Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling
Abstract
:1. Introduction
- Distinguishing macroalgae from seawater, substratum and associated non-algal organisms based on classification results from hyperspectral imagery.
- Using hyperspectral data to discriminate the main species of fucoids from green and red macroalgae.
- Testing the accuracy of supervised classification algorithms.
- Comparing field and remotely estimated cover-abundance data.
2. Materials and Methods
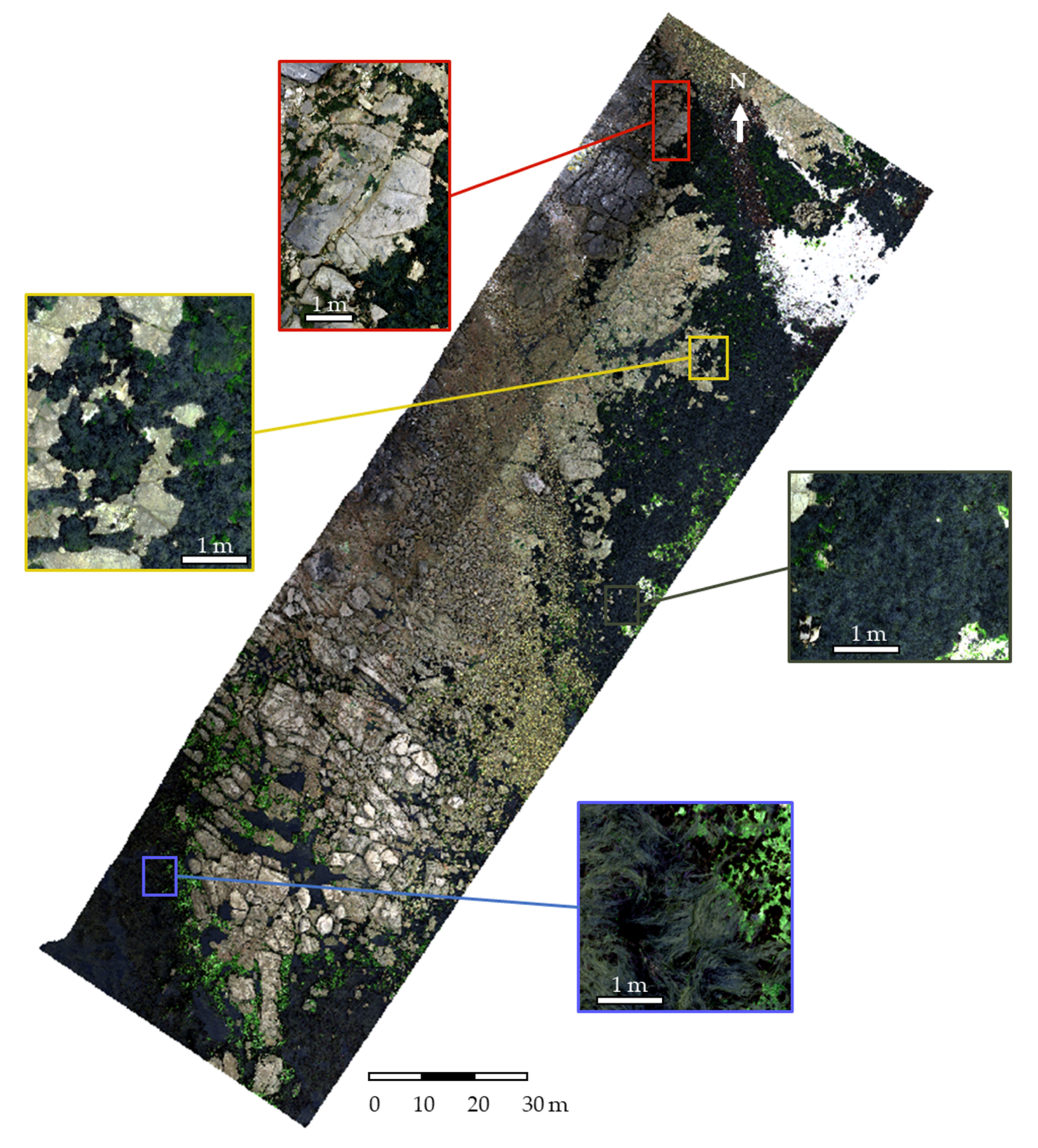
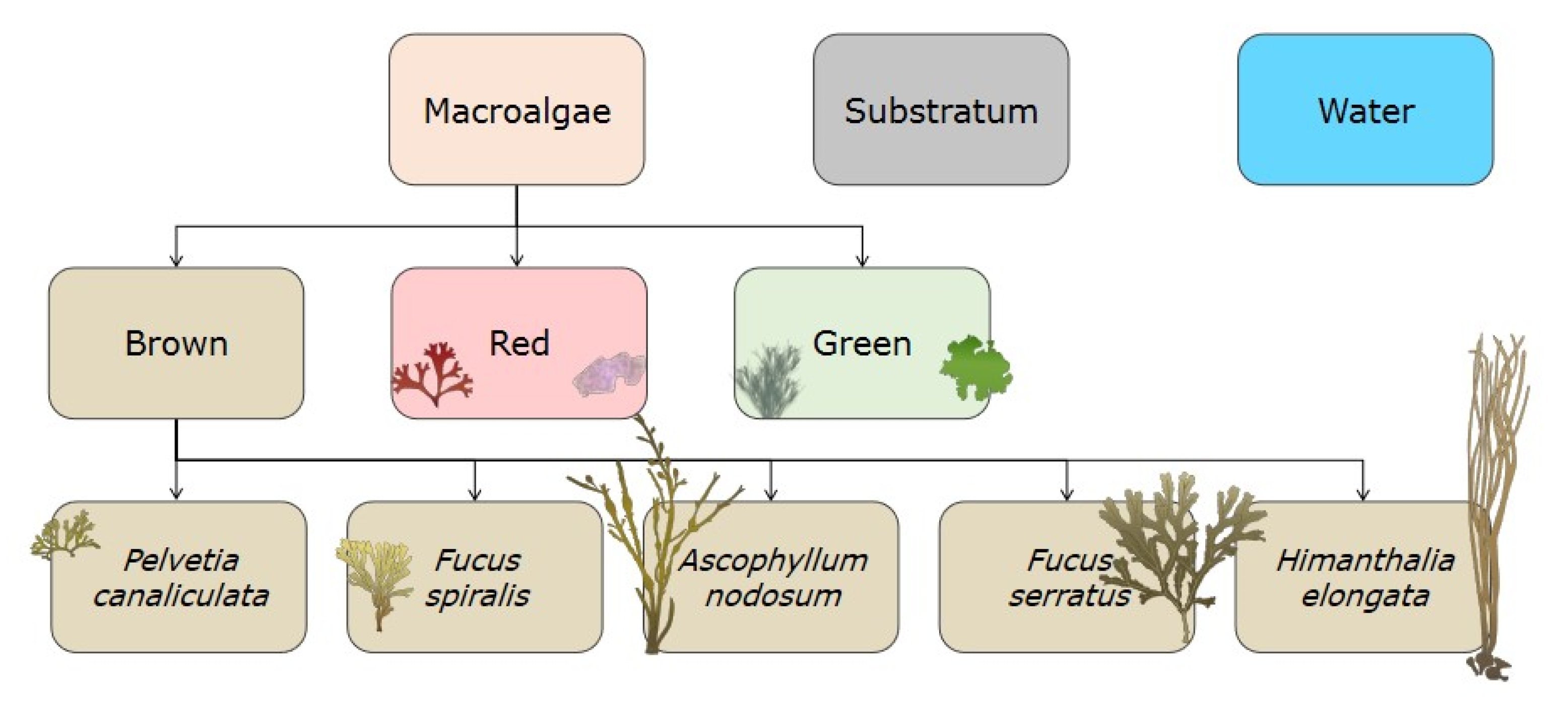
2.1. Studied Site and Communities
2.2. Sampling Method
2.3. Remote Sensing Acquisition
2.4. Pre-Processing
2.5. Data Classification
2.6. Data Analysis
3. Results
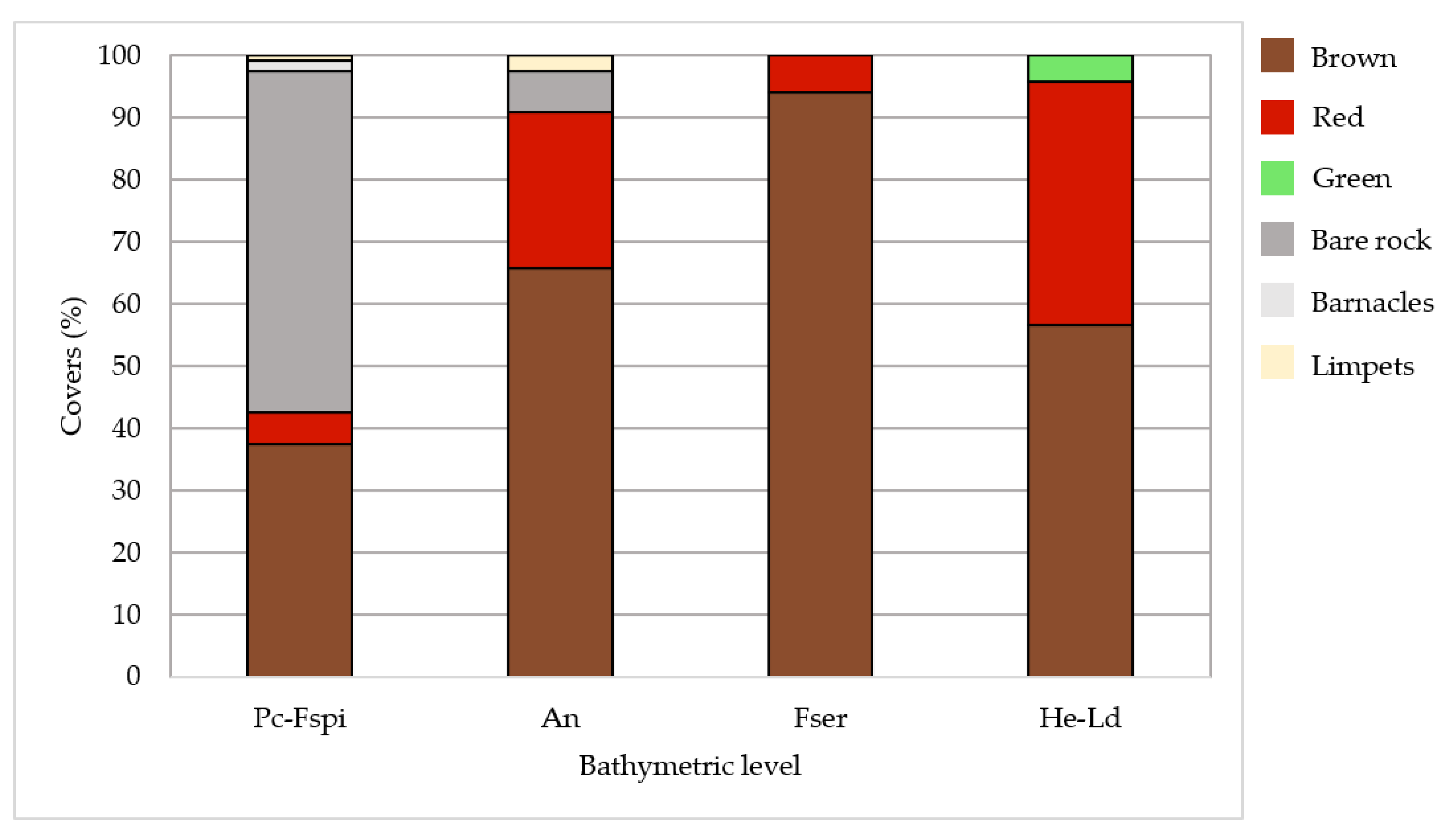
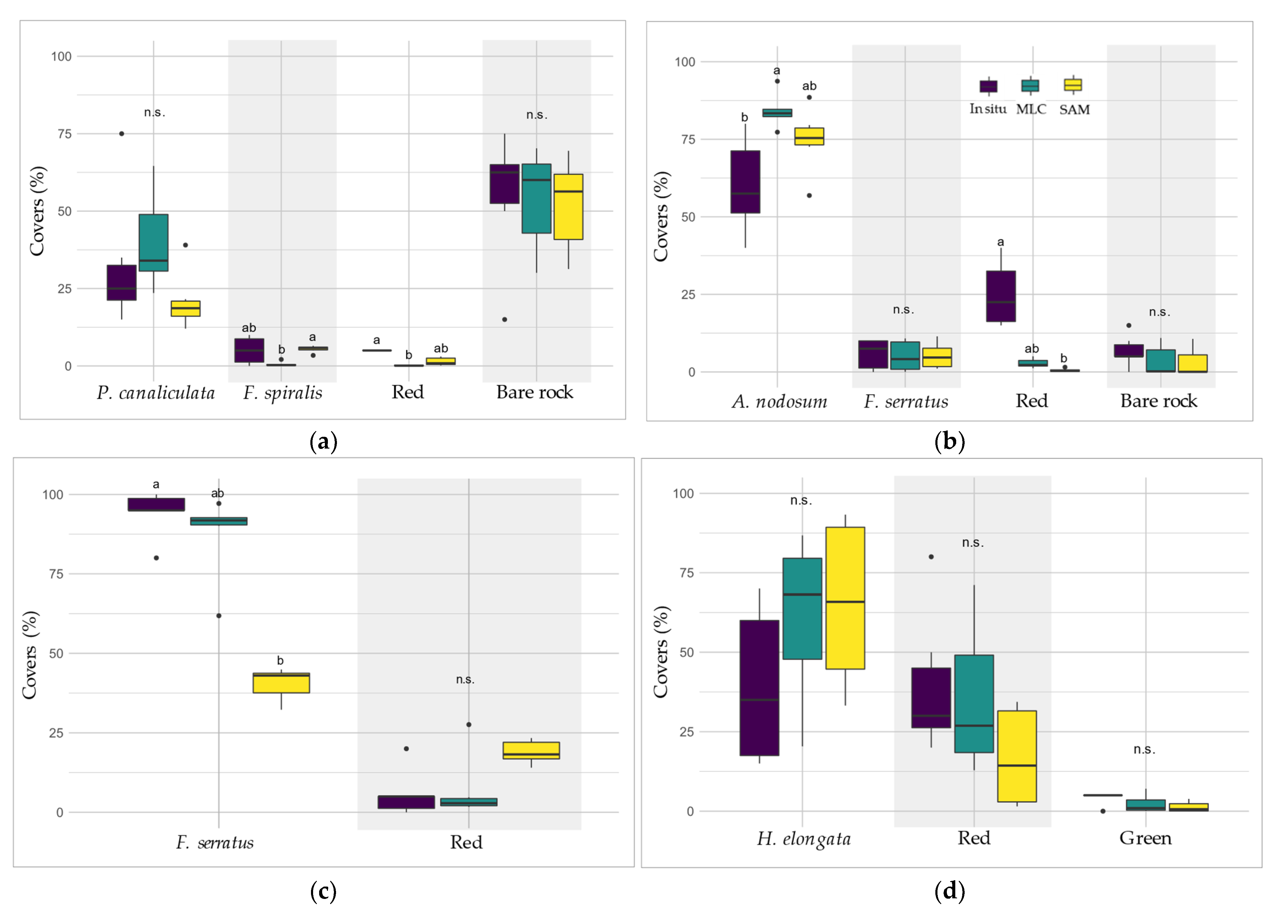
3.1. In Situ Vegetation Cover
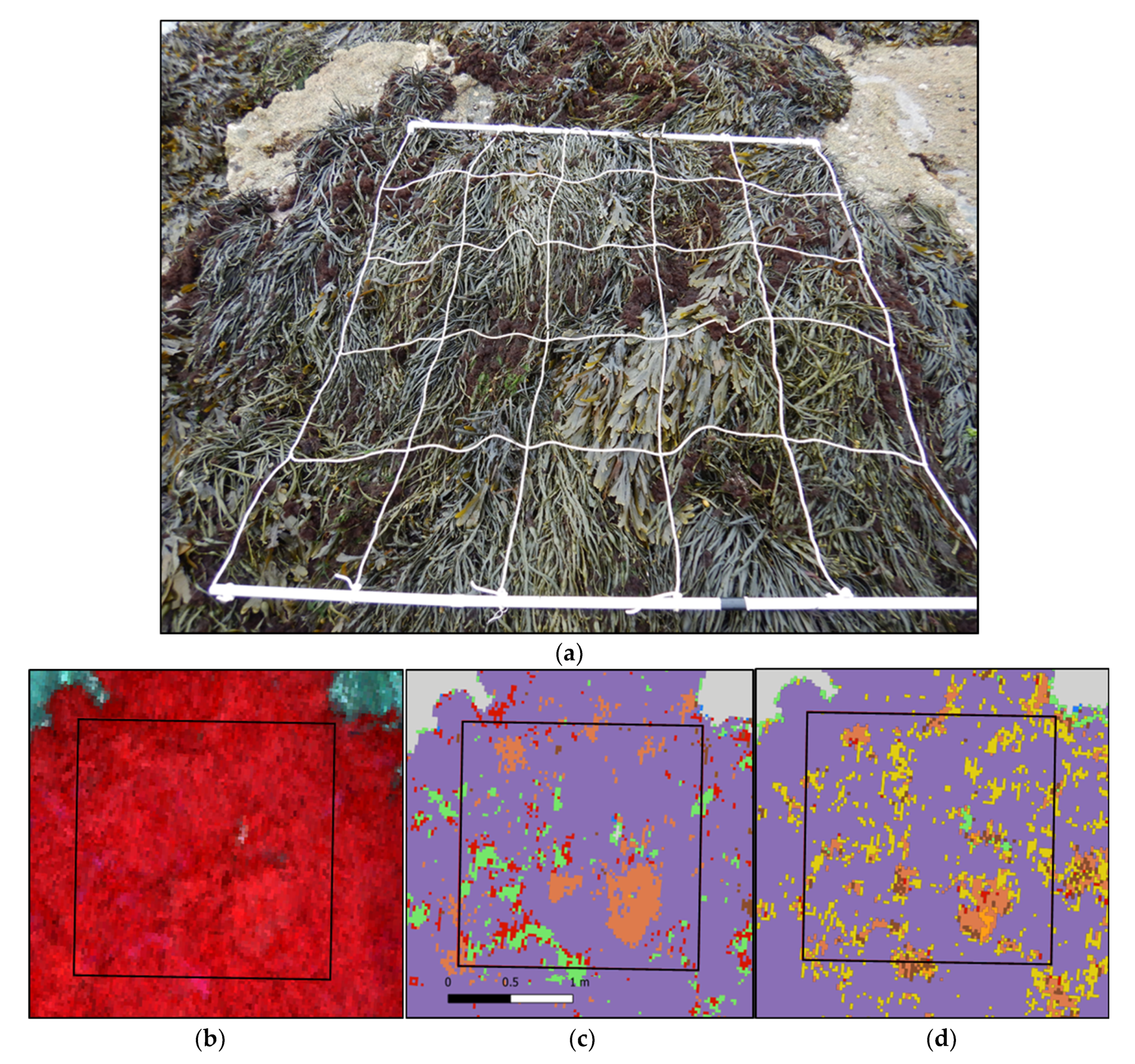
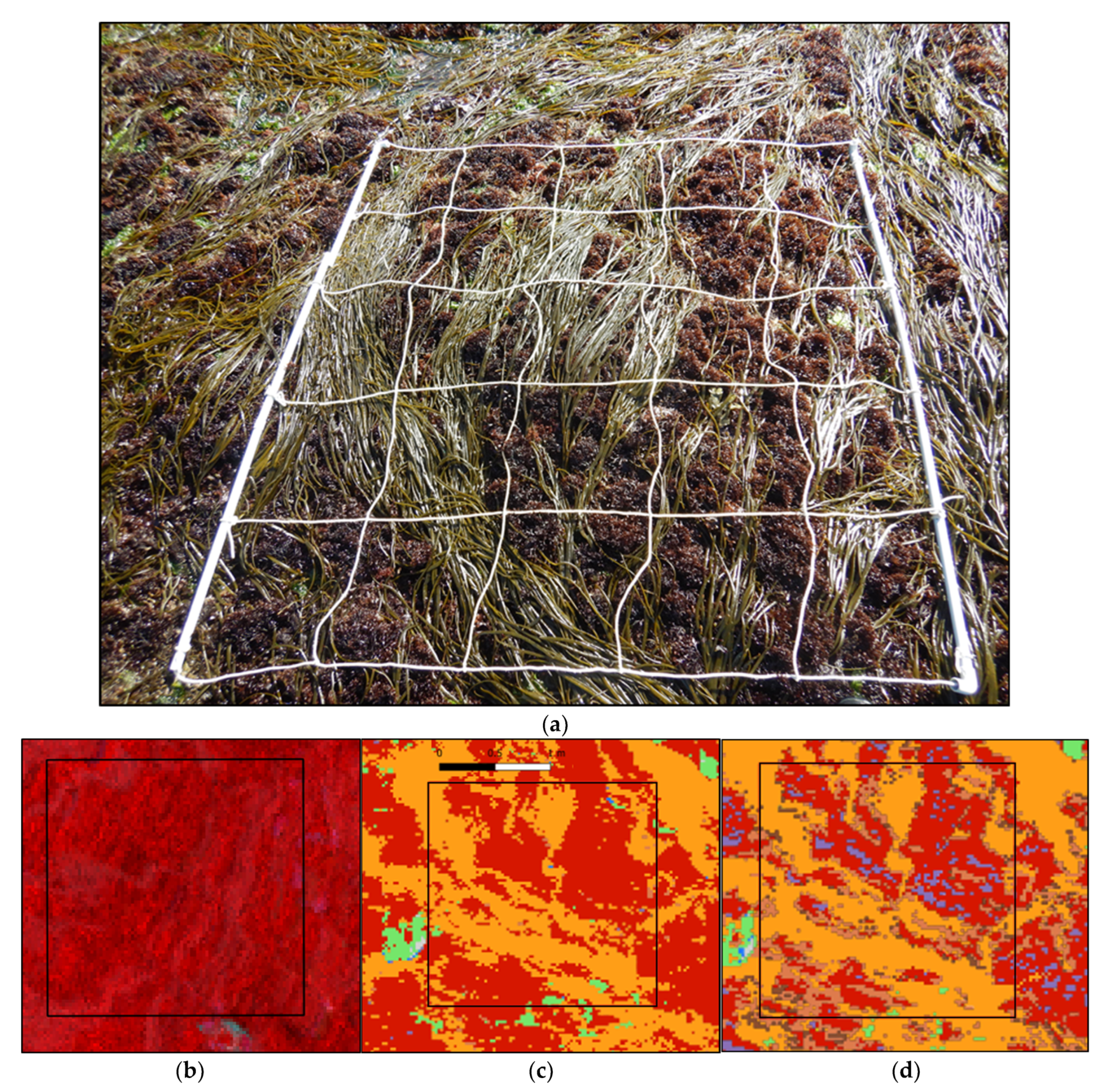
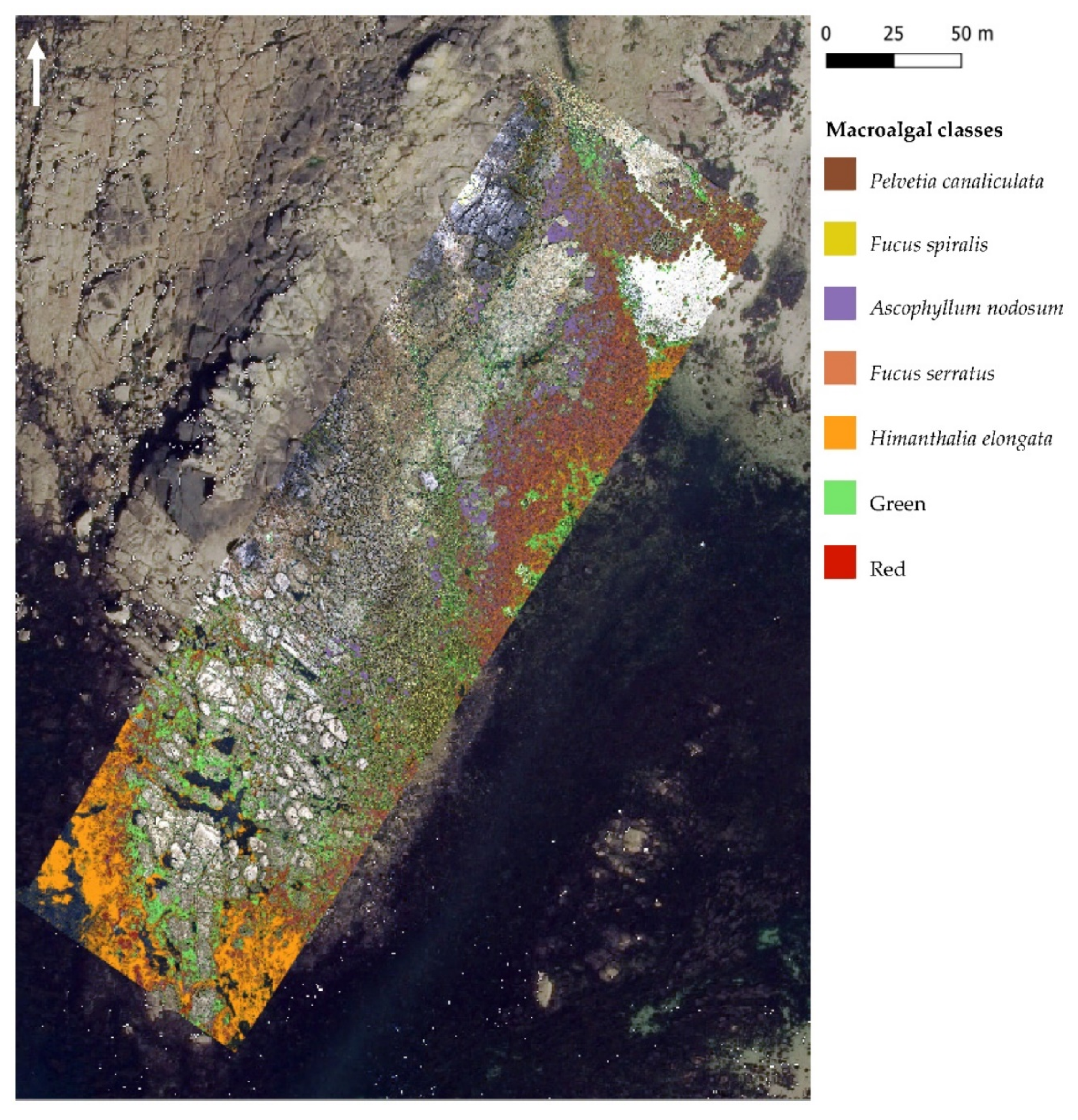
3.2. Classifications Results
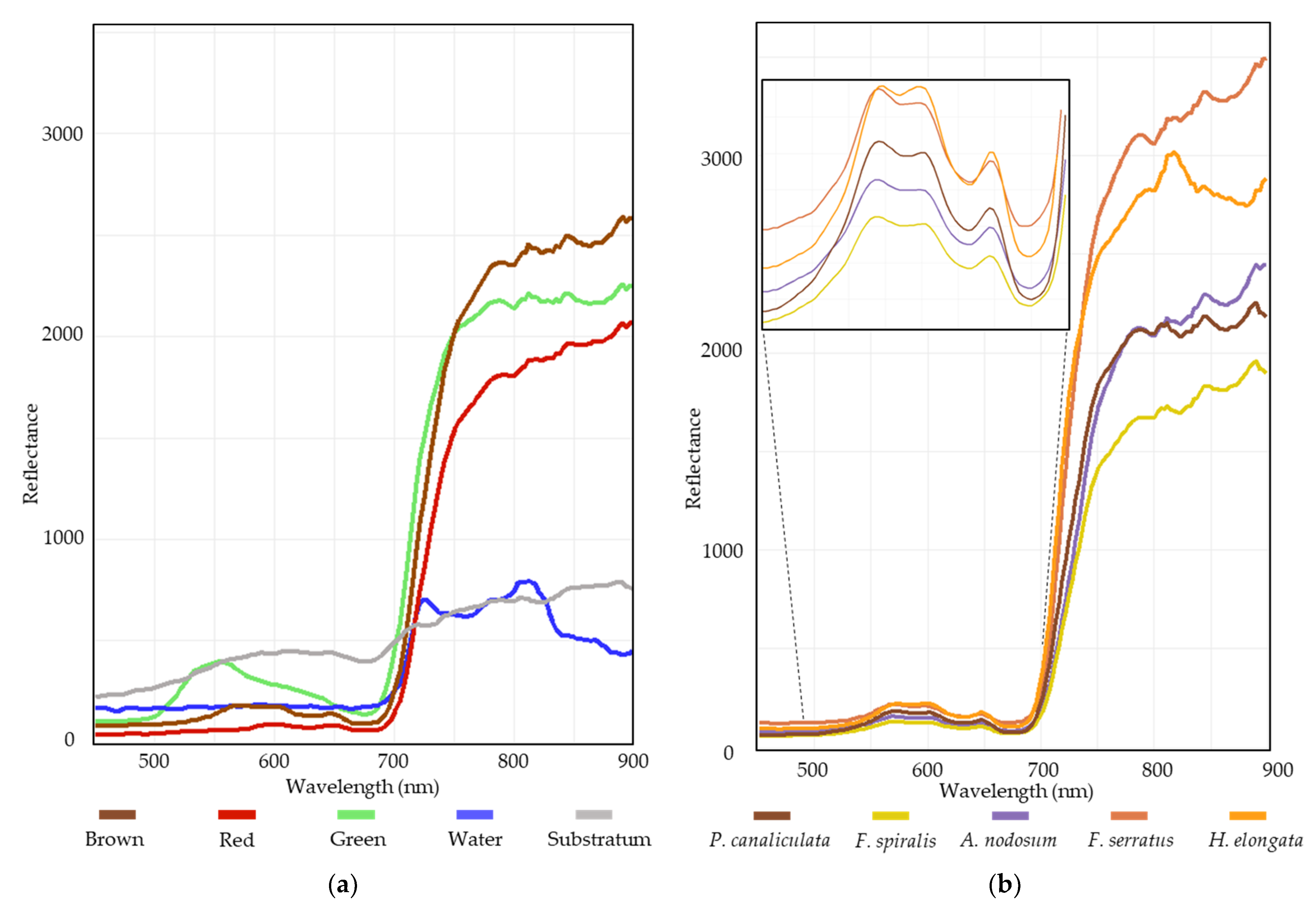
3.2.1. MLC Results
3.2.2. SAM Results
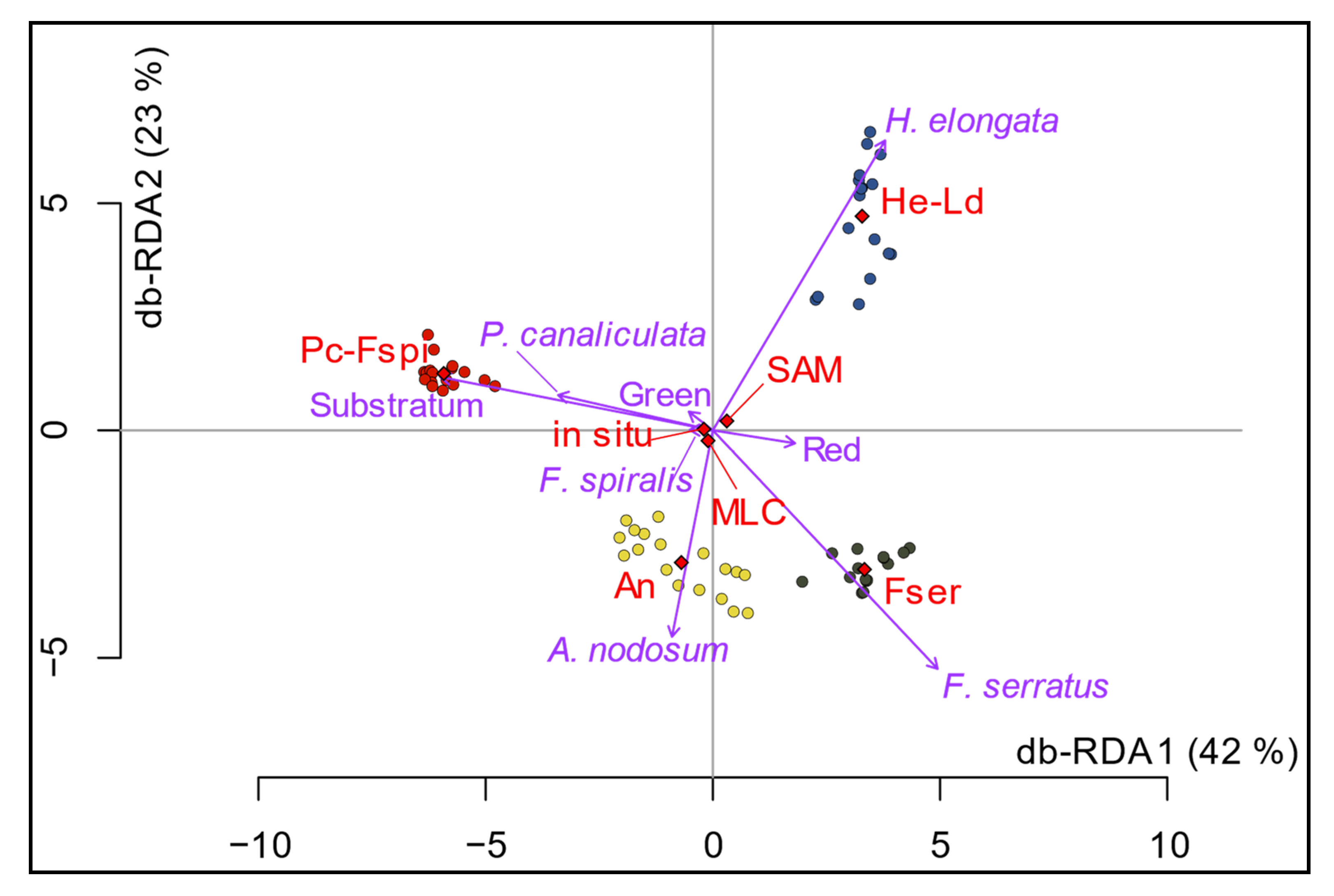
3.3. Comparison of Field Sampling and Hyperspectral Classification
4. Discussion
4.1. Habitats Characterization through Remote Sensing and Field Sampling
4.2. Comparison of the Two Classifiers
