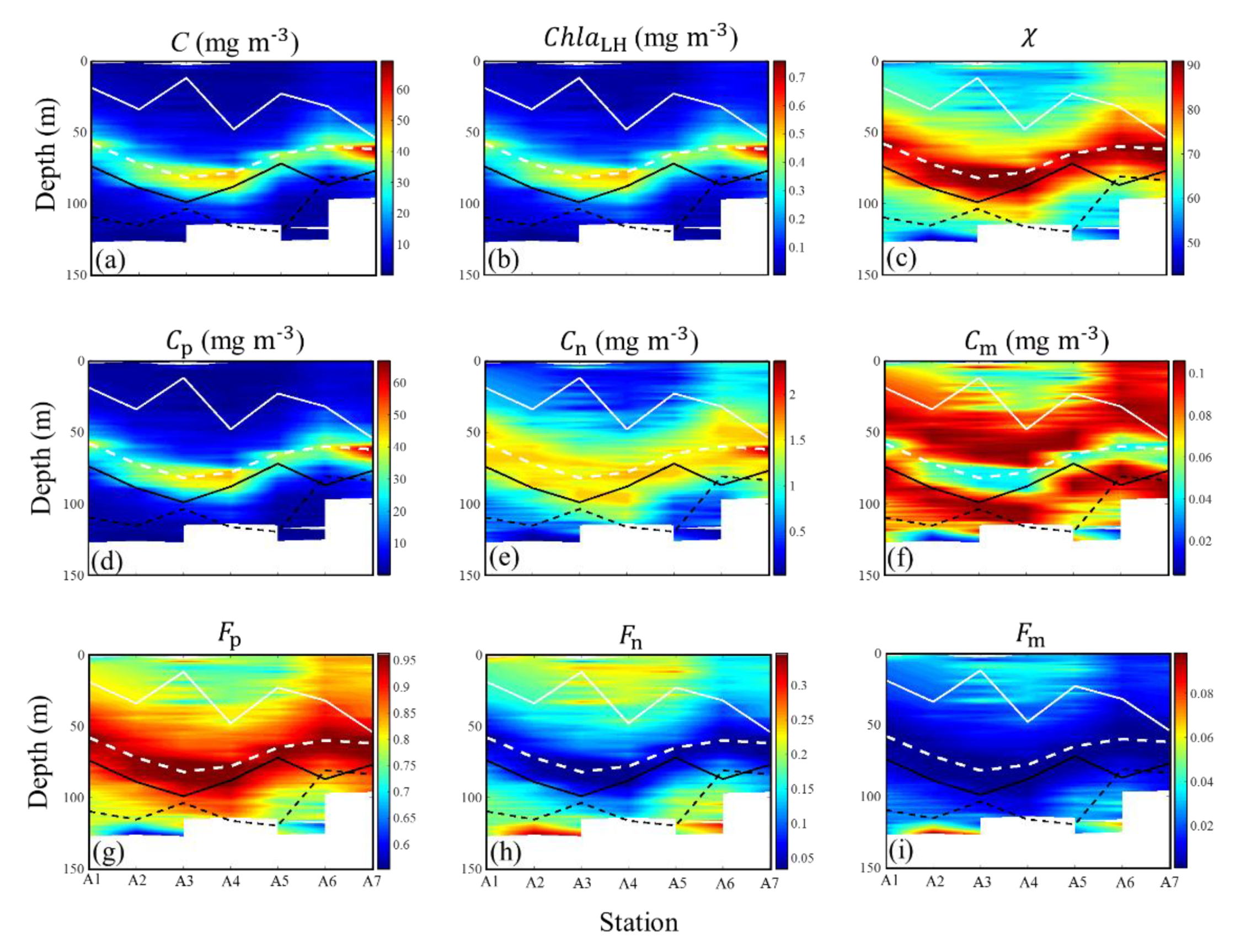
3.2. Vertical Variation of Phytoplankton Carbon and Related Bio-Parameters
3.2.1. Statistical Results from (676)
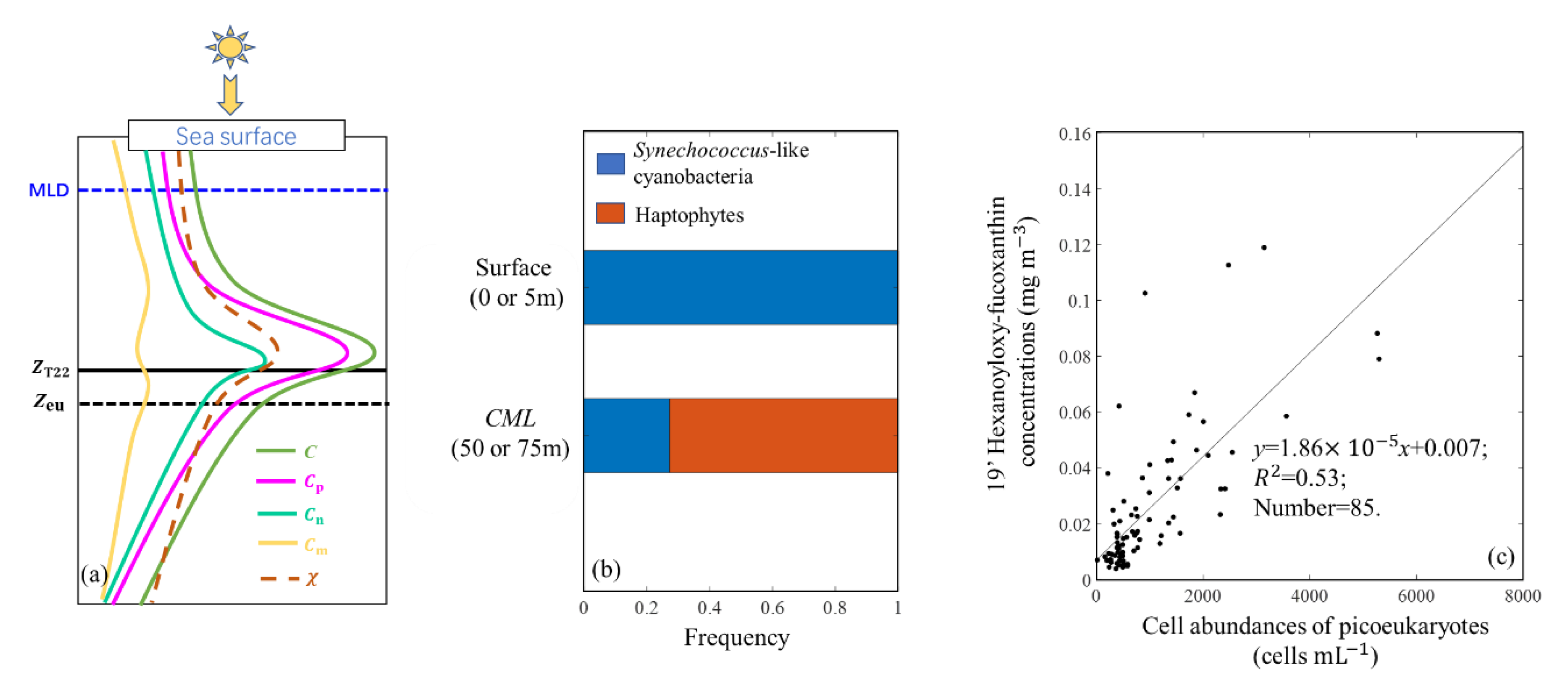
3.2.2. Vertical Profiles in the SCS Basin
Vertical Profiles at Four Discrete Depths
High Spatial Resolution Vertical Profile
4. Discussion
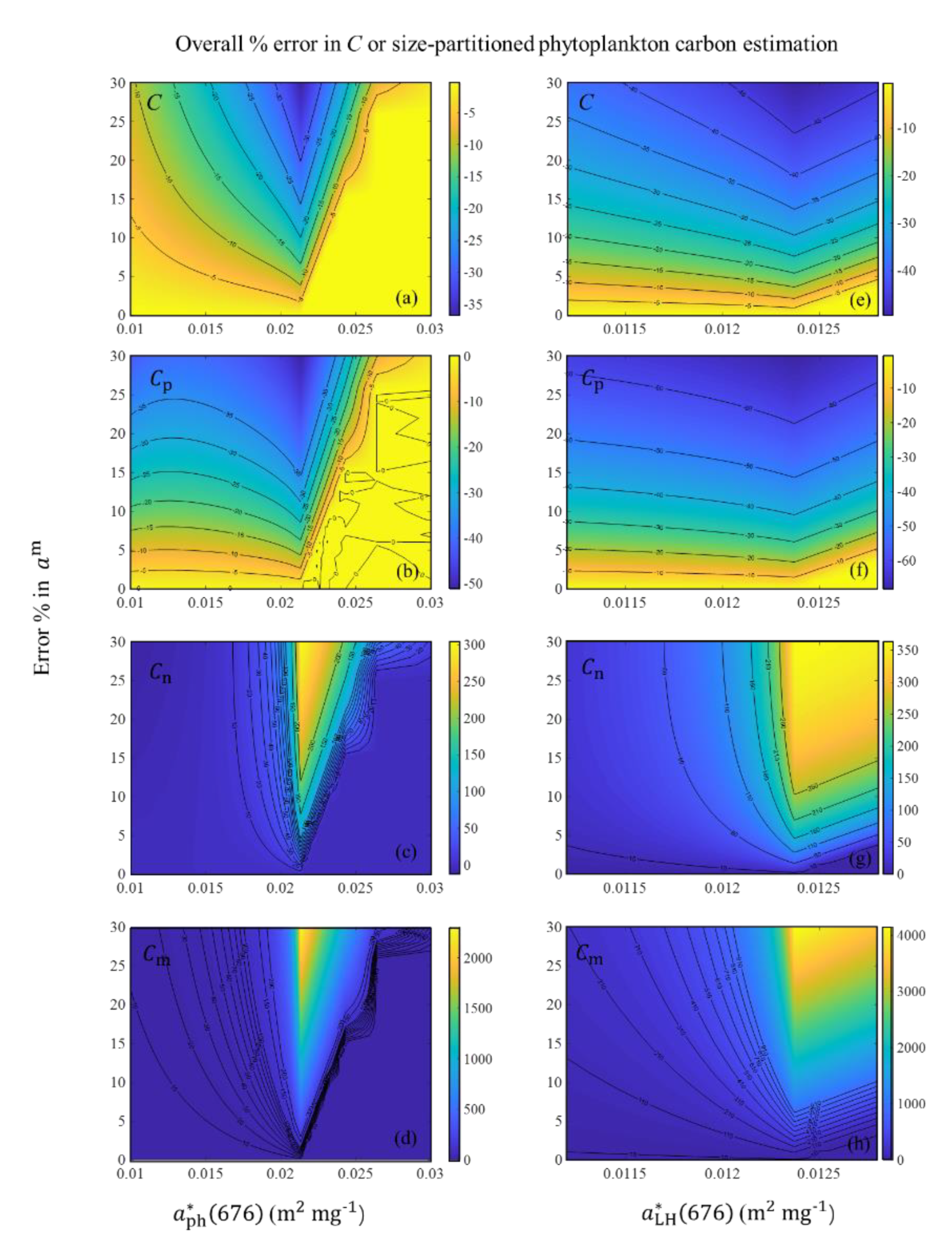
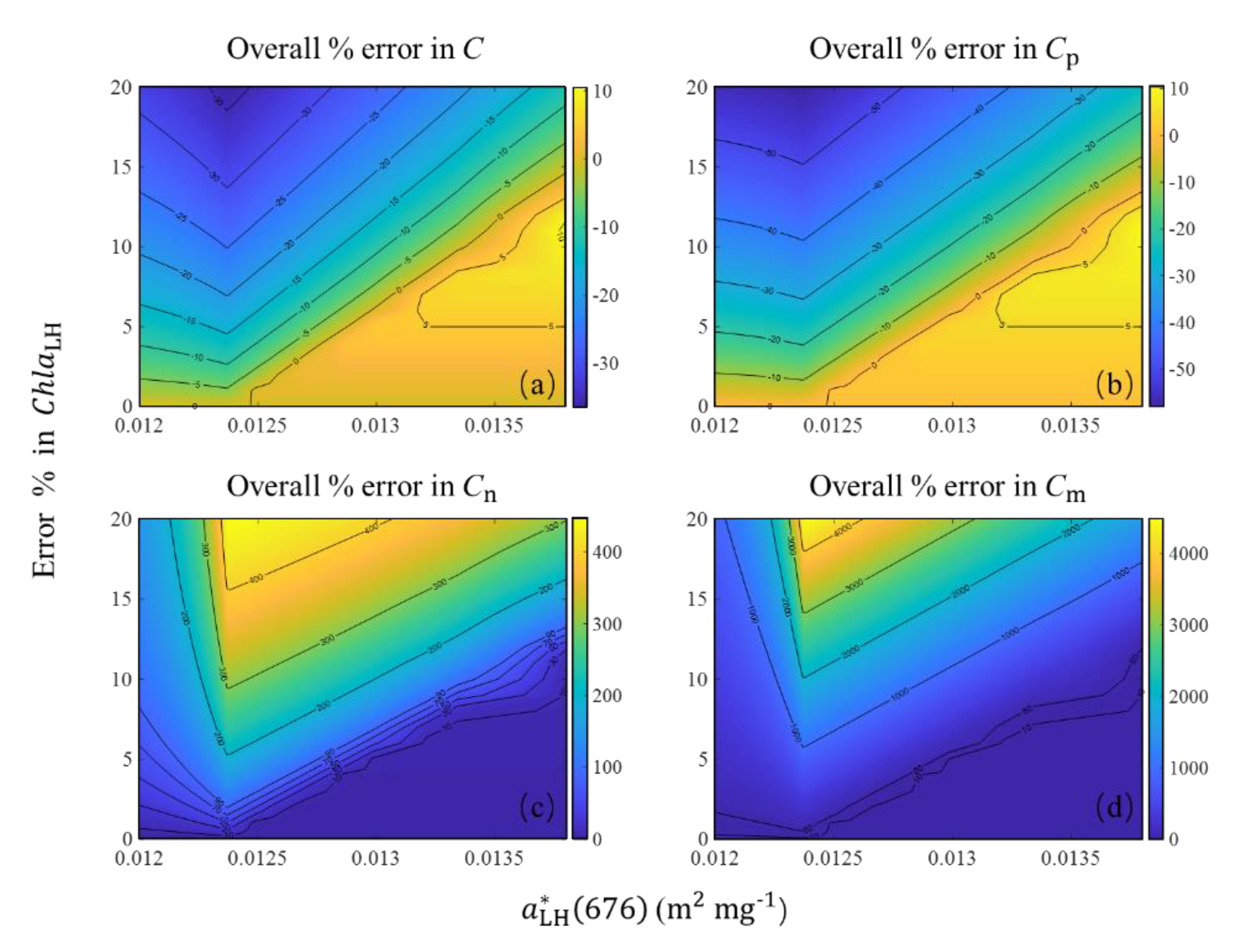
4.1. Sources of Uncertainty in the Algorithm
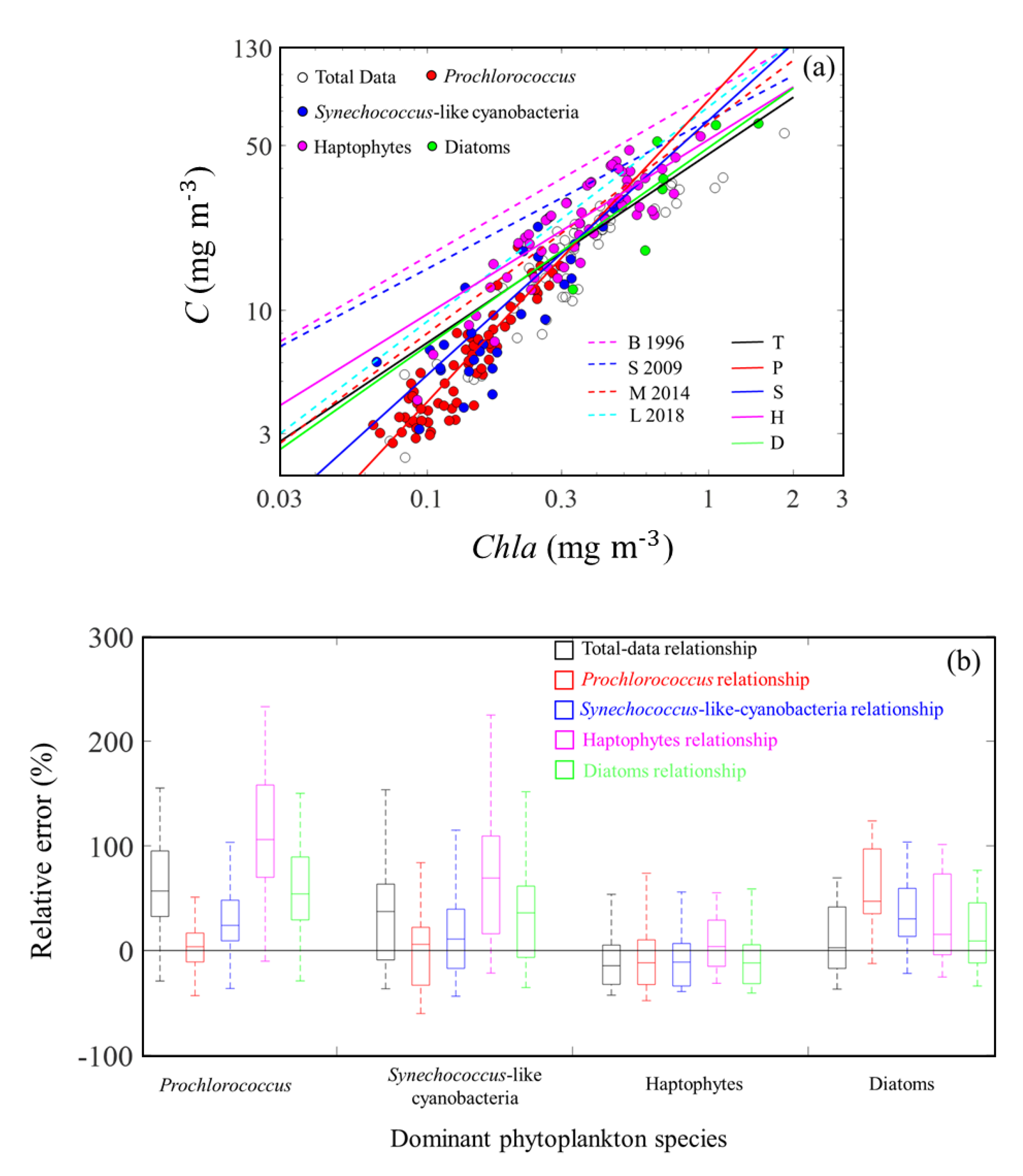
4.2. Relationship between C and Chla
4.3. General Vertical Profile of Phytoplankton Carbon in the SCS Basin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Falkowski, P. Ocean Science: The power of plankton. Nat. Cell Biol. 2012, 483, S17–S20. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Barbieux, M.; Uitz, J.; Bricaud, A.; Organelli, E.; Poteau, A.; Schmechtig, C.; Gentili, B.; Obolensky, G.; Leymarie, E.; Penkerc’H, C.; et al. Assessing the Variability in the Relationship Between the Particulate Backscattering Coefficient and the Chlorophyll a Concentration From a Global Biogeochemical-Argo Database. J. Geophys. Res. Oceans 2018, 123, 1229–1250. [Google Scholar] [CrossRef]
- Siegel, D.; Behrenfeld, M.; Maritorena, S.; McClain, C.; Antoine, D.; Bailey, S.; Bontempi, P.; Boss, E.; Dierssen, H.; Doney, S.; et al. Regional to global assessments of phytoplankton dynamics from the SeaWiFS mission. Remote Sens. Environ. 2013, 135, 77–91. [Google Scholar] [CrossRef]
- Siegel, D.A.; Maritorena, S.; Nelson, N.B.; Behrenfeld, M.J. Independence and interdependencies among global ocean color properties: Reassessing the bio-optical assumption. J. Geophys. Res. Space Phys. 2005, 110, C07011. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Siegel, D.A.; Shea, D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Glob. Biogeochem. Cycles 2005, 19, GB1006. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Milutinović, S.; Marinov, I.; Cabré, A. Carbon-based phytoplankton size classes retrieved via ocean color estimates of the particle size distribution. Ocean Sci. 2016, 12, 561–575. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Platt, T.; Kovac, Z.; Dingle, J.; Jackson, T.; Brewin, R.J.W.; Franks, P.; Maranon, E.; Kulk, G.; Bouman, H.A. Reconciling models of primary production and photoacclimation. Appl. Opt. 2020, 59, C100–C114. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Westberry, T.K.; Milligan, A.J.; Brown, M.B.; Dall’Olmo, G.; van Dongen-Vogels, V.; Reifel, K.M.; Behrenfeld, M.J. Analytical phytoplankton carbon measurements spanning diverse ecosystems. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2015, 102, 16–25. [Google Scholar] [CrossRef]
