A New Method for Extracting Individual Plant Bio-Characteristics from High-Resolution Digital Images
Abstract
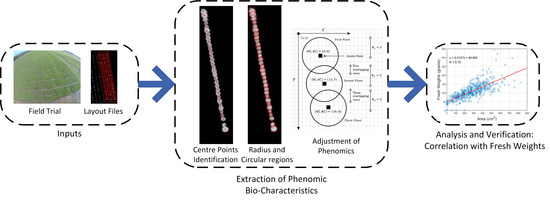
:1. Introduction
2. Problem Statements
- to identify bounding boxes with no plants;
- to calculate accurate individual plant areas, despite overlapping adjacent plants;
- to calculate accurate individual plant NDVI values, despite overlapping adjacent plants;
3. Methods
3.1. Background Correction
3.2. Center Point Calculation
3.3. Extraction of Plant Areas
3.4. Extraction of NDVI Values
3.4.1. Finding the Overlapping Pixel Rows
3.4.2. Adjusting NDVI Values at Overlapping Pixel Rows
- the maximum and minimum NDVI values of the plant are first calculated, labelled as and ; respectively;
- the whole center row is updated and will be used as a reference for the adjustment of plant pixels at overlapping rows. The step size, which is the difference of NDVI values between two adjacent pixels, is calculated as:
- Let us take a symmetric reference vector, , such that The NDVI values are adjusted as following:
3.5. Testing of the Algorithm
4. Results and Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tester, M.; Langridge, P. Breeding Technologies to Increase Crop Production in a Changing World. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the Global Food Demand of the Future by Engineering Crop Photosynthesis and Yield Potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.B.; Cheng, R.; Sirault, X.R.R.; Rungrat, T.; Murray, K.D.; Trtilek, M.; Furbank, R.T.; Badger, M.; Pogson, B.J.; O Borevitz, J. TraitCapture: Genomic and environment modelling of plant phenomic data. Curr. Opin. Plant Biol. 2014, 18, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Pratap, A.; Kumar, S. Plant Phenomics: An Overview. In Phenomics in Crop Plants: Trends, Options and Limitations; Springer: New Delhi, India, 2015; pp. 1–10. [Google Scholar]
- Sticklen, M.B. Feedstock Crop Genetic Engineering for Alcohol Fuels. Crop. Sci. 2007, 47, 2238–2248. [Google Scholar] [CrossRef] [Green Version]
- Van der Kooi, C.J.; Reich, M.; Löw, M.; De Kok, L.J.; Tausz, M. Growth and yield stimulation under elevated CO2 and drought: A meta-analysis on crops. Environ. Exp. Bot. 2017, 122, 150–157. [Google Scholar] [CrossRef]
- O’Malley, R.C.; Ecker, J.R. Linking genotype to phenotype using the Arabidopsis unimutant collection. Plant J. 2010, 61, 928–940. [Google Scholar] [CrossRef]
- Weigel, D.; Richard, M. The 1001 Genomes Project for Arabidopsis thaliana. Genome Biol. 2009, 10, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Cannon, S.B.; May, G.D.; Jackson, S.A. Three Sequenced Legume Genomes and Many Crop Species: Rich Opportunities for Translational Genomics. Plant Physiol. 2009, 151, 970–977. [Google Scholar] [CrossRef] [Green Version]
- International Brachypodium Initiative. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 2011, 463, 763–770. [Google Scholar]
- Atwell, S.; Huang, Y.S.; Vilhjálmsson, B.J.; Willems, G.; Horton, M.W.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.M.; Hu, T.T.; et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nat. Cell Biol. 2010, 465, 627–631. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, N.; Jia, T.; Leach, L.; Cockram, J.; Comadran, J.; Shaw, P.; Waugh, R.; Luo, Z. Genome-wide association mapping of agronomic and morphologic traits in highly structured populations of barley cultivars. Theor. Appl. Genet. 2012, 124, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Lucocq, J.M. Efficient quantitative morphological phenotyping of genetically altered organisms using stereology. Transgenic Res. 2007, 16, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Crane, M.M.; Lu, H. Automated on-chip rapid microscopy, phenotyping and sorting of C. elegans. Nat. Methods 2008, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, R.; Benfey, P.N. High-throughput phenotyping of multicellular organisms: Finding the link between genotype and phenotype. Genome Biol. 2011, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, N. High-throughput phenotyping. Nat. Methods 2010, 7, 1. [Google Scholar] [CrossRef]
- P-Sanz, F.; Navarro, P.J.; E-Cortines, M. Plant phenomics: An overview of image acquisition technologies and image data analysis algorithms. Gigascience 2017, 6, 1–18. [Google Scholar]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man, Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Vincent, L.; Soille, P. Watersheds in digital spaces: An efficient algorithm based on immersion simulations. IEEE Trans. Pattern Anal. Mach. Intell. 1991, 13, 583–598. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, B.P.; Spangenberg, G.; Kant, S. Fusion of Spectral and Structural Information from Aerial Images for Improved Biomass Estimation. Remote Sens. 2020, 12, 3164. [Google Scholar] [CrossRef]