4.3. Consistency of Specific Identification and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Hawkins, S.J.; Pack, K.E.; Hyder, K.; Benedetti-Cecchi, L.; Jenkins, S.R. Rocky shores as tractable test systems for experimental ecology. J. Mar. Biol. Assoc. UK 2020, 100, 1017–1041. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Bohn, K.; Firth, L.B.; Williams, G.A. Interactions in the Marine Benthos; Cambridge University Press: Cambridge, UK, 2019; ISBN 110841608X. [Google Scholar]
- Boaventura, D.M. Patterns of Distribution in Intertidal Rocky Shores: The Role of Grazing and Competition in Structuring Communities; University of Faro: Faro, Portugal, 2000. [Google Scholar]
- Graham, L.E.; Graham, J.M.; Cook, M.E.; Wilcox, L.W. Algae; LJLM Press: Madison, WI, USA, 2016. [Google Scholar]
- Raffaelli, D.G.; Hawkins, S.J. Intertidal Ecology, 2nd ed.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1999; ISBN 9780412299506. [Google Scholar]
- Juanes, J.A.; Guinda, X.; Puente, A.; Revilla, J.A. Macroalgae, a suitable indicator of the ecological status of coastal rocky communities in the NE Atlantic. Ecol. Indic. 2008, 8, 351–359. [Google Scholar] [CrossRef]
- Lüning, K. Seaweeds: Their Environment, Biogeography, and Ecophysiology; John Wiley & Sons: Hoboken, NJ, USA, 1990; ISBN 0471624349. [Google Scholar]
