Changes in the Greenness of Mountain Pine (Pinus mugo Turra) in the Subalpine Zone Related to the Winter Climate
Abstract
:1. Introduction
2. Material and Methods
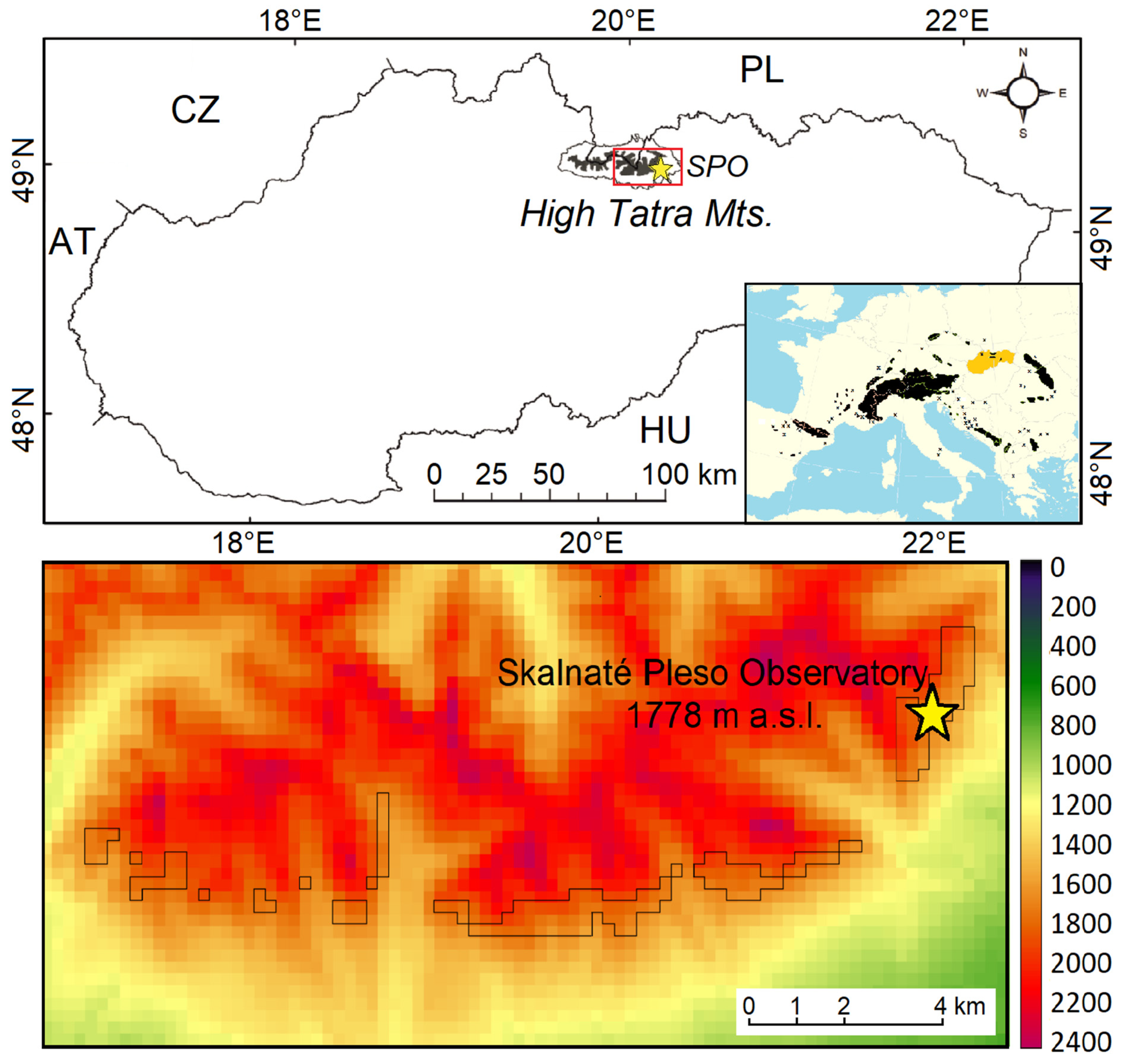
2.1. Studied Area
2.2. Meteorological Measurements and Calculations of Winter Climate Indices
- Average snow depth—ASD (cm/day) is an average of the cumulative snow depth (SDPSC) when counting for the days (dPSC) during the period of permanent snow cover (PSC).ASD = ∑SDPSC/∑dPSCThe lack of snow cover exposes the evergreen vegetation to winter desiccation and to the abrasion of sprouts by wind-blown ice. The values of winter snow depth during 2000–2020 were compared to those of the previous period, 1979–1999, to analyse changes in the snow cover.
- Winter warm spells—WWS were calculated as the incidence of five and more consecutive days when the maximum daily air temperature (TMAX) exceeded 5 °C as an interruption of the freezing period of the winter season. During this event, the increased temperature results in loss of snow cover and exposes the vegetation to initially warm and then freezing temperatures. Since the WWS can occur several times during one winter, the discrete WWS were numbered (e.g., WWS1, WWS2, etc.). To evaluate the evolution of winter warm spells, their incidence over 2000–2020 was compared to the previous periods between 1940 and 1999.
- Winter-season air temperature—TW (°C) is an average of the daily air temperatures (Td) of the winter season (W) starting on the 1st of December and ending on the 31st of March.where n is the number of days in the analysed period. The air temperature of the ith day is calculated from the term measurements at 7, 14 and 21 o’clock as defined in the following equation:TW = 1/n ∑Td,iTd,i = (T7 + T14+ 2*T21)/4Monthly air temperatures—TXII, TI, TII and TIII (°C) were calculated using the Equation (2) as averages of the daily air temperatures (Td) of December, January, February and March, respectively.
