Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Detail
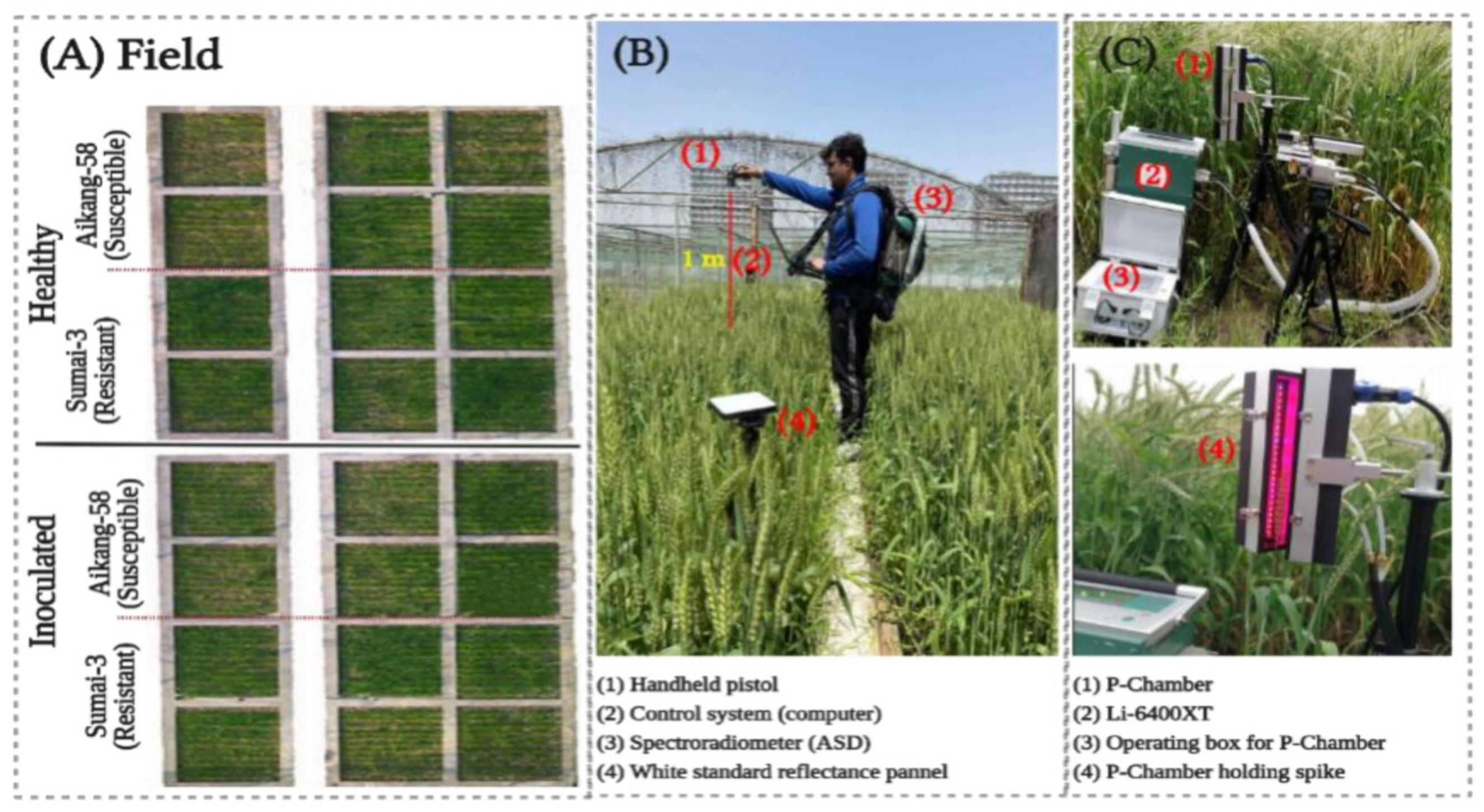
2.1.1. Study Site and Plant Material
2.1.2. Disease Inoculation and Description of Disease Severity Scaling
2.2. Data Acquisition
2.2.1. Field Reflectance Data Acquisition
2.2.2. Photosynthesis Rate (Pn)
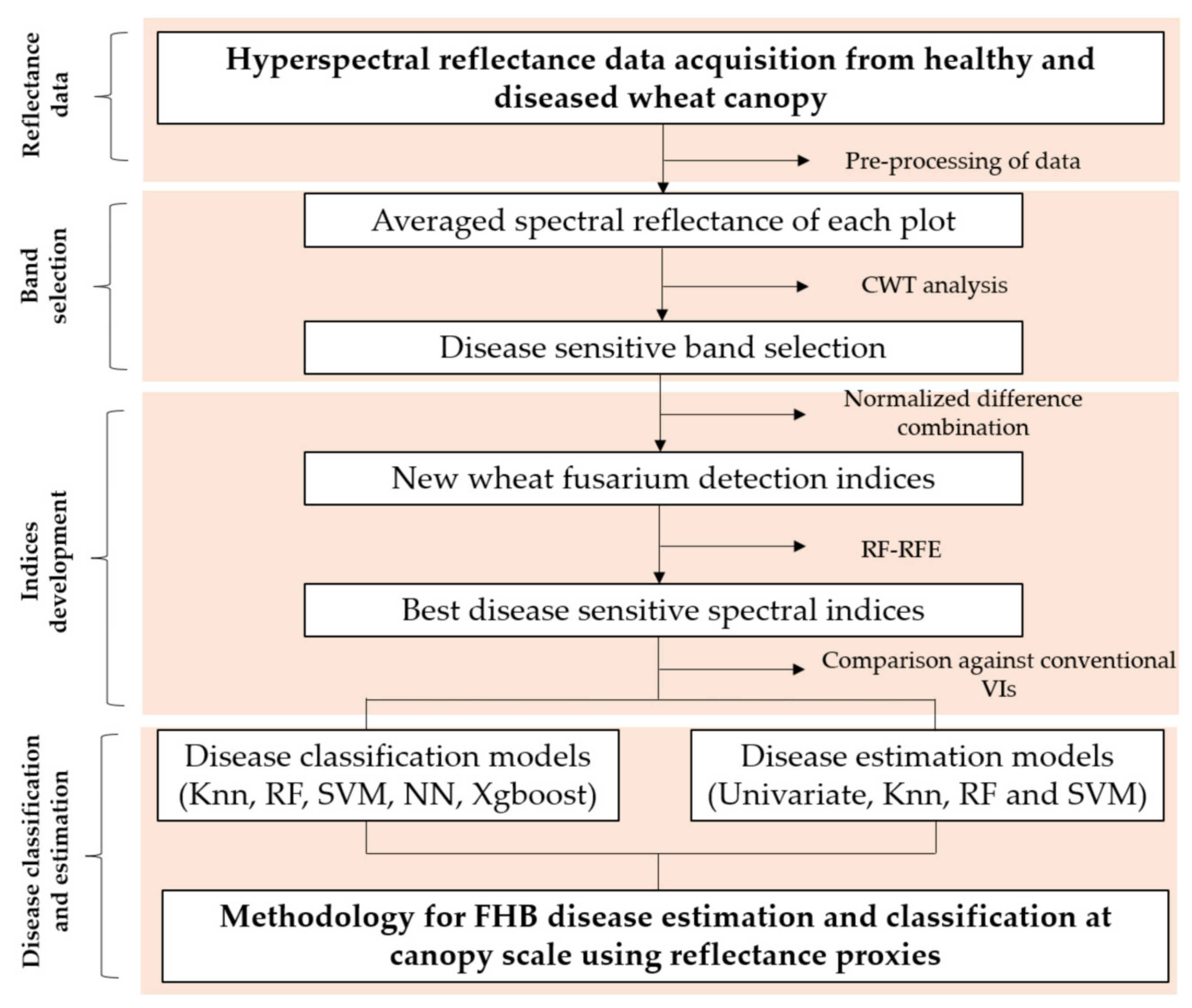
2.3. Data Analysis Interpretation
2.3.1. Methodology to Feature Selection and Indices Development
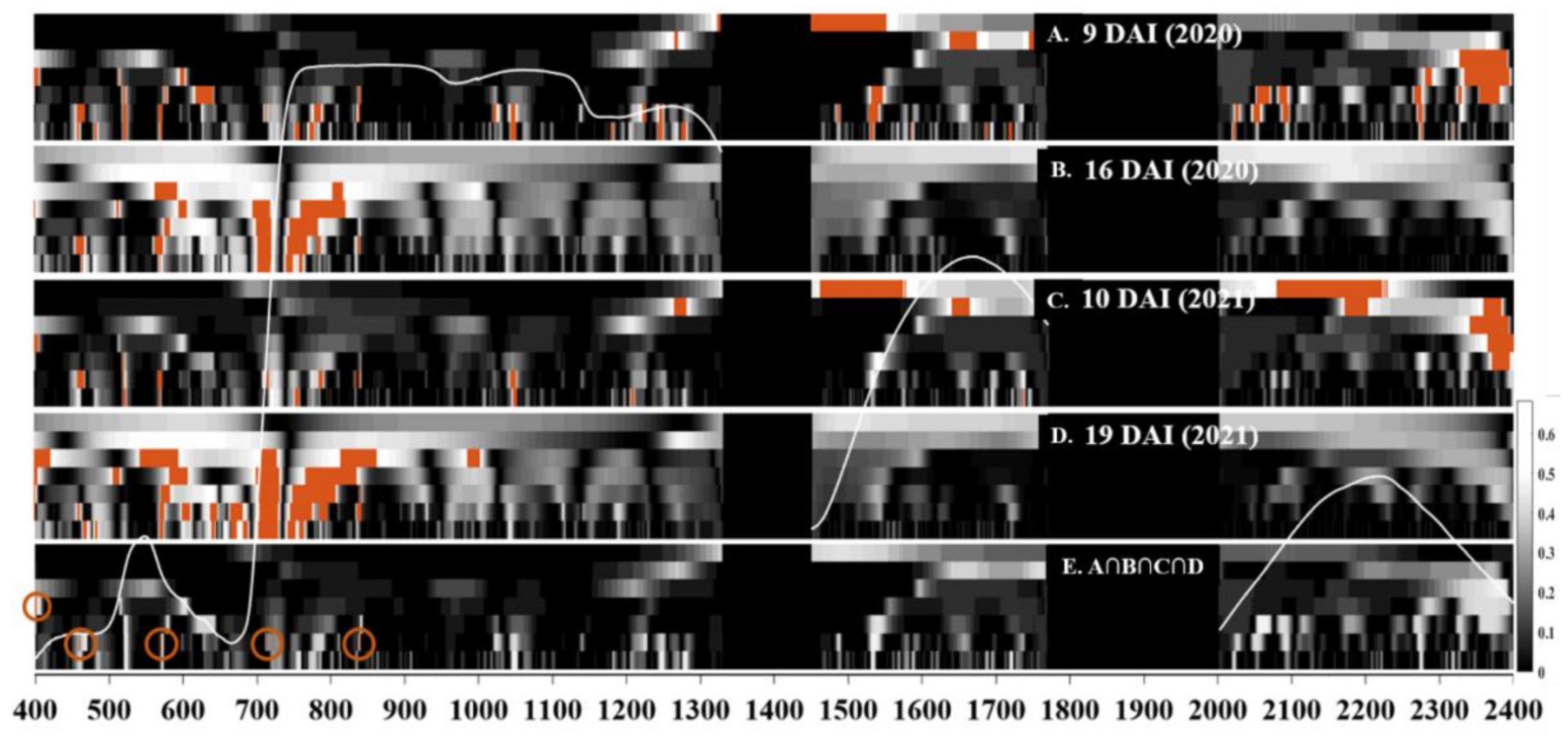
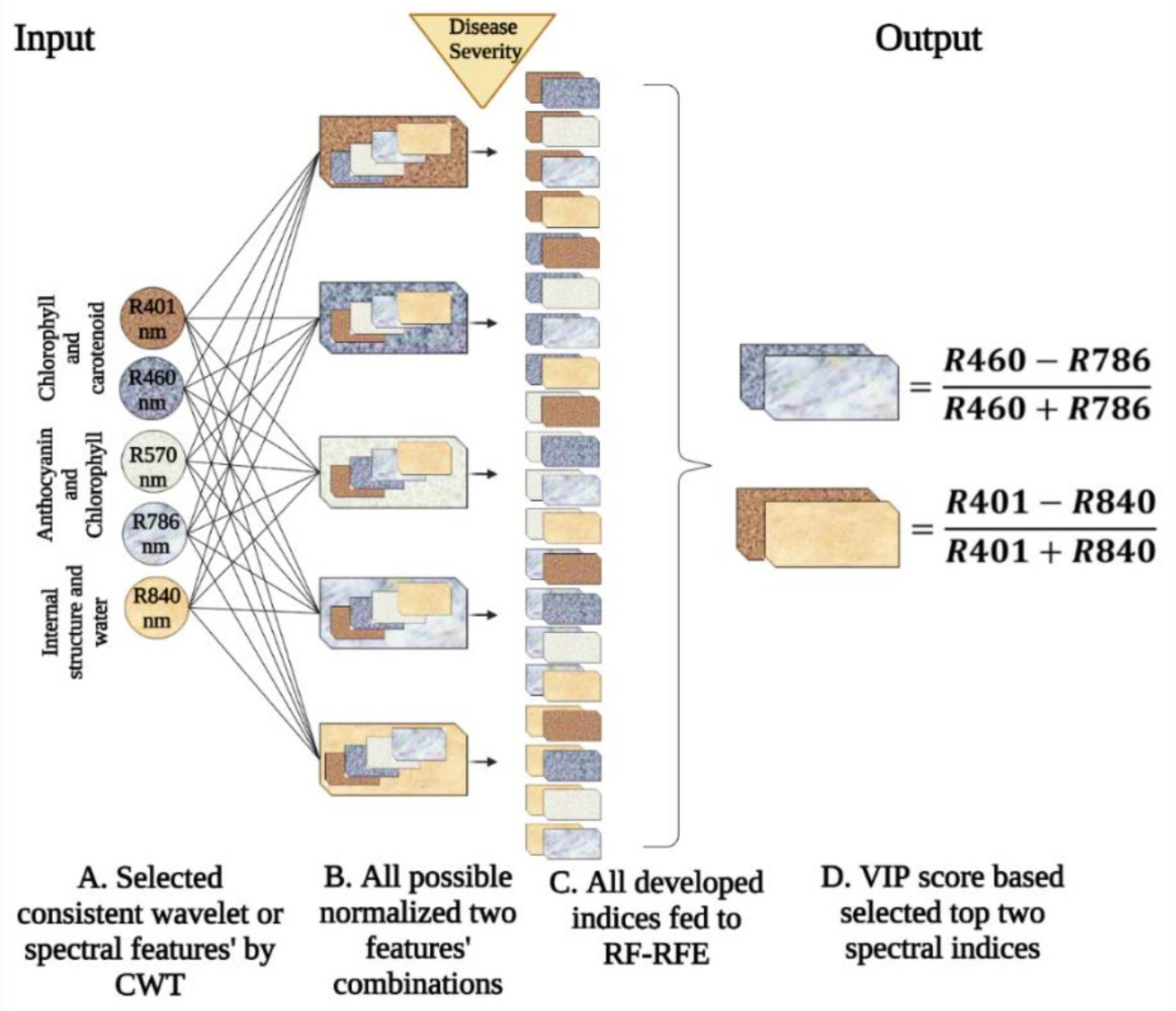
Selection of Consistent Spectral Features by Continuous Wavelet Transform (CWT)
Development of Spectral Indices
2.3.2. Selection of Consistent Vegetation Indices
2.3.3. Machine Learning Algorithms
2.3.4. Statistical Analysis of Canopy Photosynthesis and Disease Estimation
3. Results
3.1. Disease Severity Variation in Wheat Canopy
3.2. Photosynthetic Response of Wheat Canopy under Disease Invasion
3.3. Indices Development through Consistent Feature Selection
3.4. Selection of Vegetation Indices at Canopy Scale
3.5. Separability Performance of Developed Indices against Conventional Spectral Indices
3.6. Estimation of Disease Severity Using Conventional and Newly Developed Spectral Indices
4. Discussion
4.1. Spectral and Photosynthetic Variations under Different Severities of FHB in the Wheat Canopy
4.2. Interpretation of Selected Spectral Bands and Vegetation Indices Development
4.3. Disease Classification with Different Machine Learning Classifiers
4.4. Potential Constraints for Application Possibilities
5. Conclusions
- The conventional VIs (NDVI, PSNDa, PSNDb, PSNDc, LIC1, SIPI, RR4 and NDWI) were found to be highly correlated with DS (Table 1). The VIs associated with plant water and chlorophyll status were found to be negatively correlated with canopy DS. Using machine learning classifiers (MLC), including an RF model based on each consistently selected VIs, WFCI1 and WFCI2 consistently outperformed the other four MLCs (Knn, SVM, NN and Xgboost) in terms of distinguishing between healthy and infected canopies over the course of two years. RF manifested 83.33% CA at DS of 9.73% and improved to 100% CA at a DS of 10.78% at 8 DAI in the year 2020.
- The linear regression models based on WFCI1 and WFCI2 with independent data produced R2 values of 0.80, and 0.81, respectively, had root mean square errors (RMSEs) of 14.17 and 13.50, respectively. In multivariate models, the models WFCI1-WFCI2-KnnR (R2 = 0.83, RMSE = 11.61) revealed the best results for canopy scale disease estimation.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acronyms | Extended Meaning |
| ACA | Average classification accuracy |
| CSBs | Consistent spectral bands |
| CWT | Continuous wavelet transform |
| DAI | Days after inoculation |
| DWT | Discrete wavelet transform |
| FHB | Fusarium head blight |
| HR | Hyperspectral reflectance |
| HSI | Hyperspectral imaging |
| Knn | K nearest neighbor |
| KnnR | K nearest neighbor regression |
| ML | Machine learning |
| MLC | Machine learning classifiers |
| NN | Neural net |
| Pn | Photosynthesis rate |
| R2 | Coefficient of determination |