- Cabioc’h, J.; Floc’h, J.-Y.; Le Toquin, A.; Boudouresque, C.F. Guide des Algues des mers d’Europe; Delachaux & Niestlé: Paris, France, 2006; ISBN 260301384X. [Google Scholar]
- Ar Gall, E.; Le Duff, M. Development of a quality index to evaluate the structure of macroalgal communities. Estuar. Coast. Shelf Sci. 2014, 139, 99–109. [Google Scholar] [CrossRef]
- Ar Gall, E.; Le Duff, M.; Sauriau, P.G.; de Casamajor, M.N.; Gevaert, F.; Poisson, E.; Hacquebart, P.; Joncourt, Y.; Barillé, A.L.; Buchet, R.; et al. Implementation of a new index to assess intertidal seaweed communities as bioindicators for the European Water Framework Directory. Ecol. Indic. 2016, 60, 162–173. [Google Scholar] [CrossRef] [Green Version]
- Burel, T.; Le Duff, M.; Ar Gall, E. Updated check-list of the seaweeds of the French coasts, Channel and Atlantic Ocean. An Aod-Les Cahiers Naturalistes de l’Observatoire Marin 2019, 7, 1–38. [Google Scholar]
- Floc’h, J.-Y. Cartographie de la végétation marine dans l’archipel de Molène (Finistère). Revue des Travaux de l’Institut des Pêches Maritimes 1970, 34, 89–120. [Google Scholar]
- Moussa, H.B.; Viollier, M.; Belsher, T. Télédétection des algues macrophytes de l’Archipel de Molène (France) Radiomètrie de terrain et application aux données du satellite SPOT. Remote Sens. 1989, 10, 53–69. [Google Scholar] [CrossRef]