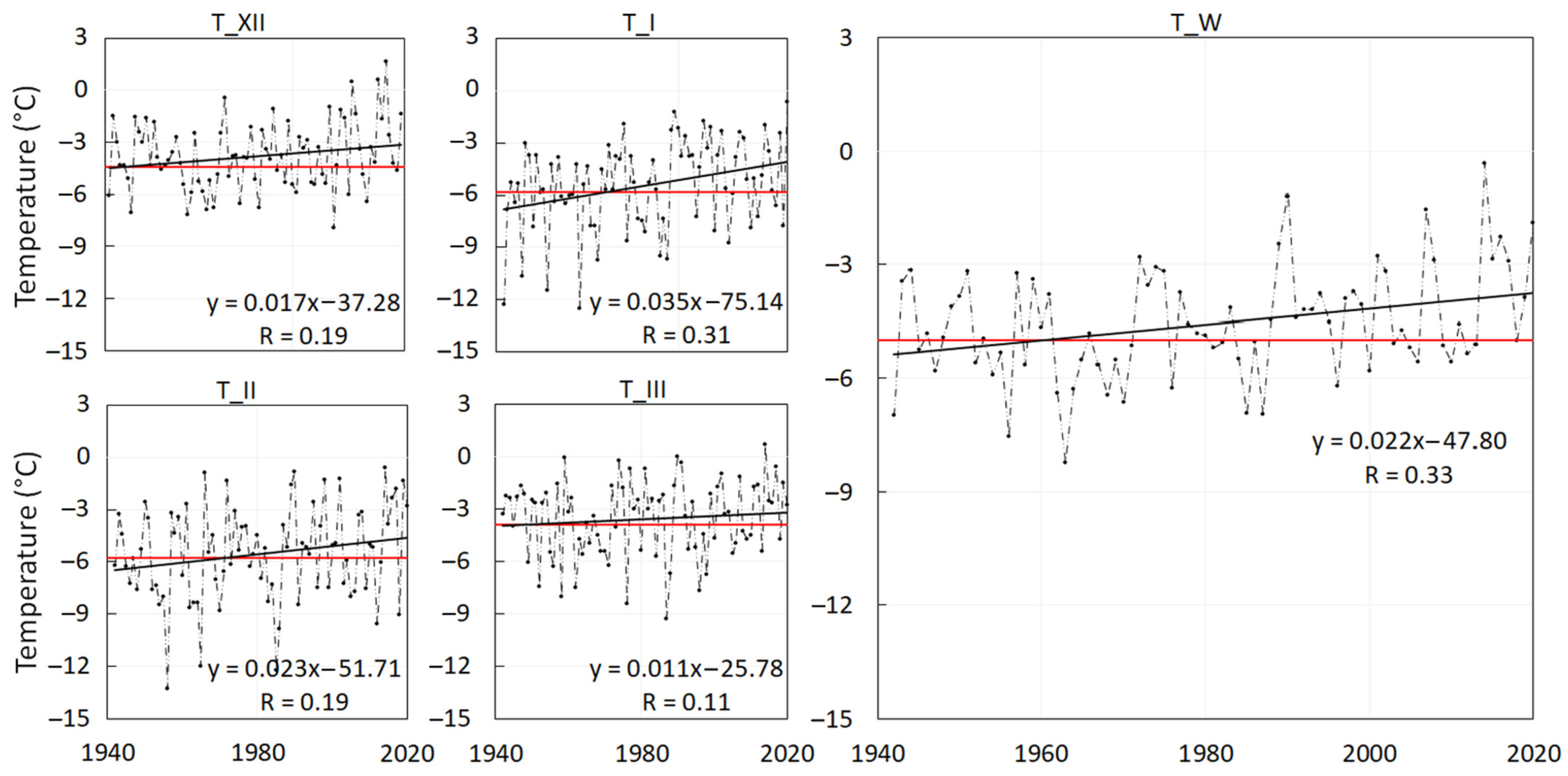
- We applied the linear trend (Pearson’s correlation) to the average monthly air temperatures and the winter-season air temperatures over the period 1941–2020,
- We compared the average monthly air temperatures and the winter-season air temperatures to corresponding periods’ climatic normal, here known as the temperature normal (TN), calculated from the daily temperatures of 1961–1990,
- Since we have two complete climatic normal periods for SPO, 1961–1990 and 1991–2020, we analysed the differences in temperatures between the two normal periods,
- We correlated the temperatures with the average snow depth.