| RF | Random forest |
| RFR | Random forest regression |
| RF-RFE | Random forest—recursive feature elimination |
| RMSE | Root mean square error |
| SCC | Spike chlorophyll contents |
| SVM | Support vector machine |
| SVMR | Support vector machine regression |
| VIP | Variable importance score |
| Xgboost | Extreme gradient boost |
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- McBeath, J.H.; McBeath, J. Plant diseases, pests and food security. In Environmental Change and Food Security in China; Springer: Berlin/Heidelberg, Germany, 2010; pp. 117–156. [Google Scholar]
- Li, W.G.; Chen, H.; Jin, Z.T.; Zhang, Z.Z.; Ge, G.X.; Ji, F.J. Remote sensing monitoring of winter wheat scab based on suitable scale selection. J. Triticeae Crops 2018, 38, 1374–1380. [Google Scholar]
- Khan, I.H.; Liu, H.; Li, W.; Cao, A.; Wang, X.; Liu, H.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W. Early Detection of Powdery Mildew Disease and Accurate Quantification of Its Severity Using Hyperspectral Images in Wheat. Remote Sens. 2021, 13, 3612. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Bao, Y.; Luo, J.; Jin, X.; Xu, X.; Song, X.; Yang, G. Exploring the best hyperspectral features for LAI estimation using partial least squares regression. Remote Sens. 2014, 6, 6221–6241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Pan, Y.; Feng, H.; Zhao, X.; Yang, X.; Ding, C.; Yang, G. Development of Fusarium head blight classification index using hyperspectral microscopy images of winter wheat spikelets. Biosyst. Eng. 2019, 186, 83–99. [Google Scholar] [CrossRef]
- Bauriegel, E.; Giebel, A.; Geyer, M.; Schmidt, U.; Herppich, W.B. Early detection of Fusarium infection in wheat using hyper-spectral imaging. Comput. Electron. Agric. 2011, 75, 304–312. [Google Scholar] [CrossRef]
- Jin, X.; Jie, L.; Wang, S.; Qi, H.J.; Li, S.W. Classifying wheat hyperspectral pixels of healthy heads and Fusarium head blight disease using a deep neural network in the wild field. Remote Sens. 2018, 10, 395. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.-K.; Alisaac, E.; Al Masri, A.; Behmann, J.; Dehne, H.-W.; Oerke, E.-C. Comparison and combination of thermal, fluorescence, and hyperspectral imaging for monitoring fusarium head blight of wheat on spikelet scale. Sensors 2019, 19, 2281. [Google Scholar] [CrossRef] [Green Version]
- Thenkabail, P.S.; Lyon, J.G. Hyperspectral Remote Sensing of Vegetation; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ma, H.; Huang, W.; Jing, Y.; Pignatti, S.; Laneve, G.; Dong, Y.; Ye, H.; Liu, L.; Guo, A.; Jiang, J. Identification of Fusarium head blight in winter wheat ears using continuous wavelet analysis. Sensors 2020, 20, 20. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wu, Z.; Huang, W.; Ma, H.; Zhao, J. Identification of fusarium head blight in winter wheat ears based on fisher’s linear discriminant analysis and a support vector machine. Appl. Sci. 2019, 9, 3894. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Cheng, T.; Zhou, M.; Li, D.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Improved estimation of rice aboveground biomass combining textural and spectral analysis of UAV imagery. Precis. Agric. 2019, 20, 611–629. [Google Scholar] [CrossRef]