- Rossi, N.; Daniel, C.; Perrot, T. Suivi de la Couverture en Macroalgues Intertidales de Substrat dur dans le Cadre du Projet REBENT. 2009, p. 76. Available online: http://www.rebent.org/ (accessed on 20 September 2021).
- Bajjouk, T. Soutien aux Actions NATURA 2000 de la Région Bretagne-Cahier des Charges Pour la Cartographie D’habitats des sites Natura 2000 Littoraux: Guide Méthodologique; RST/IFREMER/DYNECO/AG/09-01/TB/NATURA2000: 107 p.+ Annexes pp. 2009. Available online: https://wwz.ifremer.fr/natura2000/ (accessed on 20 September 2021).
- Bajjouk, T.; Rochette, S.; Laurans, M.; Ehrhold, A.; Hamdi, A.; Le Niliot, P. Multi-approach mapping to help spatial planning and management of the kelp species L. digitata and L. hyperborea: Case study of the Molène Archipelago, Brittany. J. Sea Res. 2015, 100, 2–21. [Google Scholar] [CrossRef]
- OF. Hytech Imaging. TBM Environment Réalisation d’une Cartographie des Habitats Intertidaux de L’archipel de Molène et de la Côte Nord du parc. 2020. Available online: https://parc-marin-iroise.fr/ (accessed on 20 September 2021).
- EC Council. Directive for a legislative frame and actions for the water policy, 2000/60/EC. Off. J. L 2000, 327, 1–73. [Google Scholar]
- Guinda, X.; Juanes, J.A.; Puente, A.; Revilla, J.A. Comparison of two methods for quality assessment of macroalgae assemblages, under different pollution types. Ecol. Indic. 2008, 8, 743–753. [Google Scholar] [CrossRef]