2.3. Vegetation Greenness from MODIS Remote Sensing Data
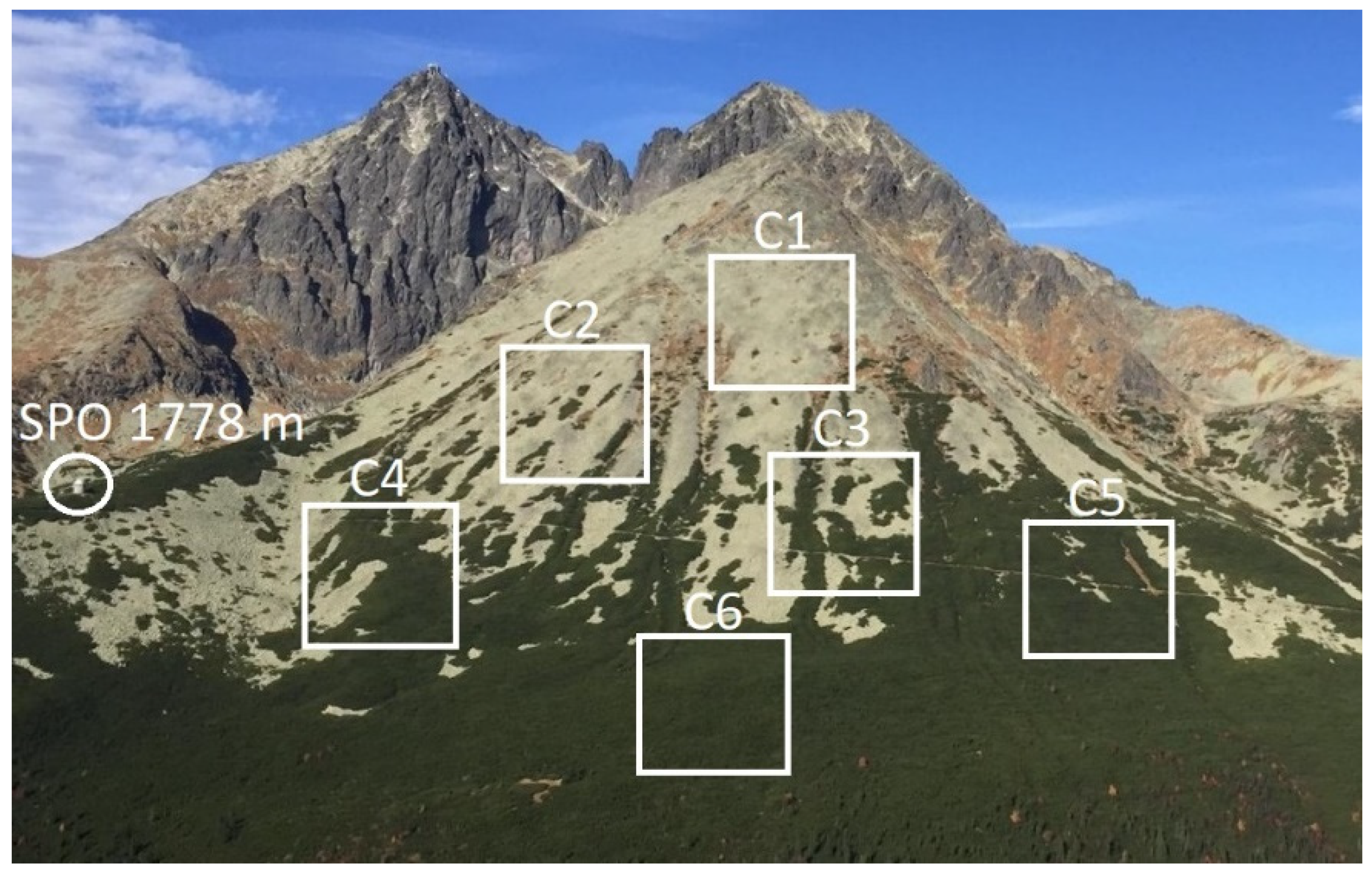
2.4. Altitude and Density of Studied P. mugo Thickets
- The highly significant correlation (p < 0.01) was exhibited between classes C2 and C3 (R = 0.75); C4 and C5 (R = 0.72); C5 and C6 (R = 0.75).
- While C1 significantly correlated with C2 (R = 0.48) and C3 (R = 0.53), no correlation was revealed between C1 and C5 (R = 0.04) and C6 (R = 0.06), and a weak correlation was found between C1 and C4 (R = 0.30). The correlation coefficient between C3 and C4 was lower than that between C4 and C5, and C4 and C6.
- Following the correlations, we created two density classes, where pixels with lower amount of greenness indicated by NDVI between 0.31–0.60 (classes C1, C2, C3) were classified as sparse thickets (density class D1) and pixels with higher amount of greenness indicated by the NDVI between 0.61–0.90 (classes C4, C5, C6) were classified as dense thickets (density class D2) (Table 3).
3. Results
3.1. Effect of Winter Climate Indices on P. mugo Greenness
- 0.17 °C per decade in December (overall 1.4 °C),
- 0.35 °C per decade in January (overall 2.8 °C),
- 0.23 °C per decade in February (overall 1.8 °C),
- 0.11 °C per decade in March (overall 0.9 °C),
- 0.22 °C per decade in the winter season (overall 1.8 °C).