- Yu, K.; Anderegg, J.; Mikaberidze, A.; Karisto, P.; Mascher, F.; McDonald, B.A.; Walter, A.; Hund, A. Hyperspectral canopy sensing of wheat septoria tritici blotch disease. Front. Plant Sci. 2018, 9, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delalieux, S.; Somers, B.; Verstraeten, W.W.; Van Aardt, J.A.N.; Keulemans, W.; Coppin, P. Hyperspectral indices to diagnose leaf biotic stress of apple plants, considering leaf phenology. Int. J. Remote Sens. 2009, 30, 1887–1912. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Camino, C.; Beck, P.S.A.; Calderon, R.; Hornero, A.; Hernández-Clemente, R.; Kattenborn, T.; Montes-Borrego, M.; Susca, L.; Morelli, M. Previsual symptoms of Xylella fastidiosa infection revealed in spectral plant-trait alterations. Nat. Plants 2018, 4, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and model inversion methods with narrowband optical indices for chlorophyll content estimation in closed forest canopies with hyperspectral data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Pu, R.; Loraamm, R.W.; Yang, G.; Wang, J. Comparison between wavelet spectral features and conventional spectral features in detecting yellow rust for winter wheat. Comput. Electron. Agric. 2014, 100, 79–87. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Rumpf, T.; Welke, P.; Dehne, H.W.; Plümer, L.; Steiner, U.; Oerke, E.C. Development of spectral indices for detecting and identifying plant diseases. Remote Sens. Environ. 2013, 128, 21–30. [Google Scholar] [CrossRef]
- Ashourloo, D.; Mobasheri, M.R.; Huete, A. Developing two spectral disease indices for detection of wheat leaf rust (Pucciniatriticina). Remote Sens. 2014, 6, 4723–4740. [Google Scholar] [CrossRef] [Green Version]
- Luedeling, E.; Hale, A.; Zhang, M.; Bentley, W.J.; Dharmasri, L.C. Remote sensing of spider mite damage in California peach orchards. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 244–255. [Google Scholar] [CrossRef]
- Liu, X.-n.; Liu, H.-k.; Huang, Y.-f.; Ye, Y.-l. Relationships between nitrogen application rate soil nitrate-nitrogen plant nitrogen concentration and wheat scab. J. Plant Nutr. Fertil. 2015, 21, 306–317. [Google Scholar]
- Chang, T.-G.; Song, Q.-F.; Zhao, H.-L.; Chang, S.; Xin, C.; Qu, M.; Zhu, X.-G. An in situ approach to characterizing photosynthetic gas exchange of rice panicle. Plant Methods 2020, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bellman, R.E. Perturbation Techniques in Mathematics, Engineering and Physics; Courier Corporation: North Chelmsford, MA, USA, 2003. [Google Scholar]
- Tian, L.; Xue, B.; Wang, Z.; Li, D.; Yao, X.; Cao, Q.; Zhu, Y.; Cao, W.; Cheng, T. Spectroscopic detection of rice leaf blast infection from asymptomatic to mild stages with integrated machine learning and feature selection. Remote Sens. Environ. 2021, 257, 112350. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, G.A.; Feng, J.; Calvo-Polanco, M. Continuous wavelet analysis for the detection of green attack damage due to mountain pine beetle infestation. Remote Sens. Environ. 2010, 114, 899–910. [Google Scholar] [CrossRef]