- Guinda, X.; Juanes, J.A.; Puente, A. The Quality of Rocky Bottoms index (CFR): A validated method for the assessment of macroalgae according to the European Water Framework Directive. Mar. Environ. Res. 2014, 102, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.M.; Gaspar, R.; Pereira, L.; Marques, J.C. Marine Macroalgae Assessment Tool (MarMAT) for intertidal rocky shores. Quality assessment under the scope of the European Water Framework Directive. Ecol. Indic. 2012, 19, 39–47. [Google Scholar] [CrossRef]
- Kuhlenkamp, R.; Schubert, P.; Bartsch, I. Water Framework Directive Monitoring-Component Macrophytobenthos N5 Helgoland, EQR Evaluation 2010. Final Report March 2011. Investigation Period: July 2010 February 2011. MMH-Report 17 for Landesamt für Landwirtschaft, Umwelt und Ländliche Räume des Landes Schleswig-Holstein; Germany (LLUR-SH). LLUR Reference number: LLUR-AZ 0608.451013. 2011, pp. 1–62. Available online: https://epic.awi.de/id/eprint/24229/ (accessed on 9 February 2022).
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Derrien-Courtel, S.; Le Gal, A. Suivi des Macroalgues Subtidales de la Façade Manche-Atlantique-Rapport Final-Convention 2010-Action 5. 2011. Available online: https://archimer.ifremer.fr/doc/00036/14735/ (accessed on 9 February 2022).