3.2. Greenness at the Beginning and End of the Studied Period 2000–2020
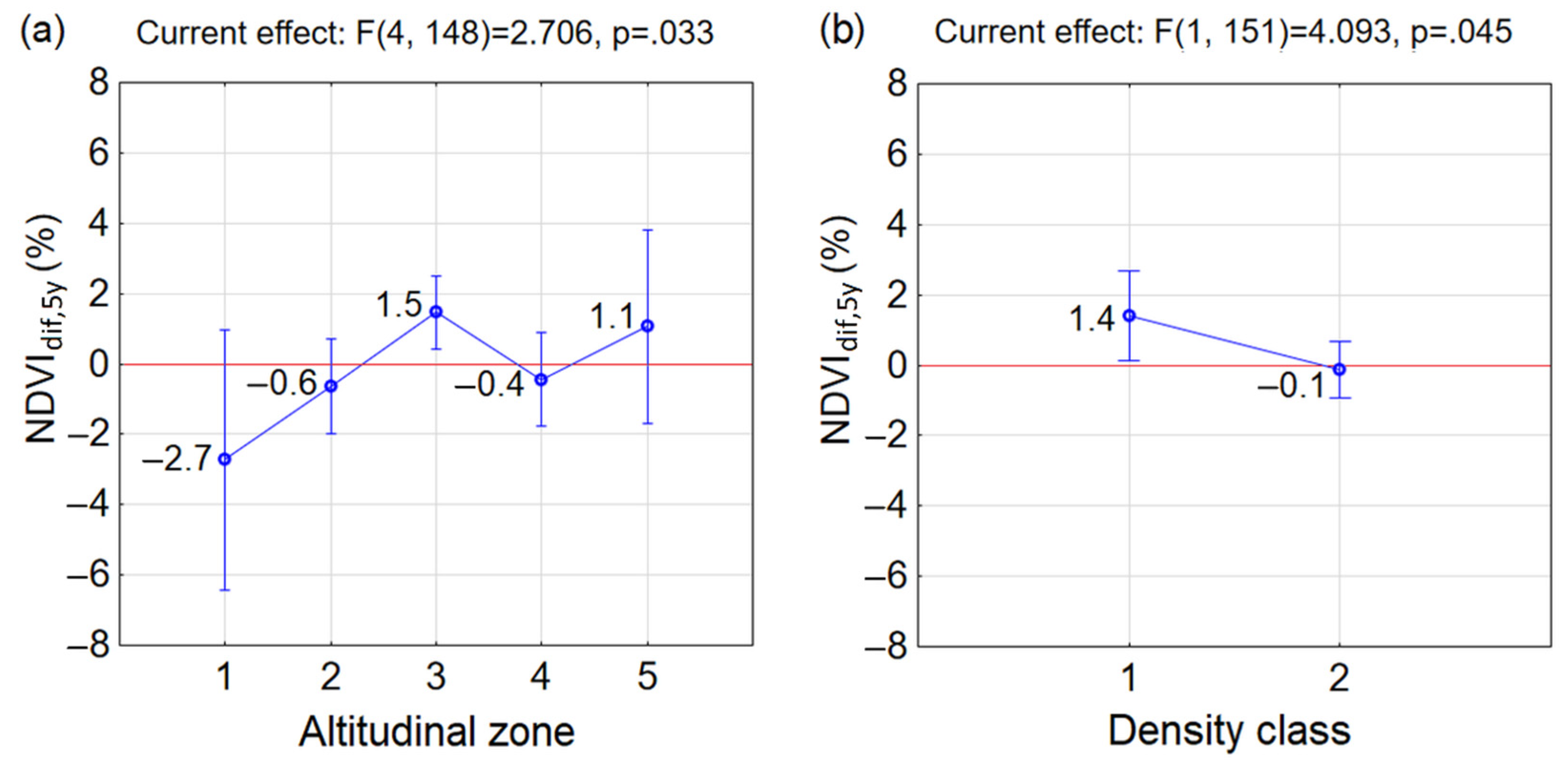
- Similar reactions of P. mugo to climate change occurred in sparse classes along altitudes (insignificant differences between combinations of D1A2, D1A3, D1A4 and D1A5). The p = 0.05 (but insignificant) between neighbouring sparse classes D1A2 and D1A3 pointed at the considerable difference between them. This may indicate, that the reaction to the climate change in sparse thickets was negative up to altitudinal zone A2, above which it became positive. This confirmed negative mean NDVIdif,5y in D1A2 and positive mean NDVIdif,5y in D1A3, D1A4 and D1A5. However, it is important to mention that only a single MODIS pixel, ND1A2 = 1, is probably not a representative sample of this group.
- In the dense class D2, significant differences occurred only between D2A3 and D2A4, while the other combinations of D2 and altitudinal zones differed insignificantly.
- Significant differences were found in combinations between different densities and altitudinal zones occurring in D2A1, D2A2 and D2A4 and D1A3. For the first three combinations the NDVIdif,5y decreased, which indicates that the changing climate influenced these dense stands negatively.
- In D1A3, the highest increase of mean NDVIdif,5y of P. mugo stands out among the other groups over the last two decades and was determined to be the most positive response to climate change. The increase of mean NDVIdif,5y under climate change was recorded also in combinations D2A3, D1A4, D1A5 and D2A5.
- Differences between D1 and D2 in individual altitudinal zones were insignificant.
4. Discussion
5. Conclusions
- Across the studied subalpine area in High Tatra Mts, we found a considerable effect of stand factors—altitude and density, on the changes of P. mugo greenness induced by the winter climate.
- We observed a negative effect of winter warm spells in dense P. mugo thickets and at lower altitudes, because the warmer weather melts the snow cover, which serves as the protection to abrasion of needles and sprouts by windblown ice, winter desiccation and photo-inhibition.
- A positive correlation was found between greenness and average snow cover depth in dense thickets, indicating that the P. mugo benefits from the protection of higher snow cover. However, after winters with low snow cover, when the protection was insufficient, P. mugo greenness decreased in the following summer.
- We also found a positive effect of the rising winter temperatures on the greenness of sparse thickets in the following summer, which we interpret as mild winters having a less deteriorating effect on the greenness of P. mugo.
- Our results revealed that the temperature increase in the first winter month—December—caused a significant increase in the greenness of P. mugo in the following summer, particularly in sparse thickets and at higher altitudes. The later onset of winter freezing temperatures provides more time for the lignification of current year fresh shoots and thus enhances their resistance to extreme winter weather events.