- Brewin, R.J.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.L.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Sauzède, R.; Claustre, H.; Jamet, C.; Uitz, J.; Ras, J.; Mignot, A.; D’Ortenzio, F. Retrieving the vertical distribution of chlorophyll a concentration and phytoplankton community composition from in situ fluorescence profiles: A method based on a neural network with potential for global-scale applications. J. Geophys. Res. Oceans 2015, 120, 451–470. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Bouman, H.; Platt, T. The global distribution of phytoplankton size spectrum and size classes from their light-absorption spectra derived from satellite data. Remote Sens. Environ. 2013, 139, 185–197. [Google Scholar] [CrossRef]
- Martínez-Vicente, V.; Evers-King, H.; Roy, S.; Kostadinov, T.S.; Tarran, G.A.; Graff, J.R.; Brewin, R.J.W.; Dall’Olmo, G.; Jackson, T.; Hickman, A.E.; et al. Intercomparison of Ocean Color Algorithms for Picophytoplankton Carbon in the Ocean. Front. Mar. Sci. 2017, 4, 378. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Stuart, V.; Nair, A.; Oka, K.; Nakane, T.; Bouman, H.; Forget, M.-H.; Maass, H.; Platt, T. Carbon-to-chlorophyll ratio and growth rate of phytoplankton in the sea. Mar. Ecol. Prog. Ser. 2009, 383, 73–84. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Platt, T. Size-partitioned phytoplankton carbon and carbon-to-chlorophyll ratio from ocean colour by an absorption-based bio-optical algorithm. Remote Sens. Environ. 2017, 194, 177–189. [Google Scholar] [CrossRef]
- Westberry, T.; Behrenfeld, M.J.; Siegel, D.A.; Boss, E. Carbon-based primary productivity modeling with vertically resolved photoacclimation. Glob. Biogeochem. Cycles 2008, 22, GB2024. [Google Scholar] [CrossRef]
- Bellacicco, M.; Volpe, G.; Briggs, N.; Brando, V.; Pitarch, J.; Landolfi, A.; Colella, S.; Marullo, S.; Santoleri, R. Global Distribution of Non-algal Particles from Ocean Color Data and Implications for Phytoplankton Biomass Detection. Geophys. Res. Lett. 2018, 45, 7672–7682. [Google Scholar] [CrossRef]