- Pauly, K.; De Clerck, O. GIS-Based Environmental Analysis, Remote Sensing, and Niche Modeling of Seaweed Communities BT—Seaweeds and their Role in Globally Changing Environments; Seckbach, J., Einav, R., Israel, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 93–114. ISBN 978-90-481-8569-6. [Google Scholar]
- Smale, D.A.; Burrows, M.T.; Moore, P.; O’Connor, N.; Hawkins, S.J. Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecol. Evol. 2013, 3, 4016–4038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, S.J.; Sugden, H.E.; Mieszkowska, N.; Moore, P.J.; Poloczanska, E.; Leaper, R.; Herbert, R.J.H.; Genner, M.J.; Moschella, P.S.; Thompson, R.C.; et al. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol. Prog. Ser. 2009, 396, 245–260. [Google Scholar] [CrossRef] [Green Version]
- Senay, G.B.; Ward, A.D.; Lyon, J.G.; Fausey, N.R.; Nokes, S.E. Manipulation of high spatial resolution aircraft remote sensing data for use in site-specific farming. Trans. ASAE 1998, 41, 489. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H.; Bradford, J.M. Yield estimation from hyperspectral imagery using spectral angle mapper (SAM). Trans. ASABE 2008, 51, 729–737. [Google Scholar] [CrossRef]
- Steneck, R.S.; Watling, L. Feeding capabilities and limitation of herbivorous molluscs: A functional group approach. Mar. Biol. 1982, 68, 299–319. [Google Scholar] [CrossRef]
- Plant, R.E.; Munk, D.S.; Roberts, B.R.; Vargas, R.L.; Rains, D.W.; Travis, R.L.; Hutmacher, R.B. Relationships between remotely sensed reflectance data and cotton growth and yield. Trans. ASAE 2000, 43, 535. [Google Scholar] [CrossRef]
- Viollier, M.; Belsher, T.; Loubersac, L. Signatures spectrales des objets du littoral. In Proceedings of the 3th International Colloquium on Spectral Signatures of Objects in Remote Sensing, Les Arcs, France, 16–20 December 1985. [Google Scholar]
- Floc’h, J.-Y. Cartographie de la Végétation Marine et Observations Écologiques dans L’archipel de MOLÈNE (Finistère). Ph.D. Thesis, Université de Rennes, Rennes, France, 1967. [Google Scholar]
- Andrefouet, S.; Zubia, M.; Payri, C. Mapping and biomass estimation of the invasive brown algae Turbinaria ornata (Turner) J. Agardh and Sargassum mangarevense (Grunow) Setchell on heterogeneous Tahitian coral reefs using 4-meter resolution IKONOS satellite data. Coral Reefs 2004, 23, 26–38. [Google Scholar] [CrossRef]
- Guillaumont, B.; Callens, L.; Dion, P. Spatial distribution and quantification of Fucus species and Ascophyllum nodosum beds in intertidal zones using spot imagery. In Fourteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1993; pp. 297–305. [Google Scholar]
- Zoffoli, M.L.; Gernez, P.; Rosa, P.; Le Bris, A.; Brando, V.E.; Barillé, A.-L.; Harin, N.; Peters, S.; Poser, K.; Spaias, L. Sentinel-2 remote sensing of Zostera noltei-dominated intertidal seagrass meadows. Remote Sens. Environ. 2020, 251, 112020. [Google Scholar] [CrossRef]
- Bajjouk, T.; Guillaumont, B.; Populus, J. Application of airborne imaging spectrometry system data to intertidal seaweed classification and mapping. In Fifteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1996; pp. 463–471. [Google Scholar]
- Brodie, J.; Ash, L.V.; Tittley, I.; Yesson, C. A comparison of multispectral aerial and satellite imagery for mapping intertidal seaweed communities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 872–881. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight unmanned aerial vehicles will revolutionize spatial ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Hamylton, S.M. Spatial Analysis of Coastal Environments; Cambridge University Press: Cambridge, UK, 2017; ISBN 1107070473. [Google Scholar]
- Crawford, C.; Harwin, S. Reassessment of Intertidal Macroalgal Communities Near to and Distant from Salmon Farms and an Evaluation of Using Drones to Survey Macroalgal Distribution; Fisheries Research and Development Corporation: Canberra, Australia, 2018. [Google Scholar]
- Tait, L.; Bind, J.; Charan-Dixon, H.; Hawes, I.; Pirker, J.; Schiel, D. Unmanned Aerial Vehicles (UAVs) for Monitoring Macroalgal Biodiversity: Comparison of RGB and Multispectral Imaging Sensors for Biodiversity Assessments. Remote Sens. 2019, 11, 2332. [Google Scholar] [CrossRef] [Green Version]
- Murfitt, S.L.; Allan, B.M.; Bellgrove, A.; Rattray, A.; Young, M.A.; Ierodiaconou, D. Applications of unmanned aerial vehicles in intertidal reef monitoring. Sci. Rep. 2017, 7, 10259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oppelt, N.M.; Schulze, F.; Doernhoefer, K.; Eisenhardt, I.; Bartsch, I. Hyperspectral classification approaches for intertidal macroalgae habitat mapping: A case study in Heligoland. Opt. Eng. 2012, 51, 111703. [Google Scholar] [CrossRef]
- Davis, S.M.; Swain, P.H. Remote Sensing: The Quantitative Approach; McGraw-Hill International Book Company: New York, NY, USA, 1978. [Google Scholar]
- Richards, J.A. Remote Sensing Digital Image Analysis; Springer: Berlin, Germany, 1986; p. 281. [Google Scholar]
- Shafri, H.Z.M.; Suhaili, A.; Mansor, S. The Performance of Maximum Likelihood, Spectral Angle Mapper, Neural Network and Decision Tree Classifiers in Hyperspectral Image Analysis. J. Comput. Sci. 2007, 3, 419–423. [Google Scholar] [CrossRef] [Green Version]
- Bolstad, P.; Lillesand, T.M. Rapid maximum likelihood classification. Photogramm. Eng. Remote Sens. 1991, 57, 67–74. [Google Scholar]
- Rossiter, T.; Furey, T.; McCarthy, T.; Stengel, D.B. Application of multiplatform, multispectral remote sensors for mapping intertidal macroalgae: A comparative approach. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1595–1612. [Google Scholar] [CrossRef]
- Bartsch, I.; Oppelt, N.; Bochow, M.; Schulze, F.; Geisler, T.; Eisenhardt, I.; Nehring, F.; Heege, T. Detection and Quantification of Marine Vegetation by Airborne Hyperspectral Remote Sensing: Case Study Helgoland. 2011. Available online: https://epic.awi.de/id/eprint/25251/ (accessed on 20 September 2021).
- Uhl, F.; Oppelt, N.; Bartsch, I. Spectral mixture of intertidal marine macroalgae around the island of Helgoland (Germany, North Sea). Aquat. Bot. 2013, 111, 112–124. [Google Scholar] [CrossRef]
- Brodie, J.; Hayes, P.K.; Barker, G.L.; Irvine, L.M. Molecular and morphological characters distinguishing two Porphyra species (Rhodophyta: Bangiophycidae). Eur. J. Phycol. 1996, 31, 303–308. [Google Scholar] [CrossRef]
- Casal, G.; Kutser, T.; Domínguez-Gómez, J.A.; Sánchez-Carnero, N.; Freire, J. Assessment of the hyperspectral sensor CASI-2 for macroalgal discrimination on the Ría de Vigo coast (NW Spain) using field spectroscopy and modelled spectral libraries. Cont. Shelf Res. 2013, 55, 129–140. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Siegel, D.A.; Reed, D.C.; Dennison, P.E. Environmental controls of giant-kelp biomass in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 2011, 429, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Deysher, L.E. Evaluation of remote sensing techniques for monitoring giant kelp populations. Hydrobiologia 1993, 260, 307–312. [Google Scholar] [CrossRef]
- D’Archino, R.; Piazzi, L. Macroalgal assemblages as indicators of the ecological status of marine coastal systems: A review. Ecol. Indic. 2021, 129, 107835. [Google Scholar] [CrossRef]
- Volent, Z.; Johnsen, G.; Sigernes, F. Kelp forest mapping by use of airborne hyperspectral imager. J. Appl. Remote Sens. 2007, 1, 11503. [Google Scholar]
- Rossiter, T.; Furey, T.; McCarthy, T.; Stengel, D.B. UAV-mounted hyperspectral mapping of intertidal macroalgae. Estuar. Coast. Shelf Sci. 2020, 242, 106789. [Google Scholar] [CrossRef]
- Burel, T.; Schaal, G.; Grall, J.; Le Duff, M.; Chapalain, G.; Schmitt, B.; Gemin, M.; Boucher, O.; Ar Gall, E. Small-scale effects of hydrodynamics on the structure of intertidal macroalgal communities: A novel approach. Estuar. Coast. Shelf Sci. 2019, 226, 106290. [Google Scholar] [CrossRef]
- Eastman, J.R. IDRISI Kilimanjaro: Guide to GIS and Image Processing. 2003. Available online: https://www.academia.edu/24202322 (accessed on 7 April 2022).
- Carrasco-Escobar, G.; Manrique, E.; Ruiz-Cabrejos, J.; Saavedra, M.; Alava, F.; Bickersmith, S.; Prussing, C.; Vinetz, J.M.; Conn, J.E.; Moreno, M. High-accuracy detection of malaria vector larval habitats using drone-based multispectral imagery. PLoS Negl. Trop. Dis. 2019, 13, e0007105. [Google Scholar] [CrossRef] [Green Version]
- Congalton, R.G.; Green, K. Assessing the Accuracy of Remotely Sensed Data: Principles and Practices; CRC Press: Boca Raton, FL, USA, 1999; ISBN 0429629354. [Google Scholar]
- Foody, G.M. Status of land cover classification accuracy assessment. Remote Sens. Environ. 2002, 80, 185–201. [Google Scholar] [CrossRef]
- Colombo, P.M.; Orsenigo, M. Sea depth effects on the algal photosynthetic apparatus II. An electron microscopic study of the photosynthetic apparatus of Halimeda tuna (Chlorophyta, Siphonales) at −0.5 m and −6.0 m sea depths. Phycologia 1977, 16, 9–17. [Google Scholar] [CrossRef]
- Jia, X.; Richards, J.A. Efficient maximum likelihood classification for imaging spectrometer data sets. IEEE Trans. Geosci. Remote Sens. 1994, 32, 274–281. [Google Scholar]
- ERDAS Inc. Erdas Field Guide; Erdas Inc.: Atlanta, GA, USA, 1999; Volume 672. [Google Scholar]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The spectral image processing system (SIPS)—Interactive visualization and analysis of imaging spectrometer data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Yuhas, R.H.; Goetz, A.F.H.; Boardman, J.W. Discrimination among semi-arid landscape endmembers using the spectral angle mapper (SAM) algorithm. In Proceedings of the JPL, Summaries of the Third Annual JPL Airborne Geoscience Workshop, Pasadena, CA, USA, 1–5 June 1992; Volume 1, pp. 147–149. [Google Scholar]
- Rwanga, S.S.; Ndambuki, J.M. Accuracy assessment of land use/land cover classification using remote sensing and GIS. Int. J. Geosci. 2017, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 12 January 2022).