- In the studied period 2000–2020, we found an overall increase of greenness at the end of the period compared to its beginning, meaning that climate warming created suitable conditions for P. mugo and advanced its expansion into the alpine zone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inouye, D.W.; Wielgolaski, F.E. Phenology of High-Altitude Climates; Schwartz, M.D., Ed.; Phenology: An Integrative Environmental Science; Kluwer Academic Publishers: Alphen, The Netherlands, 2003; pp. 195–214. [Google Scholar]
- Weiss, D.J.; Walsh, S.J. Remote Sensing of Mountain Environments. Geogr. Compass 2008, 3, 1–21. [Google Scholar] [CrossRef]
- Richardson, D.M. Ecology and Biogeography of Pinus; Cambridge University Press: Cambridge, UK, 1998; p. 527. [Google Scholar]
- Šibík, J.; Šibíková, I.; Kliment, J. The subalpine Pinus mugo-communities of the Carpathians with a European perspective. Phytocoenologia 2010, 40, 155–188. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life. Functional Plant Ecology of High Mountain Ecosystems, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003; p. 344. ISBN 978-3-642-18970-8. [Google Scholar]
- Říha, V. Jihočeské rašeliny, jejich vztah k lesu a okolí [South Bohemian peatlands, their relation to the forest and its surroundings]. Sborn. Masaryk. Akad. Práce 1938, 12, 143–194. [Google Scholar]
- Solař, J.; Janiga, M. Long-term Changes in Dwarf Pine (Pinus mugo) Cover in the High Tatra Mountains, Slovakia. Mt. Res. Dev. 2013, 33, 51–62. [Google Scholar] [CrossRef]
- Wild, J.; Winkler, E. Krummholz and grassland coexistence above the forest-line in the Krkonoše Mountains: Grid-based model of shrub dynamics. Ecol. Model. 2008, 213, 293–307. [Google Scholar] [CrossRef]
- Treml, V.; Wild, J.; Chuman, T.; Potůčková, M. Assessing the Change in Cover of Non-Indigenous Dwarf-Pine Using Aerial Photographs, a Case Study from the Hrubý Jeseník Mts., the Sudetes. J. Landsc. Ecol. 2010, 3, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Barry, R.G. Mountain Weather and Climate Third Edition; Cambridge University Press (CUP): Cambridge, UK, 2008; p. 506. [Google Scholar]
- Marty, C.; Meister, R. Long-term snow and weather observations at Weissfluhjoch and its relation to other high-altitude observatories in the Alps. Theor. Appl. Clim. 2012, 110, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Fan, X.; Wang, M. Evidence of high-elevation amplification versus Arctic amplification. Sci. Rep. 2016, 6, 19219. [Google Scholar] [CrossRef] [Green Version]
- Ohmura, A. Enhanced temperature variability in high-altitude climate change. Theor. Appl. Clim. 2012, 110, 499–508. [Google Scholar] [CrossRef]
- Mountain Research Initiative EDW Working Group; Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Steger, C.; Kotlarski, S.; Jonas, T.; Schär, C. Alpine snow cover in a changing climate: A regional climate model perspective. Clim. Dyn. 2012, 41, 735–754. [Google Scholar] [CrossRef] [Green Version]
- Jiménez Cisneros, B.E.; Oki, T.; Arnell, N.W.; Benito, G.; Cogley, J.G.; Döll, P.; Jiang, T.; Mwakalila, S.S. Freshwater Resources, In Climate Change: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 229–269. [Google Scholar]
- Räisänen, J. Warmer climate: Less or more snow? Clim. Dyn. 2008, 30, 307–319. [Google Scholar] [CrossRef]
- DeMaria, E.M.C.; Roundy, J.K.; Wi, S.; Palmer, R.N. The Effects of Climate Change on Seasonal Snowpack and the Hydrology of the Northeastern and Upper Midwest United States. J. Clim. 2016, 29, 6527–6541. [Google Scholar] [CrossRef] [Green Version]
- Croce, P.; Formichi, P.; Landi, F.; Mercogliano, P.; Bucchignani, E.; Dosio, A.; Dimova, S. The snow load in Europe and the climate change. Clim. Risk Manag. 2018, 20, 138–154. [Google Scholar] [CrossRef]
- Räisänen, J.; Eklund, J. 21st Century changes in snow climate in Northern Europe: A high-resolution view from ENSEMBLES regional climate models. Clim. Dyn. 2012, 38, 2575–2591. [Google Scholar] [CrossRef]
- Stolina, M. Ochrana Lesa (Forest Protection); Priroda: Bratislava, Slovakia, 1985; p. 473. [Google Scholar]
- Sonesson, M.; Callaghan, T.V. Strategies of survival in plants of the Fennoscandian Arctic. Arctic 1991, 44, 95–105. [Google Scholar] [CrossRef]
- Kershaw, G.P.; Jones, H.G.; Pomeroy, J.W.; Walker, D.A.; Hoham, R.W. Snow Ecology: An Interdisciplinary Examination of Snow-Covered Ecosystems. Arctic Antarct. Alp. Res. 2002, 34, 486. [Google Scholar] [CrossRef]
- Bokhorst, S.F.; Bjerke, J.W.; Tømmervik, H.; Callaghan, T.V.; Phoenix, G.K. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. J. Ecol. 2009, 97, 1408–1415. [Google Scholar] [CrossRef]