- Blackburn, G.A.; Ferwerda, J.G. Retrieval of chlorophyll concentration from leaf reflectance spectra using wavelet analysis. Remote Sens. Environ. 2008, 112, 1614–1632. [Google Scholar] [CrossRef]
- Bruce, L.M.; Li, J. Wavelets for computationally efficient hyperspectral derivative analysis. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1540–1546. [Google Scholar] [CrossRef] [Green Version]
- Rivard, B.; Feng, J.; Gallie, A.; Sanchez-Azofeifa, A. Continuous wavelets for the improved use of spectral libraries and hyperspectral data. Remote Sens. Environ. 2008, 112, 2850–2862. [Google Scholar] [CrossRef]
- Gregorutti, B.; Michel, B.; Saint-Pierre, P. Grouped variable importance with random forests and application to multiple functional data analysis. Comput. Stat. Data Anal. 2015, 90, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Lorena, A.C.; Jacintho, L.F.O.; Siqueira, M.F.; De Giovanni, R.; Lohmann, L.G.; De Carvalho, A.C.; Yamamoto, M. Comparing machine learning classifiers in potential distribution modelling. Expert Syst. Appl. 2011, 38, 5268–5275. [Google Scholar] [CrossRef]
- Gorunescu, F. Data Mining: Concepts, Models and Techniques; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; Volume 12. [Google Scholar]
- Ayyash, M. A framework for a Minkowski distance based multi metric quality of service monitoring infrastructure for mobile ad hoc networks. Int. J. Electr. Eng. Inform. 2012, 4, 289. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-J. Training v-support vector classifiers: Theory and algorithms. Neural Comput. 2001, 13, 2119–2147. [Google Scholar] [CrossRef]
- Belgiu, M.; Drăguţ, L. Random forest in remote sensing: A review of applications and future directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Gurney, K. An Introduction to Neural Networks; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the KDD ’16: Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar]
- Schliep, K.; Hechenbichler, K.; Lizee, A. kknn: Weighted k-Nearest Neighbors, version 1.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: https://cran.r-project.org/web/packages/kknn/kknn.pdf (accessed on 3 April 2022).
- RcolorBrewer, S.; Liaw, M.A. Package ‘randomForest’, version 4.6-14; University of California: Berkeley, CA, USA, 2018. [Google Scholar]
- Meyer, D.; Dimitriadou, E.; Hornik, K.; Weingessel, A.; Leisch, F. e1071: Misc Functions of the Department of Statistics, Probability Theory Group (Formerly: E1071), R package version 1.7-2; Vienna University of Technology: Vienna, Austria, 2020. [Google Scholar]
- Fritsch, S.; Guenther, F.; Guenther, M.F. Training of Neural Networks—Package ‘neuralnet’, version 1.44.2; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://cran.r-project.org/web/packages/neuralnet/neuralnet (accessed on 7 February 2022).
- Chen, T.; He, T.; Benesty, M.; Khotilovich, V. Extreme Gradient Boosting–Package ‘xgboost’; R Version 1.6.0.1; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://cran.r-project.org/web/packages/xgboost/vignettes/xgboost (accessed on 25 June 2021).