- Bellacicco, M.; Cornec, M.; Organelli, E.; Brewin, R.J.W.; Neukermans, G.; Volpe, G.; Barbieux, M.; Poteau, A.; Schmechtig, C.; D’Ortenzio, F.; et al. Global Variability of Optical Backscattering by Non-algal particles from a Biogeochemical-Argo Data Set. Geophys. Res. Lett. 2019, 46, 9767–9776. [Google Scholar] [CrossRef]
- Kostadinov, T.S.; Siegel, D.A.; Maritorena, S. Retrieval of the particle size distribution from satellite ocean color observations. J. Geophys. Res. Space Phys. 2009, 114, C09015. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Song, S.; Huang, B.; Sun, J.; Liu, H. Comparisons of picophytoplankton abundance, size, and fluorescence between summer and winter in northern South China Sea. Cont. Shelf Res. 2011, 31, 1527–1540. [Google Scholar] [CrossRef]
- Xing, X.; Qiu, G.; Boss, E.; Wang, H. Temporal and Vertical Variations of Particulate and Dissolved Optical Properties in the South China Sea. J. Geophys. Res. Oceans 2019, 124, 3779–3795. [Google Scholar] [CrossRef]
- Wong, G.T.; Ku, T.-L.; Mulholland, M.; Tseng, C.-M.; Wang, D.-P. The SouthEast Asian Time-series Study (SEATS) and the biogeochemistry of the South China Sea—An overview. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1434–1447. [Google Scholar] [CrossRef]
- Wu, J.F.; Chung, L.S.; Wen, K.K.; Liu, Y.L.L.; Chen, H.Y.C.; Karl, D.M. Dissolved Inorganic Phosphorus, Dissolved Iron, and Trichodesmium in the Oligotrophic South China Sea. Global. Biogeochemical. Cycles. 2003, 17, 1008. [Google Scholar] [CrossRef]
- Zhang, W.-Z.; Wang, H.; Chai, F.; Qiu, G. Physical drivers of chlorophyll variability in the open South China Sea. J. Geophys. Res. Oceans 2016, 121, 7123–7140. [Google Scholar] [CrossRef]
- Wang, L.; Koblinsky, C.; Howden, S.; Huang, N. Interannual variability in the South China Sea from expendable bathythermograph data. J. Geophys. Res. Space Phys. 1999, 104, 23509–23523. [Google Scholar] [CrossRef]
- Xue, H.; Chai, F.; Pettigrew, N.; Xu, D.; Shi, M.; Xu, J. Kuroshio intrusion and the circulation in the South China Sea. J. Geophys. Res. Space Phys. 2004, 109. [Google Scholar] [CrossRef]
- Gong, X.; Shi, J.; Gao, H. Modeling seasonal variations of subsurface chlorophyll maximum in South China Sea. J. Ocean. Univ. China 2014, 13, 561–571. [Google Scholar] [CrossRef]