- Tomczyk, A.M.; Sulikowska, A.; Bednorz, E.; Półrolniczak, M. Atmospheric circulation conditions during winter warm spells in Central Europe. Nat. Hazards 2019, 96, 1413–1428. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, M.R.F.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Chang. 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Kopáček, J.; Kaňa, J.; Bičárová, S.; Brahney, J.; Navrátil, T.; Norton, S.A.; Porcal, P.; Stuchlík, E. Climate change accelerates recovery of the Tatra Mountain lakes from acidification and increases their nutrient and chlorophyll a concentrations. Aquat. Sci. 2019, 81, 70. [Google Scholar] [CrossRef] [Green Version]
- Kerr, J.T.; Ostrovsky, M. From space to species: Ecological applications for remote sensing. Trends Ecol. Evol. 2003, 18, 299–305. [Google Scholar] [CrossRef]
- Beck, P.S.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Dunn, A.H.; De Beurs, K.M. Land surface phenology of North American mountain environments using moderate resolution imaging spectroradiometer data. Remote Sens. Environ. 2011, 115, 1220–1233. [Google Scholar] [CrossRef]
- Hmimina, G.; Dufrêne, E.; Pontailler, J.-Y.; Delpierre, N.; Aubinet, M.; Caquet, B.; De Grandcourt, A.; Burban, B.; Flechard, C.R.; Granier, A.; et al. Evaluation of the potential of MODIS satellite data to predict vegetation phenology in different biomes: An investigation using ground-based NDVI measurements. Remote Sens. Environ. 2013, 132, 145–158. [Google Scholar] [CrossRef]
- Bucha, T.; Koreň, M. Phenology of the beech forests in the Western Carpathians from MODIS for 2000 2015. iForest Biogeosci. For. 2017, 10, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Lukasová, V.; Bucha, T.; Škvareninová, J.; Škvarenina, J. Validation and Application of European Beech Phenological Metrics Derived from MODIS Data along an Altitudinal Gradient. Forests 2019, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Palombo, C.; Van Gils, H.; Rossiter, D.G.; Tognetti, R.; Luo, G. Pinus mugoKrummholz Dynamics During Concomitant Change in Pastoralism and Climate in the Central Apennines. Mt. Res. Dev. 2017, 37, 75–86. [Google Scholar] [CrossRef]
- Minďáš, J.; Lapin, M.; Škvarenina, J. Climate Change and Forest in Slovakia (in Slovak). Národný Klimatický Program SR (National Climate Program SR); MŽP SR: Bratislava, Slovakia, 1996; Volume 5, p. 96. [Google Scholar]
- Škvarenina, J.; Križová, E.; Tomlain, J. Impact of the climate change on the water balance of altitudinal vegetation stages in Slovakia. Ekológia 2004, 23, 13–29. [Google Scholar]
- Ďurský, J.; Škvarenina, J.; Minďáš, J.; Mikova, A. Regional analysis of climate change impact on Norway spruce (Picea abies L. Karst.) growth in Slovak mountain forests. J. For. Sci. 2012, 52, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Bičárová, S.; Bezák, V.; Bilčík, D.; Čepčeková, E.; Fleischer, P.; Hlavatá, H.; Holko, L.; Jakubják, O.; Mačutek, J.; Majcin, D.; et al. Observatory of SAS at Skalnaté Pleso-70 Years of Meteorological Measurements; Geophysical Institute SAS: Stará Lesná, Slovakia, 2013; p. 63. ISBN 978-80-85754-29-2. [Google Scholar]
- Franch, B.; Vermote, E.; Sobrino, J.; Fédèle, E. Analysis of directional effects on atmospheric correction. Remote Sens. Environ. 2013, 128, 276–288. [Google Scholar] [CrossRef]
- Kristof, D.; Pataki, R. Novel vector-based pre-processing of MODIS data. In Ebook Remote Sensing for a Changing Europe; IOS Press: Amsterdam, The Netherlands, 2009; pp. 483–490. [Google Scholar]
- Marchand, P.J. The Changing Snowpack. Life in the Cold, 3rd ed.; University Press of New England: Hanover, UK, 1996; pp. 11–39. [Google Scholar]
- Neuner, G.; Ambach, D.; Aichner, K. Impact of snow cover on photoinhibition and winter desiccation in evergreen Rhododendron ferrugineum leaves during subalpine winter. Tree Physiol. 1999, 19, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Bartík, M.; Holko, L.; Jančo, M.; Škvarenina, J.; Danko, M.; Kostka, Z. Influence of Mountain Spruce Forest Dieback on Snow Accumulation and Melt. J. Hydrol. Hydromech. 2019, 67, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, N.; Behera, R. Photoinhibition of Photosynthesis: Role of Carotenoids in Photoprotection of Chloroplast Constituents. Photosynthetica 2001, 39, 481–488. [Google Scholar] [CrossRef]
- Goh, C.-H.; Ko, S.-M.; Koh, S.; Kim, Y.-J.; Bae, H.-J. Photosynthesis and Environments: Photoinhibition and Repair Mechanisms in Plants. J. Plant Biol. 2011, 55, 93–101. [Google Scholar] [CrossRef]
- Wipf, S.; Stoeckli, V.; Bebi, P. Winter climate change in alpine tundra: Plant responses to changes in snow depth and snowmelt timing. Clim. Chang. 2009, 94, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, J.B.; Nunez, M.; Bridle, K.L.; Parry, J.; Gibson, N. Causes and consequences of variation in snow incidence on the high mountains of Tasmania, 1983–2013. Aust. J. Bot. 2017, 65, 214. [Google Scholar] [CrossRef]