- Bauriegel, E.; Brabandt, H.; Gärber, U.; Herppich, W.B. Chlorophyll fluorescence imaging to facilitate breeding of Bremia lactucae-resistant lettuce cultivars. Comput. Electron. Agric. 2014, 105, 74–82. [Google Scholar] [CrossRef]
- Cao, X.; Luo, Y.; Zhou, Y.; Duan, X.; Cheng, D. Detection of powdery mildew in two winter wheat cultivars using canopy hyperspectral reflectance. Crop Prot. 2013, 45, 124–131. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kanda, E.; Kitada, K.; Ishiguro, K.; Torigoe, Y. Detection of rice panicle blast with multispectral radiometer and the potential of using airborne multispectral scanners. Phytopathology 2001, 91, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Oerke, E.-C.; Herzog, K.; Toepfer, R. Hyperspectral phenotyping of the reaction of grapevine genotypes to Plasmopara viticola. J. Exp. Bot. 2016, 67, 5529–5543. [Google Scholar] [CrossRef] [Green Version]
- Mahlein, A.-K. Plant disease detection by imaging sensors–parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, H.; Huang, W.; Dong, Y.; Ye, H.; Ma, H.; Zhao, J. Identification of Fusarium head blight in wheat ears using vertical angle-based reflectance spectroscopy. Arab. J. Geosci. 2021, 14, 423. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment–An Earth Resource Perspective; Reprint edition; Pearson Education: Noida, India, 2002. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Féret, J.B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Al Masri, A.; Hau, B.; Dehne, H.W.; Mahlein, A.K.; Oerke, E.C. Impact of primary infection site of Fusarium species on head blight development in wheat ears evaluated by IR-thermography. Eur. J. Plant Pathol. 2017, 147, 855–868. [Google Scholar] [CrossRef]
- Steddom, K.; Bredehoeft, M.W.; Khan, M.; Rush, C.M. Comparison of visual and multispectral radiometric disease evaluations of Cercospora leaf spot of sugar beet. Plant Dis. 2005, 89, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajwa, S.G.; Rupe, J.C.; Mason, J. Soybean disease monitoring with leaf reflectance. Remote Sens. 2017, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Penuelas, J.; Baret, F.; Filella, I. Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Feng, Z.-H.; Wang, L.-Y.; Yang, Z.-Q.; Zhang, Y.-Y.; Li, X.; Song, L.; He, L.; Duan, J.-Z.; Feng, W. Hyperspectral Monitoring of Powdery Mildew Disease Severity in Wheat Based on Machine Learning. Front. Plant Sci. 2022, 13, 828454. [Google Scholar] [CrossRef]
- Guo, W.; Yang, Y.; Zhao, H.; Song, R.; Dong, P.; Jin, Q.; Baig, M.H.A.; Liu, Z.; Yang, Z. Winter Wheat Take-All Disease Index Estimation Model Based on Hyperspectral Data. Appl. Sci. 2021, 11, 9230. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Ding, W.; Huang, W.; Hu, T.; Zhao, J. Monitoring of wheat scab using the specific spectral index from ASD hyperspectral dataset. J. Spectrosc. 2019, 2019, 9153195. [Google Scholar] [CrossRef]