- Gebremedhin, A.; Badenhorst, P.; Wang, J.; Shi, F.; Breen, E.; Giri, K.; Spangenberg, G.C.; Smith, K. Development and Validation of a Phenotyping Computational Workflow to Predict the Biomass Yield of a Large Perennial Ryegrass Breeding Field Trial. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Silva, J.C.E.; Kerr, R.J. Accounting for competition in genetic analysis, with particular emphasis on forest genetic trials. Tree Genet. Genomes 2012, 9, 1–17. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Neumann, K.; Klukas, C.; Friedel, S.; Rischbeck, P.; Chen, D.; Entzian, A.; Stein, N.; Graner, A.; Kilian, B. Dissecting spatiotemporal biomass accumulation in barley under different water regimes using high-throughput image analysis. Plant Cell Environ. 2015, 38, 1980–1996. [Google Scholar] [CrossRef]
- Tackenberg, O. A New Method for Non-destructive Measurement of Biomass, Growth Rates, Vertical Biomass Distribution and Dry Matter Content Based on Digital Image Analysis. Ann. Bot. 2006, 99, 777–783. [Google Scholar] [CrossRef]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the Phenotypic Components of Crop Plant Growth and Drought Responses Based on High-Throughput Image Analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Czauderna, T.; Hoffmann, R.; Stein, N.; Schreiber, F. HTPheno: An image analysis pipeline for high-throughput plant phenotyping. BMC Bioinform. 2011, 12, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, R.; Spalding, E.P.; Ferrier, N.J. A high throughput robot system for machine vision based plant phenotype studies. Mach. Vis. Appl. 2012, 24, 619–636. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.D.; Parks, B.M.; Spalding, E.P. Computer-vision analysis of seedling responses to light and gravity. Plant J. 2007, 52, 374–381. [Google Scholar] [CrossRef]
- Clark, R.T.; MacCurdy, R.B.; Jung, J.K.; Shaff, J.E.; McCouch, S.R.; Aneshansley, D.J.; Kochian, L.V. Three-Dimensional Root Phenotyping with a Novel Imaging and Software Platform. Plant Physiol. 2011, 156, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, T.; Kerbyson, D. Size invariant circle detection. Image Vis. Comput. 1999, 17, 795–803. [Google Scholar] [CrossRef]
- Yuen, H.K.; Princen, J.; Dlingworth, J.; Kittler, J. A Comparative Study of Hough Transform Methods for Circle Finding. Image Vis. Comput. 1989, 8, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Gebremedhin, A.; Badenhorst, P.; Wang, J.; Giri, K.; Spangenberg, G.; Smith, K. Development and Validation of a Model to Combine NDVI and Plant Height for High-Throughput Phenotyping of Herbage Yield in a Perennial Ryegrass Breeding Program. Remote Sens. 2019, 11, 2494. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.L.; Nicewander, W.A. Thirteen Ways to Look at the Correlation Coefficient. Am. Stat. 1988, 42, 59. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Villanueva, B.; Woolliams, J.A. Accuracy of Predicting the Genetic Risk of Disease Using a Genome-Wide Approach. PLoS ONE 2008, 3, e3395. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Image Time Point | Correlation | |
|---|---|---|
| Area of Rectangular Bounding Boxes | Area of Circular Plant Regions | |
| 9 May 2017 | 0.74 | 0.75 |
| 5 July 2017 | 0.30 | 0.74 |
| 11 September 2017 | 0.28 | 0.63 |
| 20 November 2017 | 0.30 | 0.66 |
| Image Time Point | Correlation | ||
|---|---|---|---|
| Unadjusted NDVI from Rectangular Boxes | Unadjusted NDVI from Circular Plant Regions | Adjusted NDVI from Circular Plant Regions | |
| 9 May 2017 | 0.56 | 0.56 | 0.57 |
| 5 July 2017 | 0.55 | 0.58 | 0.59 |
| 11 September 2017 | 0.52 | 0.54 | 0.55 |
| 20 November 2017 | 0.51 | 0.53 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabab, S.; Breen, E.; Gebremedhin, A.; Shi, F.; Badenhorst, P.; Chen, Y.-P.P.; Daetwyler, H.D. A New Method for Extracting Individual Plant Bio-Characteristics from High-Resolution Digital Images. Remote Sens. 2021, 13, 1212. https://doi.org/10.3390/rs13061212
Rabab S, Breen E, Gebremedhin A, Shi F, Badenhorst P, Chen Y-PP, Daetwyler HD. A New Method for Extracting Individual Plant Bio-Characteristics from High-Resolution Digital Images. Remote Sensing. 2021; 13(6):1212. https://doi.org/10.3390/rs13061212
Chicago/Turabian StyleRabab, Saba, Edmond Breen, Alem Gebremedhin, Fan Shi, Pieter Badenhorst, Yi-Ping Phoebe Chen, and Hans D. Daetwyler. 2021. "A New Method for Extracting Individual Plant Bio-Characteristics from High-Resolution Digital Images" Remote Sensing 13, no. 6: 1212. https://doi.org/10.3390/rs13061212