- Laternser, M.; Schneebeli, M. Long-term snow climate trends of the Swiss Alps (1931–99). Int. J. Clim. 2003, 23, 733–750. [Google Scholar] [CrossRef]
- Scherrer, S.C.; Appenzeller, C.; Laternser, M. Trends in Swiss Alpine snow days: The role of local- and large-scale climate variability. Geophys. Res. Lett. 2004, 31, 31. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.D.; Petkova, N. Snow cover variability in Bulgarian mountainous regions, 1931–2000. Int. J. Clim. 2007, 27, 1215–1229. [Google Scholar] [CrossRef]
- Kapnick, S.; Hall, A. Causes of recent changes in western North American snowpack. Clim. Dyn. 2011, 38, 1885–1899. [Google Scholar] [CrossRef]
- McCabe, G.J.; Wolock, D.M. Long-term variability in Northern Hemisphere snow cover and associations with warmer winters. Clim. Chang. 2009, 99, 141–153. [Google Scholar] [CrossRef]
- Minder, J.R. The Sensitivity of Mountain Snowpack Accumulation to Climate Warming. J. Clim. 2010, 23, 2634–2650. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, S.H.; Liston, G.E.; Tamstorf, M.P.; Westergaard-Nielsen, A.; Schmidt, N.M. Quantifying Episodic Snowmelt Events in Arctic Ecosystems. Ecosystems 2015, 18, 839–856. [Google Scholar] [CrossRef]
- Hadley, J.L.; Smith, W.K. Influence of Wind Exposure on Needle Desiccation and Mortality for Timberline Conifers in Wyoming, U.S.A. Arctic Alp. Res. 1983, 15, 127–135. [Google Scholar] [CrossRef]
- Mayr, S.; Schmid, P.; Rosner, S. Winter Embolism and Recovery in the Conifer Shrub Pinus mugo L. Forests 2019, 10, 941. [Google Scholar] [CrossRef] [Green Version]
- Kromka, M. Influence of Expected Climatic Changes on Mineralization of Soil Organic Matter (in Slovak); Národný klimatický program SR (National Climate Program SR); MŽP SR: Bratislava, Slovakia, 2001; Volume 11, pp. 88–102. [Google Scholar]
- Hůnová, I.; Novotný, R.; Uhlířová, H.; Vráblík, T.; Horálek, J.; Lomský, B.; Šrámek, V. The impact of ambient ozone on mountain spruce forests in the Czech Republic as indicated by malondialdehyde. Environ. Pollut. 2010, 158, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Sicard, P.; Dalstein-Richier, L.; Vas, N. Annual and seasonal trends of ambient ozone concentration and its impact on forest vegetation in Mercantour National Park (South-eastern France) over the 2000–2008 period. Environ. Pollut. 2011, 159, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Büker, P.; Pleijel, H.; Emberson, L.; Karlsson, P.E.; Uddling, J. A unifying explanation for variation in ozone sensitivity among woody plants. Glob. Chang. Biol. 2017, 24, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Bičárová, S.; Shashikumar, A.; Dalstein-Richier, L.; Lukasová, V.; Adamčíková, K.; Pavlendová, H.; Sitková, Z.; Buchholcerová, A.; Bilčík, D. The response of Pinus species to ozone uptake in different climate regions of Europe. Cent. Eur. For. J. 2020, 66, 255–268. [Google Scholar] [CrossRef]
- Parobeková, Z.; Bugala, M.; Kardoš, M.; Dovciak, M.; Lukáčik, I.; Saniga, M. Long-Term Changes in Dwarf Pine (Pinus mugo Turra) Cover and Growth in the Orava Beskid Mountains, Slovakia. Mt. Res. Dev. 2018, 38, 342–353. [Google Scholar] [CrossRef]
- Jørgensen, H. NOBANIS–Invasive Alien Species Fact Sheet–Pinus Mugo. Online Database of the European Network on Invasive Alien Species–NOBANIS 2010. Available online: https://www.nobanis.org/globalassets/speciesinfo/p/pinus-mugo/pinus_mugo.pdf (accessed on 19 March 2020).
- Kuras, T.; Beneš, J.; Konvička, M. Behaviour and within-habitat distribution of adult Erebia sudetica sudetica, endemic of the Hruby Jeseník Mts., Czech Republic (Nymphalidae, Satyrinae). Nota Lepidopterol. 2001, 24, 69–83. [Google Scholar]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent Plant Diversity Changes on Europe’s Mountain Summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef]
- Winkler, M.; Lamprecht, A.; Steinbauer, K.; Hülber, K.; Theurillat, J.-P.; Breiner, F.; Choler, P.; Ertl, S.; Girón, A.G.; Rossi, G.; et al. The rich sides of mountain summits—A pan-European view on aspect preferences of alpine plants. J. Biogeogr. 2016, 43, 2261–2273. [Google Scholar] [CrossRef]




| Bit No. | Parameter Name | Bit Combination—Parameter State |
|---|---|---|
| 0–1 | Cloud state | 00—Clear; 01—Cloudy; 10—Mixed; 11—Not set, assumed clear |
| 2 | Cloud shadow | 1—Yes; 0—No |
| 3–5 | Characteristics of land cover: land/water | 000—shallow ocean; 001—land; 010—ocean coast or lake shore; 011—Shallow inland water; 100—ephemeral water; 101—Deep inland water; 110—continental/moderate ocean; 111—Deep Ocean |
| 6–7 | Aerosol quantity | 00—Climatology; 01—Low; 10—Average; 11—High |