- Escobar-Briones, E.G.; Díaz, C.; Legendre, P. Meiofaunal community structure of the deep-sea Gulf of Mexico: Variability due to the sorting methods. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 2627–2633. [Google Scholar] [CrossRef]
- Marçal, A.R.S.; Borges, J.S.; Gomes, J.A.; Da Costa, J.F.P. Land cover update by supervised classification of segmented ASTER images. Int. J. Remote Sens. 2005, 26, 1347–1362. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. In Ecosystem Management; Springer: Berlin/Heidelberg, Germany, 1994; pp. 130–147. [Google Scholar]
- Teagle, H.; Hawkins, S.J.; Moore, P.J.; Smale, D.A. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J. Exp. Mar. Bio. Ecol. 2017, 492, 81–98. [Google Scholar] [CrossRef]
- Belsher, T. Apport du Satellite SPOT à la Cartographie des Végétaux Marins. Halieutique, Océanographie Télédétection Contrib. Française aux Colloq. Fr. Thème Télédétection, 3–13 Octobre 1988, Tokyo Shimizu, Japan. 1990, Volume 6, p. 61. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers20-07/31498.pdf#page=65 (accessed on 20 September 2021).
- Bell, T.W.; Allen, J.G.; Cavanaugh, K.C.; Siegel, D.A. Three decades of variability in California’s giant kelp forests from the Landsat satellites. In Proceedings of the AGU Fall Meeting Abstracts, San Francisco, CA, USA, 5–9 December 2011; Volume 2018, p. B31L-2645. [Google Scholar]
- Gomes, I.; Peteiro, L.; Bueno-Pardo, J.; Albuquerque, R.; Pérez-Jorge, S.; Oliveira, E.R.; Alves, F.L.; Queiroga, H. What’s a picture really worth? On the use of drone aerial imagery to estimate intertidal rocky shore mussel demographic parameters. Estuar. Coast. Shelf Sci. 2018, 213, 185–198. [Google Scholar] [CrossRef]
- Barbosa, R.V. Assessing the Effect of Microhabitat Features on the Dynamics of a Benthic Intertidal Species: Use of Remote Sensing and Biophysical Modeling. Université de Bretagne Occidentale. 2022. Available online: http://www.theses.fr/s210110 (accessed on 8 March 2022).
- Brunier, G.; Oiry, S.; Gruet, Y.; Dubois, S.F.; Barillé, L. Topographic Analysis of Intertidal Polychaete Reefs (Sabellaria alveolata) at a Very High Spatial Resolution. Remote Sens. 2022, 14, 307. [Google Scholar] [CrossRef]
- Connan, S. Etude de la Diversité Spécifique des Macroalgues de la Pointe de Bretagne et Analyse des Composés Phénoliques des Phéophycées Dominantes. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2004. [Google Scholar]
- Burel, T. Effet de L’hydrodynamisme sur la Structure des Communautés Macroalgales et sur les Interactions Macroflore/Macrofaune en Zone Intertidale. Ph.D. Thesis, Université de Bretagne Occidentale, Brest, France, 2020. [Google Scholar]
- Douay, F.; Verpoorter, C.; Duong, G.; Spilmont, N.; Gevaert, F. New Hyperspectral Procedure to Discriminate Intertidal Macroalgae. Remote Sens. 2022, 14, 346. [Google Scholar] [CrossRef]
- Olmedo-Masat, O.M.; Raffo, M.P.; Rodríguez-Pérez, D.; Arijón, M.; Sánchez-Carnero, N. How Far Can We Classify Macroalgae Remotely? An Example Using a New Spectral Library of Species from the South West Atlantic (Argentine Patagonia). Remote Sens. 2020, 12, 3870. [Google Scholar] [CrossRef]
- Kutser, T.; Miller, I.; Jupp, D.L.B. Mapping coral reef benthic substrates using hyperspectral space-borne images and spectral libraries. Estuar. Coast. Shelf Sci. 2006, 70, 449–460. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, G.; Troy, A.; Cadenasso, M.L. Object-based land cover classification of shaded areas in high spatial resolution imagery of urban areas: A comparison study. Remote Sens. Environ. 2009, 113, 1769–1777. [Google Scholar] [CrossRef]
- Stengel, D.B.; Dring, M.J. Seasonal variation in the pigment content and photosynthesis of different thallus regions of Ascophyllum nodosum (Fucales, Phaeophyta) in relation to position in the canopy. Phycologia 1998, 37, 259–268. [Google Scholar] [CrossRef]
- Stengel, D.B.; Wilkes, R.J.; Guiry, M.D. Seasonal growth and recruitment of Himanthalia elongata (Fucales, Phaeophycota) in different habitats on the Irish west coast. Eur. J. Phycol. 1999, 34, 213–221. [Google Scholar] [CrossRef]
- Burel, T.; Grall, J.; Schaal, G.; Le Duff, M.; Ar Gall, E. Wave height vs. elevation effect on macroalgal dominated shores: An intercommunity study. J. Appl. Phycol. 2020, 32, 2523–2534. [Google Scholar] [CrossRef]
- Petropoulos, G.P.; Vadrevu, K.P.; Kalaitzidis, C. Spectral angle mapper and object-based classification combined with hyperspectral remote sensing imagery for obtaining land use/cover mapping in a Mediterranean region. Geocarto Int. 2013, 28, 114–129. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, J.; Wang, A.; Chen, J. Object- and pixel-based classifications of macroalgae farming area with high spatial resolution imagery. Geocarto Int. 2017, 33, 1048–1063. [Google Scholar] [CrossRef]
- Liu, D.; Xia, F. Assessing object-based classification: Advantages and limitations. Remote Sens. Lett. 2010, 1, 187–194. [Google Scholar] [CrossRef]
- Foody, G.M. Explaining the unsuitability of the kappa coefficient in the assessment and comparison of the accuracy of thematic maps obtained by image classification. Remote Sens. Environ. 2020, 239, 111630. [Google Scholar] [CrossRef]
- Hobley, B.; Arosio, R.; French, G.; Bremner, J.; Dolphin, T.; Mackiewicz, M. Semi-Supervised Segmentation for Coastal Monitoring Seagrass Using RPA Imagery. Remote Sens. 2021, 13, 1741. [Google Scholar] [CrossRef]
- Kumar, P.; Gupta, D.K.; Mishra, V.N.; Prasad, R. Comparison of support vector machine, artificial neural network, and spectral angle mapper algorithms for crop classification using LISS IV data. Int. J. Remote Sens. 2015, 36, 1604–1617. [Google Scholar] [CrossRef]
- Otukei, J.R.; Blaschke, T. Land cover change assessment using decision trees, support vector machines and maximum likelihood classification algorithms. Int. J. Appl. Earth Obs. Geoinf. 2010, 12, S27–S31. [Google Scholar] [CrossRef]
- Zheng, Y.; Duarte, C.M.; Chen, J.; Li, D.; Lou, Z.; Wu, J. Remote sensing mapping of macroalgal farms by modifying thresholds in the classification tree. Geocarto Int. 2019, 34, 1098–1108. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Wang, K.; Zhang, S.; Li, J.; Sun, M. Assessment of intertidal seaweed biomass based on RGB imagery. PLoS ONE 2022, 17, e0263416. [Google Scholar] [CrossRef]
- Thibaut, T.; Pinedo, S.; Torras, X.; Ballesteros, E. Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, North-western Mediterranean). Mar. Pollut. Bull. 2005, 50, 1472–1489. [Google Scholar] [CrossRef]
- Jonsson, P.R.; Kotta, J.; Andersson, H.C.; Herkül, K.; Virtanen, E.; Sandman, A.N.; Johannesson, K. High climate velocity and population fragmentation may constrain climate-driven range shift of the key habitat former Fucus vesiculosus. Divers. Distrib. 2018, 24, 892–905. [Google Scholar] [CrossRef] [Green Version]
- Le Roux, A. Les patelles (Patella vulgata L.), agents de la destruction de la couverture algale des estrans rocheux du Golfe du Morbihan. Bull. De La Société Des Sci. Nat. De L’ouest De La Fr. 2008, 30, 162–180. [Google Scholar]
- Richardson, J.P.; Lefcheck, J.S.; Orth, R.J. Warming temperatures alter the relative abundance and distribution of two co-occurring foundational seagrasses in Chesapeake Bay, USA. Mar. Ecol. Prog. Ser. 2018, 599, 65–74. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Mondardini, L.; Alestra, T.; Gerrity, S.; Tait, L.; South, P.M.; Lilley, S.A.; Schiel, D.R. Local extinction of bull kelp (Durvillaea spp.) due to a marine heatwave. Front. Mar. Sci. 2019, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Beas-Luna, R.; Micheli, F.; Woodson, C.B.; Carr, M.; Malone, D.; Torre, J.; Boch, C.; Caselle, J.E.; Edwards, M.; Freiwald, J. Geographic variation in responses of kelp forest communities of the California Current to recent climatic changes. Glob. Chang. Biol. 2020, 26, 6457–6473. [Google Scholar] [CrossRef]
- Smale, D.A. Impacts of ocean warming on kelp forest ecosystems. New Phytol. 2020, 225, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.G.; Coops, N.C.; Sharma, T. Exploring the utility of hyperspectral imagery and LiDAR data for predicting Quercus garryana ecosystem distribution and aiding in habitat restoration. Restor. Ecol. 2011, 19, 245–256. [Google Scholar] [CrossRef]
- Valle, M.; Pala, V.; Lafon, V.; Dehouck, A.; Garmendia, J.M.; Borja, A.; Chust, G. Mapping estuarine habitats using airborne hyperspectral imagery, with special focus on seagrass meadows. Estuar. Coast. Shelf Sci. 2015, 164, 433–442. [Google Scholar] [CrossRef]
- Israel, A.; Einav, R.; Seckbach, J.; Israel, A.; Einav, R.; Seckbach, J. Seaweeds and Their Role in Globally Changing Environments; Springer Science & Business Media: New York, NY, USA, 2010; Volume 15, ISBN 9048185696. [Google Scholar]
- Bordeyne, F.; Migné, A.; Plus, M.; Davoult, D. Modelling the annual primary production of an intertidal brown algal community based on in situ measurements. Mar. Ecol. Prog. Ser. 2020, 656, 95–107. [Google Scholar] [CrossRef]
- Golléty, C.; Migné, A.; Davoult, D. Benthic metabolism on a sheltered rocky shore: Role of the canopy in the carbon budget. J. Phycol. 2008, 44, 1146–1153. [Google Scholar] [CrossRef]
- Dugdale, S.J. An Evaluation of Imagery from an Unmanned Aerial Vehicle (UAV) for the Mapping of Intertidal Macroalgae on Seal Sands, Tees Estuary, UK. Master’s Thesis, Durham University, Durham, UK, 2007. [Google Scholar]
- Ben Moussa, H. Contribution de la Télédétection Satellitaire à la Cartographie des Végétaux Marins: Archipel de Molène (Bretagne/France). 1987. Available online: https://archimer.ifremer.fr/doc/00104/21524/ (accessed on 24 January 2022).