- Lu, Z.; Gan, J.; Dai, M.; Cheung, A.Y. The influence of coastal upwelling and a river plume on the subsurface chlorophyll maximum over the shelf of the northeastern South China Sea. J. Mar. Syst. 2010, 82, 35–46. [Google Scholar] [CrossRef]
- Ning, X.; Chai, F.; Xue, H.; Cai, Y.; Liu, C.; Shi, J. Physical-biological oceanographic coupling influencing phytoplankton and primary production in the South China Sea. J. Geophys. Res. Space Phys. 2004, 109, C10005. [Google Scholar] [CrossRef]
- Wang, G.; Cao, W.; Yang, D.; Zhao, J. Partitioning particulate absorption coefficient into contributions of phytoplankton and nonalgal particles: A case study in the northern South China Sea. Estuar. Coast. Shelf Sci. 2008, 78, 513–520. [Google Scholar] [CrossRef]
- Stramski, D.; Reynolds, R.A.; Kaczmarek, S.; Uitz, J.; Zheng, G. Correction of pathlength amplification in the filter-pad technique for measurements of particulate absorption coefficient in the visible spectral region. Appl. Opt. 2015, 54, 6763–6782. [Google Scholar] [CrossRef]
- Sullivan, J.M.; Twardowski, M.S.; Zaneveld, J.R.V.; Moore, C.M.; Barnard, A.H.; Donaghay, P.L.; Rhoades, B. Hyperspectral temperature and salt dependencies of absorption by water and heavy water in the 400–750 nm spectral range. Appl. Opt. 2006, 45, 5294–5309. [Google Scholar] [CrossRef] [PubMed]
- Zaneveld, R.V.; Kitchen, J.C.; Moore, C. The Scattering Error-Correction of Reflecting-Tube Absorption Meters. Ocean Opt. 1994, Xii, 44–55. [Google Scholar]
- Hamilton, E. A manual of chemical & biological methods for seawater analysis. Mar. Pollut. Bull. 1984, 15, 419–420. [Google Scholar] [CrossRef]
- Knap, A.; Michaels, A.; Close, A. Protocols for the Joint Global Ocean Flux Study (Jgofs) Core Measurements; UNESCO: Paris, France, 1996. [Google Scholar]
- Vidussi, F.; Claustre, H.; Bustillos Guzman, J.; Cailliau, C.; Marty, J.C. Determination of Chlorophylls and Carotenoids of Marine Phytoplankton: Separation of Chlorophyll a from Divinyl-Chlorophyll a and Zeaxanthin from Lutein. J. Plankton Res. 1996, 18, 2377–2382. [Google Scholar] [CrossRef]
- Alvain, S.; Moulin, C.; Dandonneau, Y.; Bréon, F.-M. Remote sensing of phytoplankton groups in case 1 waters from global SeaWiFS imagery. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 1989–2004. [Google Scholar] [CrossRef]