- Abdurrahman, G.; Sintawati, M. Implementation of xgboost for classification of parkinson’s disease. J. Phys. Conf. Ser. 2020, 1538, 012024. [Google Scholar] [CrossRef]
- Aydin, Z.E.; Ozturk, Z.K. XGBoost feature selection on Chronic kidney disease diagnosis. In Proceedings of the IV International Conference on Data Science and Applications (ICONDATA’20), Eskisehir, Turkey, 4–6 June 2021. [Google Scholar]
- Alisaac, E.; Behmann, J.; Kuska, M.T.; Dehne, H.W.; Mahlein, A.K. Hyperspectral quantification of wheat resistance to Fusarium head blight: Comparison of two Fusarium species. Eur. J. Plant Pathol. 2018, 152, 869–884. [Google Scholar] [CrossRef]
- Ashourloo, D.; Aghighi, H.; Matkan, A.A.; Mobasheri, M.R.; Rad, A.M. An investigation into machine learning regression techniques for the leaf rust disease detection using hyperspectral measurement. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2016, 9, 4344–4351. [Google Scholar] [CrossRef]
- Huang, W.; Guan, Q.; Luo, J.; Zhang, J.; Zhao, J.; Liang, D.; Huang, L.; Zhang, D. New optimized spectral indices for identifying and monitoring winter wheat diseases. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 2516–2524. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Blackburn, G.A. Spectral indices for estimating photosynthetic pigment concentrations: A test using senescent tree leaves. Int. J. Remote Sens. 1998, 19, 657–675. [Google Scholar] [CrossRef]
- Blackburn, G.A. Relationships between spectral reflectance and pigment concentrations in stacks of deciduous broadleaves. Remote Sens. Environ. 1999, 70, 224–237. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey Iii, J.E. Ratio analysis of reflectance spectra (RARS): An algorithm for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the vernal advancement and retrogradation (green wave effect) of natural vegetation. In NASA/GSFC Type III Final Report; NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Maccioni, A.; Agati, G.; Mazzinghi, P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J. Photochem. Photobiol. B Biol. 2001, 61, 52–61. [Google Scholar] [CrossRef]
- Dash, J.; Curran, P.J. The MERIS Terrestrial Chlorophyll Index; Taylor & Francis: Abingdon, UK, 2004. [Google Scholar]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a + b, and total carotenoid content in eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using Eucalyptus leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Signature analysis of leaf reflectance spectra: Algorithm development for remote sensing of chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Féret, J.-B.; François, C.; Gitelson, A.; Asner, G.P.; Barry, K.M.; Panigada, C.; Richardson, A.D.; Jacquemoud, S. Optimizing spectral indices and chemometric analysis of leaf chemical properties using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2742–2750. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Suárez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Garrity, S.R.; Bohrer, G.; Maurer, K.D.; Mueller, K.L.; Vogel, C.S.; Curtis, P.S. A comparison of multiple phenology data sources for estimating seasonal transitions in deciduous forest carbon exchange. Agric. For. Meteorol. 2011, 151, 1741–1752. [Google Scholar] [CrossRef]
- Peñuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Hardisky, M.A.; Klemas, V.; Smart, R.M. The influence of soil-salinity, growth form, and leaf moisture on the spectral radiance of spartina–alterniflora canopies. Photogramm. Eng. Remote Sens. 1983, 49, 77–83. [Google Scholar]
- Gitelson, A.A.; Yacobi, Y.Z.; Schalles, J.F.; Rundquist, D.C.; Han, L.; Stark, R.; Etzion, D. Remote estimation of phytoplankton density in productive waters. Adv. Limnol. Stuttg. 2000, 55, 121–136. [Google Scholar]
- Calderón, R.; Navas-Cortés, J.A.; Lucena, C.; Zarco-Tejada, P.J. High-resolution airborne hyperspectral and thermal imagery for early detection of Verticillium wilt of olive using fluorescence, temperature and narrow-band spectral indices. Remote Sens. Environ. 2013, 139, 231–245. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; De Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Lang, M.; Sowinska, M.; Heisel, F.; Miehe, J.A. Detection of vegetation stress via a new high resolution fluorescence imaging system. J. Plant Physiol. 1996, 148, 599–612. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and airborne canopy-level measurements with hyperspectral data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]


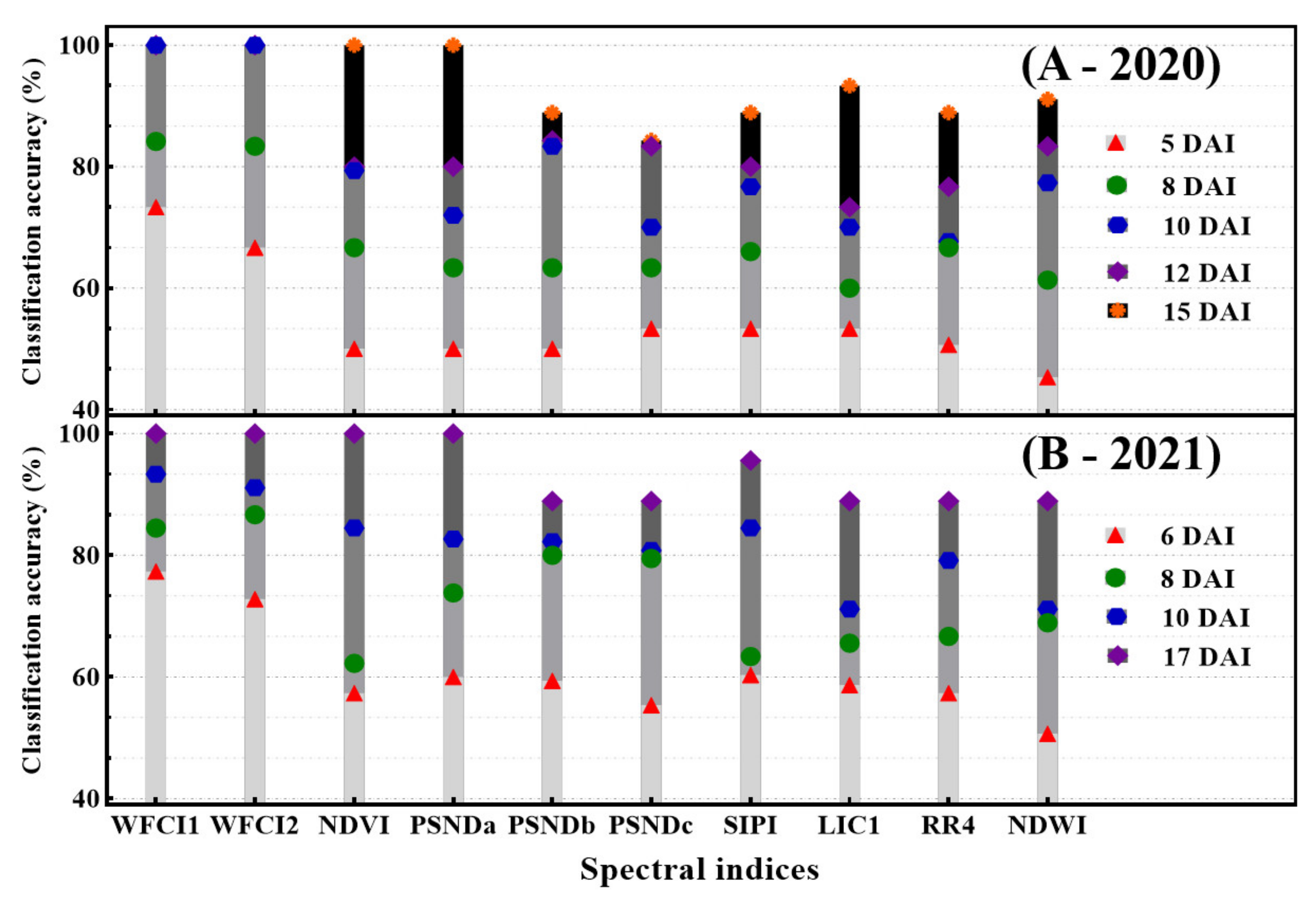
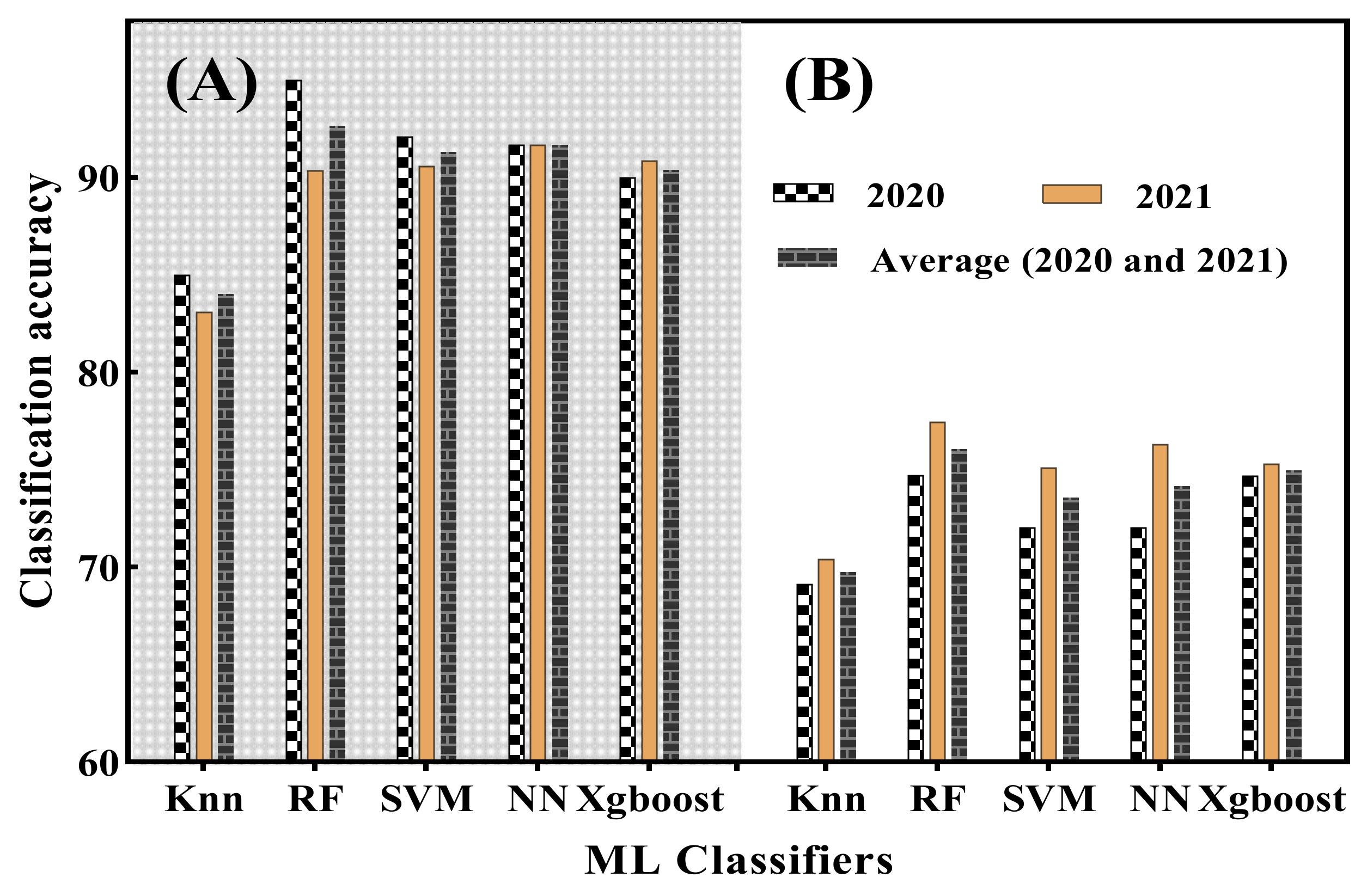
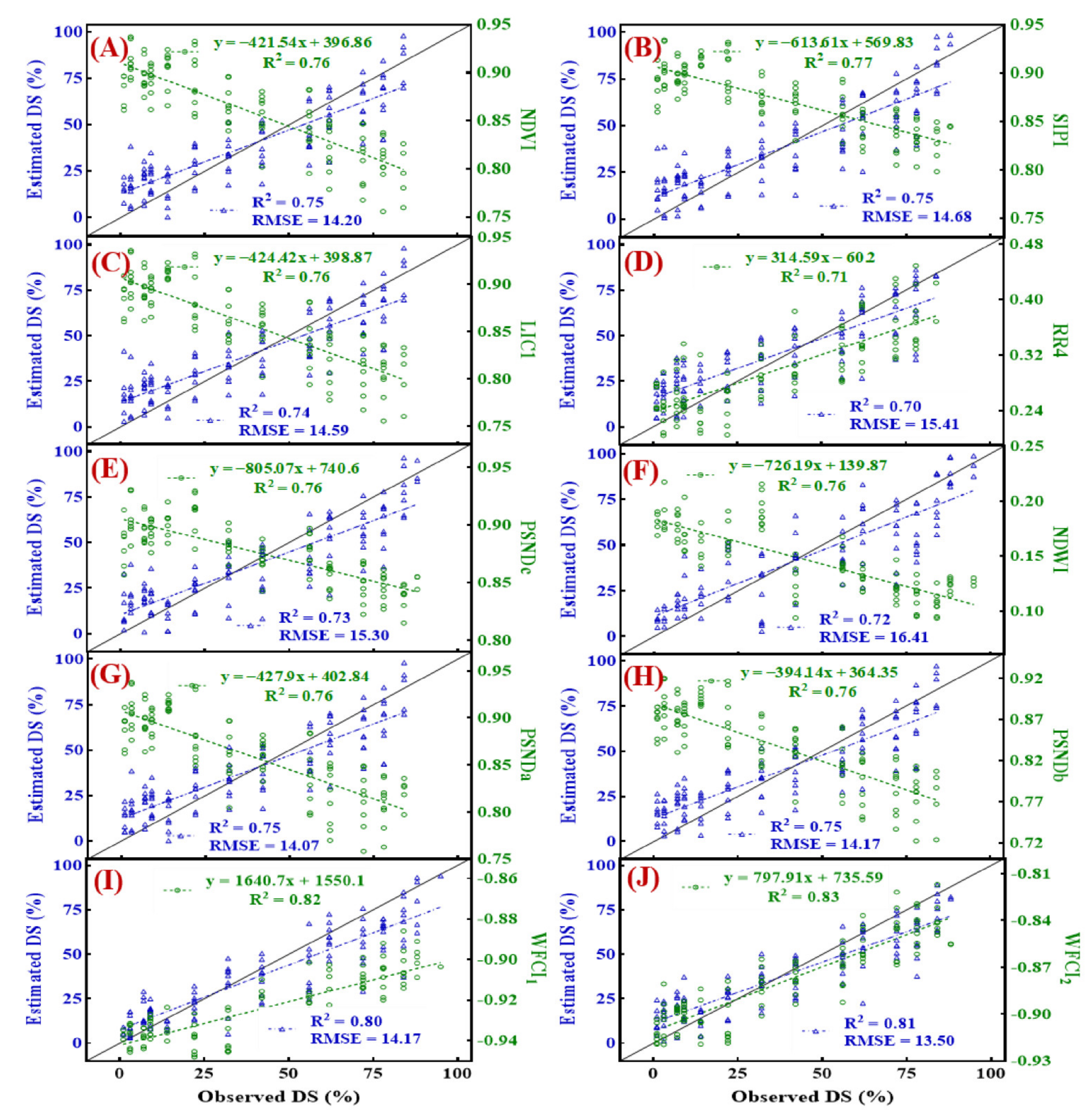
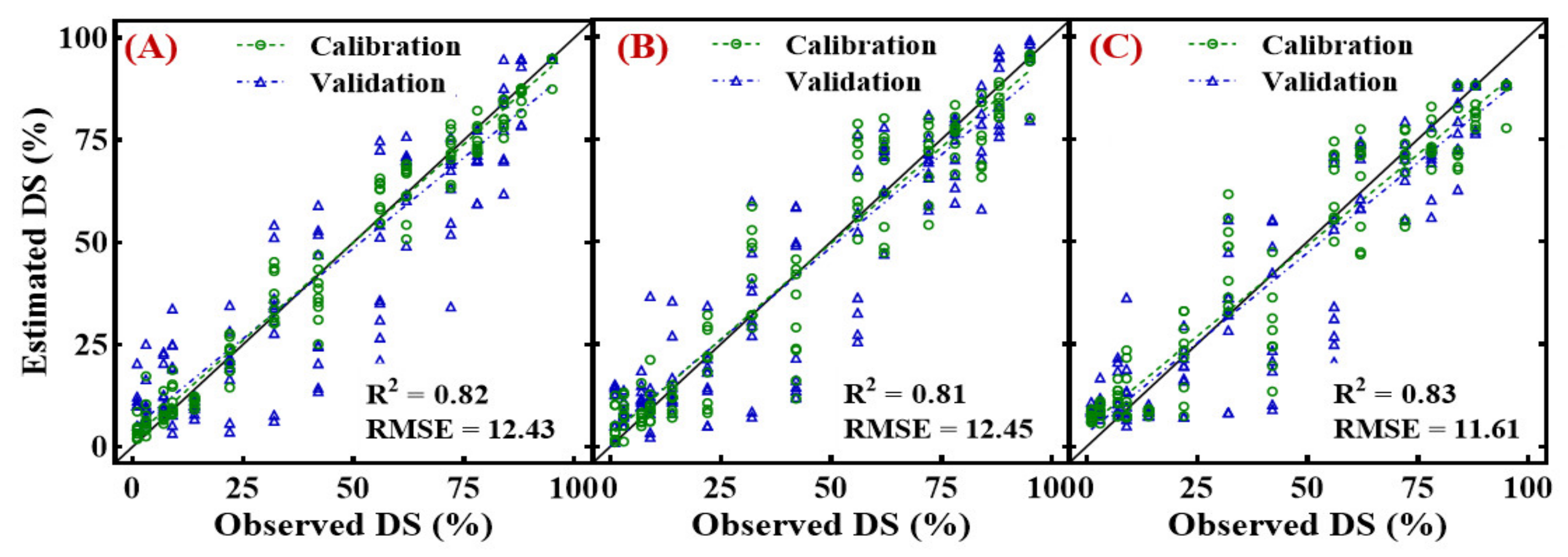

| Rank | Highly Correlated for 2020 | R | Highly Correlated for 2021 | R | Average | R |
|---|---|---|---|---|---|---|
| 1 | WFCI1 | 0.95 | WFCI1 | 0.85 | WFCI1 | 0.90 |
| 2 | WFCI2 | 0.94 | WFCI2 | 0.84 | WFCI2 | 0.89 |
| 3 | NDVI | −0.94 | PSNDc | −0.83 | PSNDb | −0.88 |
| 4 | PSNDa | −0.94 | SIPI | −0.82 | NDVI | −0.87 |
| 5 | PSNDb | −0.94 | PSNDb | −0.81 | PSNDa | −0.87 |
| 6 | LIC1 | −0.94 | NDWI | −0.81 | PSNDc | −0.87 |
| 7 | PSNDc | −0.91 | RR4 | 0.81 | LIC1 | −0.87 |
| 8 | SIPI | −0.91 | NDVI | −0.80 | SIPI | −0.86 |
| 9 | RR4 | 0.91 | PSNDa | −0.80 | NDWI | −0.86 |
| 10 | NDWI | −0.90 | LIC1 | −0.80 | RR4 | 0.86 |
| Overall Classification Accuracy (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| This Classification Accuracy (CA) Is for Test Data Set (Train = 70, Test = 30) | ||||||||||
| Year | 2020 | 2021 | ||||||||
| DAI | 5 | 8 | 10 | 12 | 15 | 6 | 8 | 10 | 17 | |
| DP | 9.73 | 10.78 | 18 | 24.12 | 30.12 | 8.21 | 12.41 | 19.34 | 28.13 | |
| (A) | Knn | 66.67 | 66.67 | 100.0 | 100.0 | 100.0 | 73.33 | 77.78 | 88.89 | 100.0 |
| WFCI1 | RF | 83.33 | 100.0 | 100.0 | 100.0 | 100.0 | 73.33 | 77.78 | 100.0 | 100.0 |
| SVM | 66.67 | 87.50 | 100.0 | 100.0 | 100.0 | 73.33 | 88.89 | 100.0 | 100.0 | |