| Spectral Range | Spatial Pixels | Spectral Resolution | Spectral Sampling | Number of Bands | FOV Across Track | iFOV Across/ 3Along Track | Coding |
|---|---|---|---|---|---|---|---|
| 0.4–1 µm | 1240 | 4.5 nm | 3 nm | 200 | 20° | 0.27/0.27 mrad | 12 bits |
| Flight Altitude | Ground Sampling Distance | Swath | Mapped Area | Viewing Angle | Flight Lines |
|---|---|---|---|---|---|
| 64 m | 2 cm | 23 m | 1.76 ha | 20° | 4 |
| Class | Number of ROIs | Number of Pixels |
|---|---|---|
| P. canaliculata | 76 | 29,899 |
| F. spiralis | 10 | 551 |
| A. nodosum | 233 | 334,002 |
| F. serratus | 227 | 894,910 |
| H. elongata | 145 | 353,825 |
| Green | 482 | 73,592 |
| Red | 509 | 41,808 |
| Substratum | 408 | 1,834,496 |
| Water | 235 | 1,073,044 |
| Class | P. canaliculata | F. spiralis | A. nodosum | F. serratus | H. elongata | Green | Red | Substratum | Water | Total | User Acc. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unclassified | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - |
| P. canaliculata | 97.82 | 44.13 | 7.04 | 0.01 | 0.02 | 0 | 3.04 | 0.90 | 0.03 | 1.28 | 26.04 |
| F. spiralis | 0.13 | 39.00 | 0.23 | 0 | 0.07 | 0 | 0.85 | 0.09 | 0.24 | 0.14 | 6.23 |
| A. nodosum | 0.79 | 14.81 | 89.32 | 5.59 | 0.03 | 0.27 | 0.78 | 0.04 | 0.02 | 5.63 | 91.93 |
| F. serratus | 0.01 | 0 | 1.56 | 91.71 | 0.03 | 0.01 | 0.04 | 0 | 0 | 6.80 | 98.61 |
| H. elongata | 0.09 | 0.15 | 0.08 | 0.38 | 93.53 | 0.18 | 2.60 | 0 | 4.00 | 9.69 | 90.72 |
| Green | 0.17 | 0.15 | 0.73 | 0.35 | 0.07 | 96.92 | 0.70 | 0.11 | 0.21 | 1.66 | 88.85 |
| Red | 0.01 | 1.76 | 0.75 | 1.78 | 3.14 | 1.64 | 90.54 | 0.02 | 0.22 | 1.68 | 67.03 |
| Substratum | 0.67 | 0 | 0.14 | 0.03 | 0 | 0.22 | 0.25 | 96.59 | 0.15 | 51.78 | 99.90 |
| Water | 0.30 | 0 | 0.15 | 0.15 | 3.10 | 0.75 | 1.20 | 2.25 | 95.15 | 21.34 | 92.76 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - |
| Prod. Acc. | 97.82 | 39.00 | 89.32 | 91.71 | 93.53 | 96.92 | 90.54 | 96.59 | 95.15 | - | - |
| Class | P. canaliculata | F. spiralis | A. nodosum | F. serratus | H. elongata | Green | Red | Substratum | Water | Total | User Acc. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unclassified | 0 | 0 | 0 | 0 | 0.06 | 0 | 0 | 0 | 0.24 | 0.06 | - |
| P. canaliculata | 65.22 | 14.37 | 4.62 | 9.604 | 0.72 | 6.32 | 3.97 | 0.03 | 0.04 | 1.44 | 15.44 |
| F. spiralis | 9.44 | 25.95 | 18.76 | 2.78 | 0 | 0.33 | 0.70 | 0.01 | 0 | 1.35 | 0.43 |
| A. nodosum | 3.02 | 37.54 | 67.06 | 17.37 | 0.04 | 0.32 | 22.84 | 0.01 | 0.01 | 5.48 | 70.97 |
| F. serratus | 1.97 | 8.80 | 7.35 | 38.80 | 1.36 | 1.44 | 8.21 | 0.01 | 0.04 | 3.54 | 80.26 |
| H. elongata | 9.49 | 1.47 | 0.44 | 11.78 | 95.01 | 10.76 | 7.26 | 0.01 | 7.92 | 11.76 | 75.96 |
| Green | 7.72 | 6.45 | 0.77 | 0.75 | 0.86 | 77.33 | 1.42 | 0.64 | 0.73 | 1.89 | 62.07 |
| Red | 2.56 | 5.13 | 0.93 | 18.86 | 0.60 | 0.23 | 54.82 | 0 | 0.02 | 2.19 | 31.09 |
| Substratum | 0.29 | 0 | 0.05 | 0.01 | 0 | 0.43 | 0.06 | 99.04 | 8.41 | 54.81 | 96.79 |
| Water | 0.30 | 0.29 | 0.02 | 0.02 | 1.35 | 2.82 | 0.72 | 0.24 | 82.60 | 17.49 | 98.23 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | - |
| Prod. Acc. | 65.22 | 25.95 | 67.06 | 38.8 | 95.01 | 77.33 | 54.82 | 99.04 | 82.6 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diruit, W.; Le Bris, A.; Bajjouk, T.; Richier, S.; Helias, M.; Burel, T.; Lennon, M.; Guyot, A.; Ar Gall, E. Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling. Remote Sens. 2022, 14, 3124. https://doi.org/10.3390/rs14133124
Diruit W, Le Bris A, Bajjouk T, Richier S, Helias M, Burel T, Lennon M, Guyot A, Ar Gall E. Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling. Remote Sensing. 2022; 14(13):3124. https://doi.org/10.3390/rs14133124
Chicago/Turabian StyleDiruit, Wendy, Anthony Le Bris, Touria Bajjouk, Sophie Richier, Mathieu Helias, Thomas Burel, Marc Lennon, Alexandre Guyot, and Erwan Ar Gall. 2022. "Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling" Remote Sensing 14, no. 13: 3124. https://doi.org/10.3390/rs14133124
APA StyleDiruit, W., Le Bris, A., Bajjouk, T., Richier, S., Helias, M., Burel, T., Lennon, M., Guyot, A., & Ar Gall, E. (2022). Seaweed Habitats on the Shore: Characterization through Hyperspectral UAV Imagery and Field Sampling. Remote Sensing, 14(13), 3124. https://doi.org/10.3390/rs14133124