- Marie, D.; Partensky, F.; Vaulot, D.; Brussaard, C. Enumeration of Phytoplankton, Bacteria, and Viruses in Marine Samples. Curr. Protoc. Cytom. 1999, 10, 1–15. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, W.; Cao, W.; Yin, J.; Yang, Y.; Sun, Z.; Zhang, Y.; Zhao, J. Variation of particulate organic carbon and its relationship with bio-optical properties during a phytoplankton bloom in the Pearl River estuary. Mar. Pollut. Bull. 2011, 62, 1939–1947. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Y. A Bio-optical model for ocean photosynthetic available radiation. J. Trop. Oceanogr. 2002, 21, 47–54. (In Chinese) [Google Scholar]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Space Phys. 2006, 111, C08005. [Google Scholar] [CrossRef]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance metrics for the assessment of satellite data products: An ocean color case study. Opt. Express 2018, 26, 7404–7422. [Google Scholar] [CrossRef]
- Roy, S.; Sathyendranath, S.; Platt, T. Retrieval of phytoplankton size from bio-optical measurements: Theory and applications. J. R. Soc. Interface 2010, 8, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Roesler, C.S.; Barnard, A.H. Optical proxy for phytoplankton biomass in the absence of photophysiology: Rethinking the absorption line height. Methods Oceanogr. 2013, 7, 79–94. [Google Scholar] [CrossRef]
- Buitenhuis, E.T.; Li, W.K.W.; Vaulot, D.; Lomas, M.W.; Landry, M.R.; Partensky, F.; Karl, D.M.; Ulloa, O.; Campbell, L.; Jacquet, S.; et al. Picophytoplankton biomass distribution in the global ocean. Earth Syst. Sci. Data 2012, 4, 37–46. [Google Scholar] [CrossRef]
- Liu, H.; Chang, J.; Tseng, C.-M.; Wen, L.-S.; Liu, K.-K. Seasonal variability of picoplankton in the Northern South China Sea at the SEATS station. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 1602–1616. [Google Scholar] [CrossRef]
- Eppley, R.W.; Chavez, F.P.; Barber, R.T. Standing Stocks of Particulate Carbon and Nitrogen in the Equatorial Pacific at 150-Degrees-W. J. Geophys. Res. Ocean. 1992, 97, 655–661. [Google Scholar] [CrossRef]
- Gundersen, K.; Orcutt, K.M.; Purdie, D.A.; Michaels, A.F.; Knap, A.H. Particulate organic carbon mass distribution at the Bermuda Atlantic Time-series Study (BATS) site. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 1697–1718. [Google Scholar] [CrossRef]
- DuRand, M.D.; Olson, R.J.; Chisholm, S.W. Phytoplankton population dynamics at the Bermuda Atlantic Time-series station in the Sargasso Sea. Deep. Sea Res. II Top. Stud. Oceanogr. 2001, 48, 1983–2003. [Google Scholar] [CrossRef]