| NN | 83.33 | 83.33 | 100.0 | 100.0 | 100.0 | 80.00 | 88.89 | 88.89 | 100.0 | |
| Xgboost | 66.67 | 83.33 | 100.0 | 100.0 | 100.0 | 86.67 | 88.89 | 88.89 | 100.0 | |
| (B) | Knn | 50.00 | 66.67 | 100.0 | 100.0 | 100.0 | 70.59 | 66.67 | 87.50 | 100.0 |
| WFCI2 | RF | 83.33 | 83.33 | 100.0 | 100.0 | 100.0 | 73.33 | 88.89 | 90.00 | 100.0 |
| SVM | 66.67 | 100.0 | 100.0 | 100.0 | 100.0 | 73.33 | 100.0 | 88.89 | 100.0 | |
| NN | 66.67 | 83.33 | 100.0 | 100.0 | 100.0 | 73.33 | 88.89 | 88.89 | 100.0 | |
| Xgboost | 66.67 | 83.33 | 100.0 | 100.0 | 100.0 | 73.33 | 88.89 | 100.0 | 100.0 | |
| (C) | Knn | 33.33 | 66.67 | 83.33 | 83.33 | 100.0 | 60.00 | 55.56 | 88.89 | 100.0 |
| NDVI | RF | 33.33 | 66.67 | 100.0 | 66.67 | 100.0 | 53.33 | 66.67 | 77.78 | 100.0 |
| SVM | 66.67 | 66.67 | 83.33 | 83.33 | 100.0 | 53.33 | 55.56 | 88.89 | 100.0 | |
| NN | 50.00 | 66.67 | 100.0 | 83.33 | 100.0 | 53.33 | 66.67 | 88.89 | 100.0 | |
| Xgboost | 66.67 | 66.67 | 100.0 | 83.33 | 100.0 | 66.67 | 66.67 | 77.78 | 100.0 | |
| (D) | Knn | 33.33 | 50.00 | 66.67 | 83.33 | 100.0 | 50.00 | 66.67 | 80.00 | 100.0 |
| PSNDa | RF | 66.67 | 66.67 | 66.67 | 66.67 | 100.0 | 66.67 | 80.00 | 77.78 | 100.0 |
| SVM | 50.00 | 66.67 | 60.00 | 83.33 | 100.0 | 50.00 | 66.67 | 88.89 | 100.0 | |
| NN | 50.00 | 66.67 | 83.33 | 83.33 | 100.0 | 66.67 | 88.89 | 88.89 | 100.0 | |
| Xgboost | 50.00 | 66.67 | 83.33 | 83.33 | 100.0 | 66.67 | 66.67 | 77.78 | 100.0 | |
| (E) | Knn | 50.00 | 66.67 | 83.33 | 83.33 | 88.88 | 50.00 | 77.78 | 77.78 | 88.88 |
| PSNDb | RF | 50.00 | 50.00 | 83.33 | 83.33 | 88.88 | 73.33 | 77.78 | 77.78 | 88.88 |
| SVM | 50.00 | 66.67 | 83.33 | 83.33 | 88.88 | 73.33 | 77.78 | 88.89 | 88.88 | |
| NN | 50.00 | 66.67 | 83.33 | 83.33 | 88.88 | 50.00 | 77.78 | 77.78 | 88.88 | |
| Xgboost | 50.00 | 66.67 | 83.33 | 83.33 | 88.88 | 50.00 | 88.89 | 88.89 | 88.88 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, G.; Zheng, H.; Khan, I.H.; Tian, L.; Jia, H.; Li, G.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y.; et al. Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning. Remote Sens. 2022, 14, 2784. https://doi.org/10.3390/rs14122784
Mustafa G, Zheng H, Khan IH, Tian L, Jia H, Li G, Cheng T, Tian Y, Cao W, Zhu Y, et al. Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning. Remote Sensing. 2022; 14(12):2784. https://doi.org/10.3390/rs14122784
Chicago/Turabian StyleMustafa, Ghulam, Hengbiao Zheng, Imran Haider Khan, Long Tian, Haiyan Jia, Guoqiang Li, Tao Cheng, Yongchao Tian, Weixing Cao, Yan Zhu, and et al. 2022. "Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning" Remote Sensing 14, no. 12: 2784. https://doi.org/10.3390/rs14122784
APA StyleMustafa, G., Zheng, H., Khan, I. H., Tian, L., Jia, H., Li, G., Cheng, T., Tian, Y., Cao, W., Zhu, Y., & Yao, X. (2022). Hyperspectral Reflectance Proxies to Diagnose In-Field Fusarium Head Blight in Wheat with Machine Learning. Remote Sensing, 14(12), 2784. https://doi.org/10.3390/rs14122784