| 8–9 | Cirrus detection | 00—None; 01—Small; 10—Average; 11—High |
| 10 | Internal cloud algorithm | 1—Cloud; 0—No cloud |
| 11 | Internal fire algorithm | 1—fire; 0—No fire |
| 12 | Snow/ice | 1—Yes; 0—No |
| 13 | Pixel is adjacent to cloud | 1—Yes; 0—No |
| 14 | BRDF Correction performed */Salt pan ** | 1—Yes; 0—No |
| 15 | Internal snow mask | 1—snow; 0—No snow |
| Bit No. | 15 | 14 | 13 | 12 | 11 | 10 | 9 | 8 | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suitable state | 0 | 0 | 0/1 | 0 | 0 | 0 | 0 | 0 | 0/1 | 0/1 | 0 | 0 | 1 | 0/1 | 0 | 0 |
| DN value | 32,768 | 16,384 | 8192 | 4096 | 2048 | 1024 | 512 | 256 | 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 |
| NDVI Class | NDVI Range | Coverage of Shrubs | Density Class |
|---|---|---|---|
| C1 | 0.31–0.40 | incidence of individual P. mugo shrubs or very small groups | Sparse—D1 |
| C2 | 0.41–0.50 | individual groups of P. mugo | |
| C3 | 0.51–0.60 | half of the ground covered by P. mugo | |
| C4 | 0.61–0.70 | large fields of P. mugo | Dense—D2 |
| C5 | 0.71–0.80 | the area of P. mugo thickets is interrupted by small rocky fields | |
| C6 | 0.81–0.90 | P. mugo covers the whole area of pixel |
| Climate Index | All Pixels | Density Class | Altitudinal Zone (m a.s.l.) | |||||
|---|---|---|---|---|---|---|---|---|
| D1 (Sparse) | D2 (Dense) | A1 (1500–1600) | A2 (1600–1700) | A3 (1700–1800) | A4 (1800–1900) | A5 (1900–2000) | ||
| WWS | −0.32 | 0.04 | −0.47 | 0.10 | −0.54 | −0.54 | −0.06 | 0.31 |
| ASD | 0.34 | 0.03 | 0.45 | 0.16 | 0.50 | 0.22 | 0.31 | 0.06 |
| TW | 0.31 | 0.50 | 0.10 | −0.23 | −0.03 | 0.12 | 0.49 | 0.53 |
| TXII | 0.47 | 0.57 | 0.28 | 0.01 | 0.16 | 0.28 | 0.63 | 0.44 |
| TI | 0.12 | 0.11 | 0.09 | 0.13 | 0.00 | 0.06 | 0.12 | 0.26 |
| TII | 0.08 | 0.38 | −0.13 | −0.32 | −0.22 | −0.10 | 0.27 | 0.51 |
| TIII | −0.02 | 0.27 | −0.20 | −0.41 | −0.25 | −0.08 | 0.23 | 0.03 |
| Years with Occurrence of WWS | N 1 | WWS1 | WWS2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Duration (days) | TMAX (°C) | Snow Melt (%) | Remain SD 2 (mm) | Duration (Days) | TMAX (°C) | Snow Melt (%) | Remain SD (mm) | ||
| 2002 | 2 | 7 (31.1–6.2) | 12.5 | 47 | 8 | 5 (11–15.3) | 8.6 | 38 | 21 |
| 2004 | 1 | 8 (14–21.3) | 12.6 | 51 | 21 | - | - | - | - |
| 2008 | 1 | 6 (22–27.2) | 9.5 | 37 | 37 | - | - | - | - |
| 2010 | 1 | 5 (23–27.3) | 6.5 | 54 | 19 | - | - | - | - |
| 2012 | 1 | 5 (21–25.3) | 9.1 | 25 | 5 | - | - | - | - |
| 2014 | 2 | 6 (5–10.1) | 9.2 | 100 | 0 | 6 (10–15.3) | 8.9 | 34 | 11 |
| 2019 | 1 | 5 (15–19.2) | 11.2 | 7 | 26 | - | - | - | - |
| 2020 | 1 | 5 (14–18.1) | 10.6 | 29 | 10 | - | - | - | - |
| D | D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 | D1 | D2 | Mean NDVIdif,5y | Conf. interval | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | A | A1 | A1 | A2 | A2 | A3 | A3 | A4 | A4 | A5 | A5 | N | St.dev | −95% | 95% | |
| D1 | A1 | - | - | - | - | - | - | - | - | - | - | 0 | - | - | - | - |
| D2 | A1 | - | 0.56 | 0.27 | 0.01 | 0.06 | 0.10 | 0.61 | 0.13 | 0.23 | 5 | −2.7 | 1.9 | −6.4 | 0.9 | |
| D1 | A2 | - | 0.25 | 0.05 | 0.13 | 0.15 | 0.38 | 0.16 | 0.18 | 1 | −5.4 | 4.2 | −13.6 | 2.8 | ||
| D2 | A2 | - | 0.01 | 0.11 | 0.30 | 0.34 | 0.40 | 0.51 | 36 | −0.5 | 0.7 | −1.9 | 0.9 | |||
| D1 | A3 | - | 0.11 | 0.12 | 0.00 | 0.30 | 0.65 | 16 | 2.9 | 1.0 | 0.9 | 5.0 | ||||
| D2 | A3 | - | 0.81 | 0.02 | 0.98 | 0.86 | 47 | 1.0 | 0.6 | −0.2 | 2.2 | |||||
| D1 | A4 | - | 0.08 | 0.90 | 0.80 | 20 | 0.7 | 0.9 | −1.1 | 2.5 | ||||||
| D2 | A4 | - | 0.16 | 0.31 | 19 | −1.6 | 1.0 | −3.5 | 0.2 | |||||||
| D1 | A5 | - | 0.87 | 7 | 0.9 | 1.6 | −2.2 | 4.0 | ||||||||
| D2 | A5 | - | 2 | 1.5 | 2.9 | −4.3 | 7.3 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukasová, V.; Bucha, T.; Mareková, Ľ.; Buchholcerová, A.; Bičárová, S. Changes in the Greenness of Mountain Pine (Pinus mugo Turra) in the Subalpine Zone Related to the Winter Climate. Remote Sens. 2021, 13, 1788. https://doi.org/10.3390/rs13091788
Lukasová V, Bucha T, Mareková Ľ, Buchholcerová A, Bičárová S. Changes in the Greenness of Mountain Pine (Pinus mugo Turra) in the Subalpine Zone Related to the Winter Climate. Remote Sensing. 2021; 13(9):1788. https://doi.org/10.3390/rs13091788
Chicago/Turabian StyleLukasová, Veronika, Tomáš Bucha, Ľubica Mareková, Anna Buchholcerová, and Svetlana Bičárová. 2021. "Changes in the Greenness of Mountain Pine (Pinus mugo Turra) in the Subalpine Zone Related to the Winter Climate" Remote Sensing 13, no. 9: 1788. https://doi.org/10.3390/rs13091788
APA StyleLukasová, V., Bucha, T., Mareková, Ľ., Buchholcerová, A., & Bičárová, S. (2021). Changes in the Greenness of Mountain Pine (Pinus mugo Turra) in the Subalpine Zone Related to the Winter Climate. Remote Sensing, 13(9), 1788. https://doi.org/10.3390/rs13091788