- Oubelkheir, K.; Claustre, H.; Sciandra, A.; Babin, M. Bio-optical and biogeochemical properties of different trophic regimes in oceanic waters. Limnol. Oceanogr. 2005, 50, 1795–1809. [Google Scholar] [CrossRef]
- Liang, W.; Tang, D.; Luo, X. Phytoplankton size structure in the western South China Sea under the influence of a ‘jet-eddy system’. J. Mar. Syst. 2018, 187, 82–95. [Google Scholar] [CrossRef]
- Marañón, E.; Cermeño, P.; Huete-Ortega, M.; López-Sandoval, D.C.; Mouriño-Carballido, B.; Rodríguez-Ramos, T. Resource Supply Overrides Temperature as a Controlling Factor of Marine Phytoplankton Growth. PLoS ONE 2014, 9, e99312. [Google Scholar] [CrossRef]
- Buck, K.; Chavezt, F.; Campbell, L. Basin-wide distributions of living carbon components and the inverted trophic pyramid of the central gyre of the North Atlantic Ocean, summer 1993. Aquat. Microb. Ecol. 1996, 10, 283–298. [Google Scholar] [CrossRef]
- Loisel, L.D.H.; Dessailly, S.; Sathyendranath, H.E.; Vantrepotte, S.; Thomalla, A.M.; D’andon, O.H.F. A Satellite View of the Particulate Organic Carbon and Its Algal and Non-Algal Carbon Pools. In Proceedings of the Ocean Optics XXIV, Dubrovnik, Croatia, 7–12 October 2018. [Google Scholar]
- Gong, X.; Shi, J.; Gao, H.W.; Yao, X.H. Steady-state solutions for subsurface chlorophyll maximum in stratified water columns with a bell-shaped vertical profile of chlorophyll. Biogeosciences 2015, 12, 905–919. [Google Scholar] [CrossRef]
- Wang, L.; Huang, B.; Chiang, K.-P.; Liu, X.; Chen, B.; Xie, Y.; Xu, Y.; Hu, J.; Dai, M. Physical-Biological Coupling in the Western South China Sea: The Response of Phytoplankton Community to a Mesoscale Cyclonic Eddy. PLoS ONE 2016, 11, e0153735. [Google Scholar] [CrossRef]
- Andersen, R.A.; Bidigare, R.R.; Keller, M.D.; Latasa, M. A comparison of HPLC pigment signatures and electron microscopic observations for oligotrophic waters of the North Atlantic and Pacific Oceans. Deep. Sea Res. II Top. Stud. Oceanogr. 1996, 43, 517–537. [Google Scholar] [CrossRef]
- Moon-van der Staay, S.Y.; van der Staay, G.W.M.; Guillou, L.; Vaulot, D.; Claustre, H.; Medlin, L.K. Abundance and Diversity of Prymnesiophytes in the Picoplankton Community from the Equatorial Pacific Ocean Inferred from 18s Rdna Sequences. Limnol. Oceanogr. 2000, 45, 98–109. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Pan, X.; Yang, H.-H.; George, T.W.; Shiah, F.-K. Controls on temporal and spatial variations of phytoplankton pigment distribution in the Northern South China Sea. Deep. Sea Res. II Top. Stud. Oceanogr. 2015, 117, 65–85. [Google Scholar] [CrossRef]


| Abbreviations | Definition | Unit |
|---|---|---|
| aph() | The absorption coefficient of phytoplankton | m−1 |
| () | The ratio of the absorption coefficient of phytoplankton to chlorophyll-a concentration (the specific-absorption coefficient of phytoplankton) | m2 mg−1 |
| apg() | The non-watery beam absorption | m−1 |
| aLH(676) | The absorption line height at 676 nm | m−1 |
| (676) | The specific-absorption coefficient of phytoplankton at 676 nm derived from apg() | m2 mg−1 |
| am | The maximum (676) or (676) determined from in situ measurements | m2 mg−1 |
| (676) | The observed specific absorption coefficient of chlorophyll-a at 676 nm derived from (676) or (676) | m2 mg−1 |
| The theoretical specific absorption coefficient of chlorophyll-a at 676 nm | m2 mg−1 | |
| Chla | Total chlorophyll-a concentration | mg m−3 |
| ChlaLH | The value of Chla derived from apg() based on the absorption line height method | mg m−3 |
| The exponent of the phytoplankton size spectra | Dimensionless | |
| C | Total phytoplankton carbon biomass | mg m−3 |
| Cp | The picophytoplankton carbon biomass | mg m−3 |
| Cn | The nanophytoplankton carbon biomass | mg m−3 |
| Cm | The microphytoplankton carbon biomass | mg m−3 |
| Fp | The fractions of carbon biomass associated with picophytoplankton | Dimensionless |
| Fn | The fractions of carbon biomass associated with nanophytoplankton | Dimensionless |
| Fm | The fractions of carbon biomass associated with microphytoplankton | Dimensionless |
| The ratio of Cp to the chlorophyll-a concentration associated with picophytoplankton | Dimensionless | |
| The ratio of Cn to the chlorophyll-a concentration associated with nanophytoplankton | Dimensionless | |
| The ratio of Cm to the chlorophyll-a concentration associated with microphytoplankton | Dimensionless | |
| The ratio of C to Chla (C:Chla) | Dimensionless | |
| POC | Particulate organic carbon concentration | mg m−3 |
| MLD | The mixed layer depth | m |
| Zeu | The depth of the euphotic zone | m |
| ZT22 | The depth of 22 | m |
| CML | The C maximum layer | none |
| Parameters | Units | Mean | Max | Min | SD | Num |
|---|---|---|---|---|---|---|
| C | mg m−3 | 16.53 | 62.02 | 2.38 | 12.57 | 215 |
| Cp | mg m−3 | 11.91 | 47.21 | 0.62 | 10.47 | 215 |
| Cn | mg m−3 | 3.69 | 25.70 | 0.21 | 3.46 | 215 |
| Cm | mg m−3 | 0.93 | 13.45 | 0.0056 | 1.36 | 215 |
| None | 53.77 | 91.44 | 25.85 | 17.55 | 215 | |
| None | 83.09 | 96.36 | 72.15 | 5.96 | 215 | |
| None | 33.85 | 39.26 | 29.39 | 2.43 | 215 | |
| None | 17.44 | 17.91 | 17.04 | 0.21 | 215 | |
| POC | mg m−3 | 60.84 | 185.48 | 27.23 | 32.59 | 83 |
| C:POC | % | 41.27 | 95.32 | 5.54 | 25.80 | 83 |
| Empirical Relationship | Reference/Dominated Phytoplankton Species | Num | R | RMSE | Bias | MAE |
|---|---|---|---|---|---|---|
| C = | Buck et al. (1996) [54] | 215 | 0.90 | 19.96 | 2.53 | 2.53 |
| C = | Sathyendranath et al. (2009) [14] | 215 | 0.90 | 13.29 | 2.13 | 2.13 |
| C = | Marañón et al. (2014) [53] | 215 | 0.88 | 8.19 | 1.42 | 1.51 |
| C = | Loisel et al. (2018) [55] | 215 | 0.88 | 11.50 | 1.63 | 1.66 |
| C = | This study/All data | 215 | 0.89 | 5.85 | 1.20 | 1.40 |
| C = | This study/Prochlorococcus | 65 | 0.91 | 1.60 | 1.02 | 1.19 |
| C = | This study/Synechococcus-like cyanobacteria | 29 | 0.82 | 4.25 | 1.08 | 1.36 |
| C = | This study/Haptophytes | 60 | 0.82 | 6.21 | 1.06 | 1.24 |
| C = | This study/Diatoms | 7 | 0.84 | 9.97 | 1.09 | 1.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Zhou, W.; Cao, W.; Liu, Y.; Wang, G.; Deng, L.; Li, C.; Zhang, Y.; Zeng, K. Vertical Variability of Total and Size-Partitioned Phytoplankton Carbon in the South China Sea. Remote Sens. 2021, 13, 993. https://doi.org/10.3390/rs13050993
Zheng W, Zhou W, Cao W, Liu Y, Wang G, Deng L, Li C, Zhang Y, Zeng K. Vertical Variability of Total and Size-Partitioned Phytoplankton Carbon in the South China Sea. Remote Sensing. 2021; 13(5):993. https://doi.org/10.3390/rs13050993
Chicago/Turabian StyleZheng, Wendi, Wen Zhou, Wenxi Cao, Yupeng Liu, Guifen Wang, Lin Deng, Cai Li, Yu Zhang, and Kai Zeng. 2021. "Vertical Variability of Total and Size-Partitioned Phytoplankton Carbon in the South China Sea" Remote Sensing 13, no. 5: 993. https://doi.org/10.3390/rs13050993
APA StyleZheng, W., Zhou, W., Cao, W., Liu, Y., Wang, G., Deng, L., Li, C., Zhang, Y., & Zeng, K. (2021). Vertical Variability of Total and Size-Partitioned Phytoplankton Carbon in the South China Sea. Remote Sensing, 13(5), 993. https://doi.org/10.3390/rs13050993








