Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Eddy Covariance Climate and Carbon Flux Data
2.3. MOD13A2 Vegetation Indices Data
2.4. MOD17A2 GPP Data
2.5. Statistical Processing
3. Results
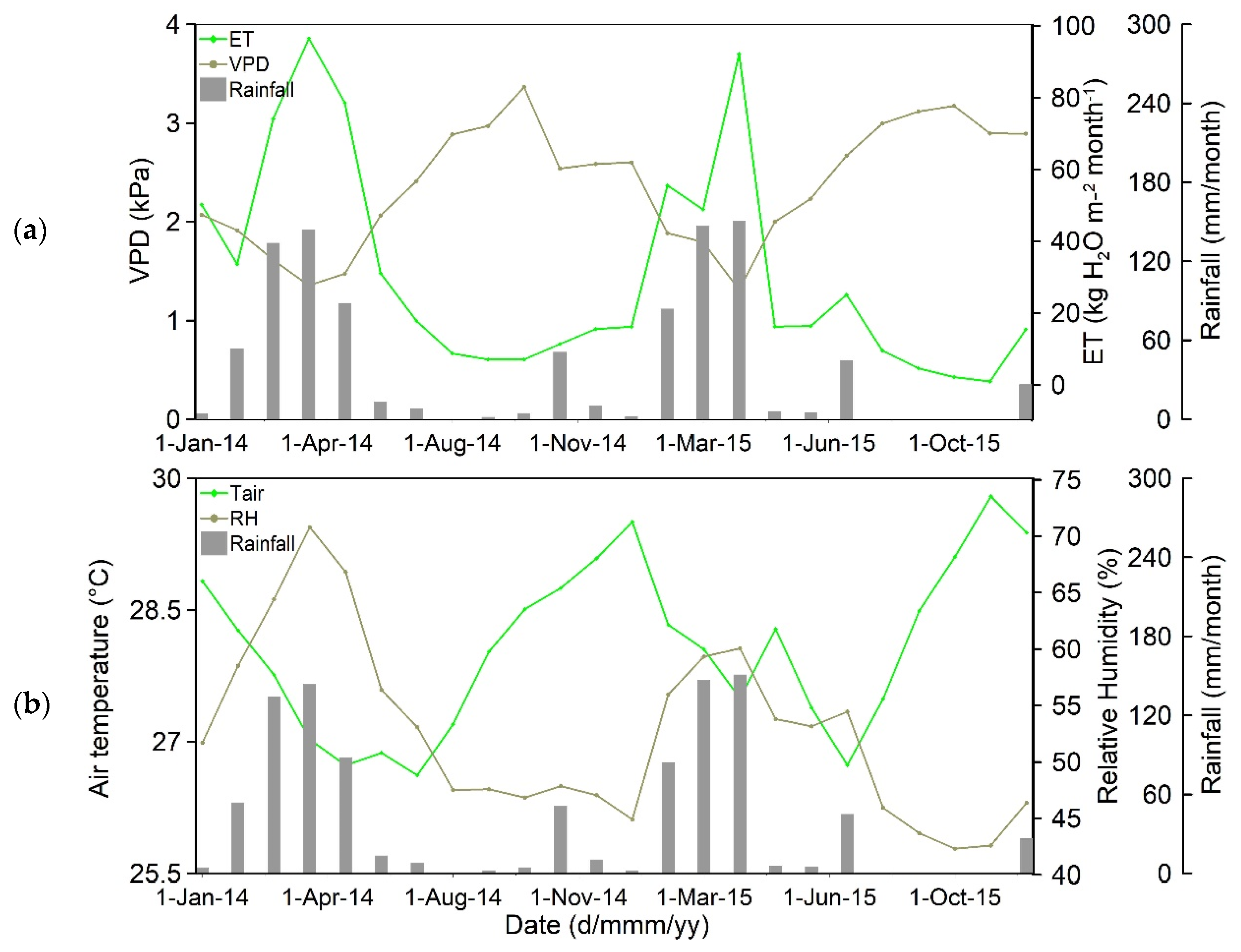
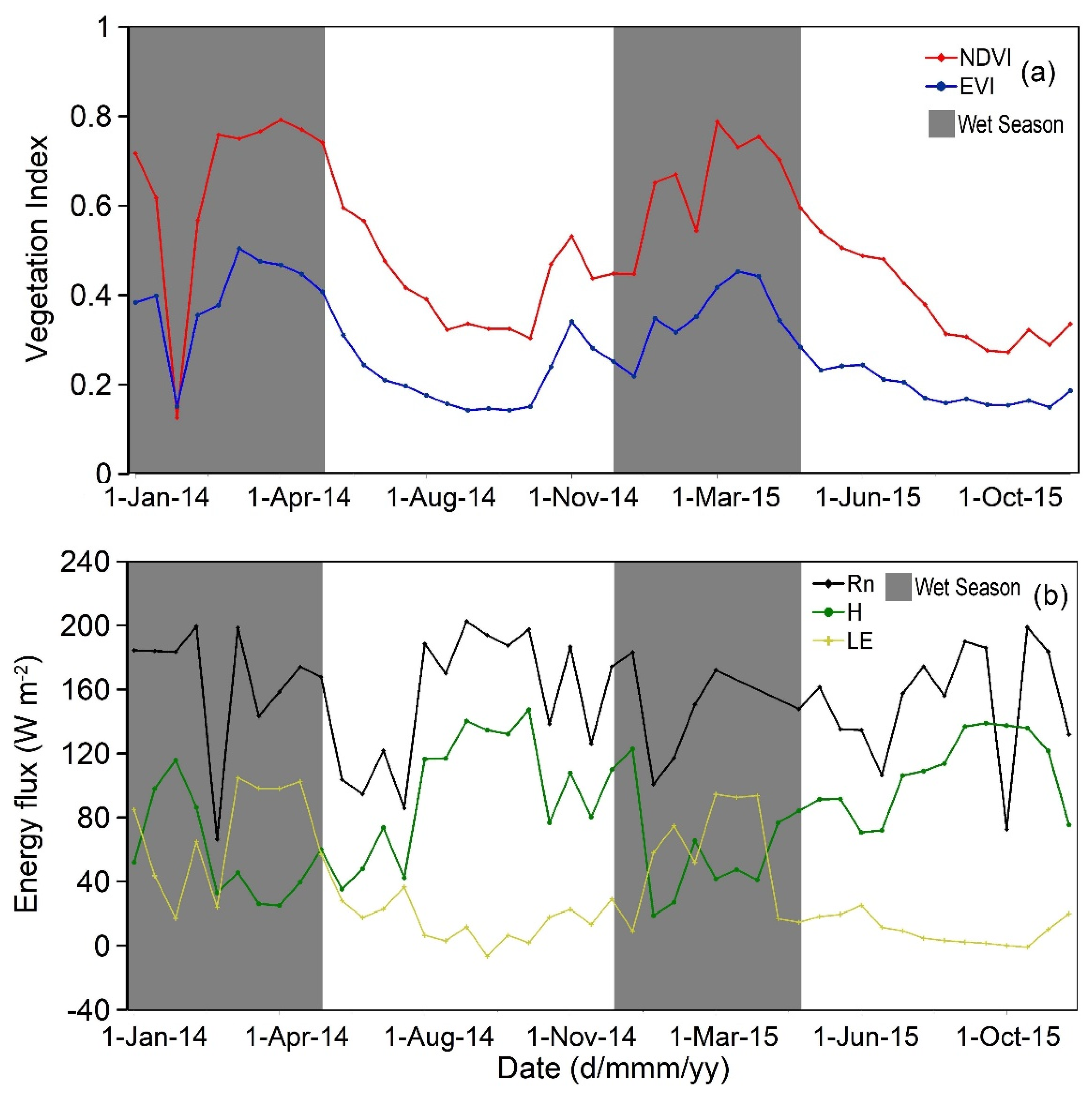
3.1. Seasonal Dynamics of Climate, Vegetation Indices, Carbon, and Energy Fluxes
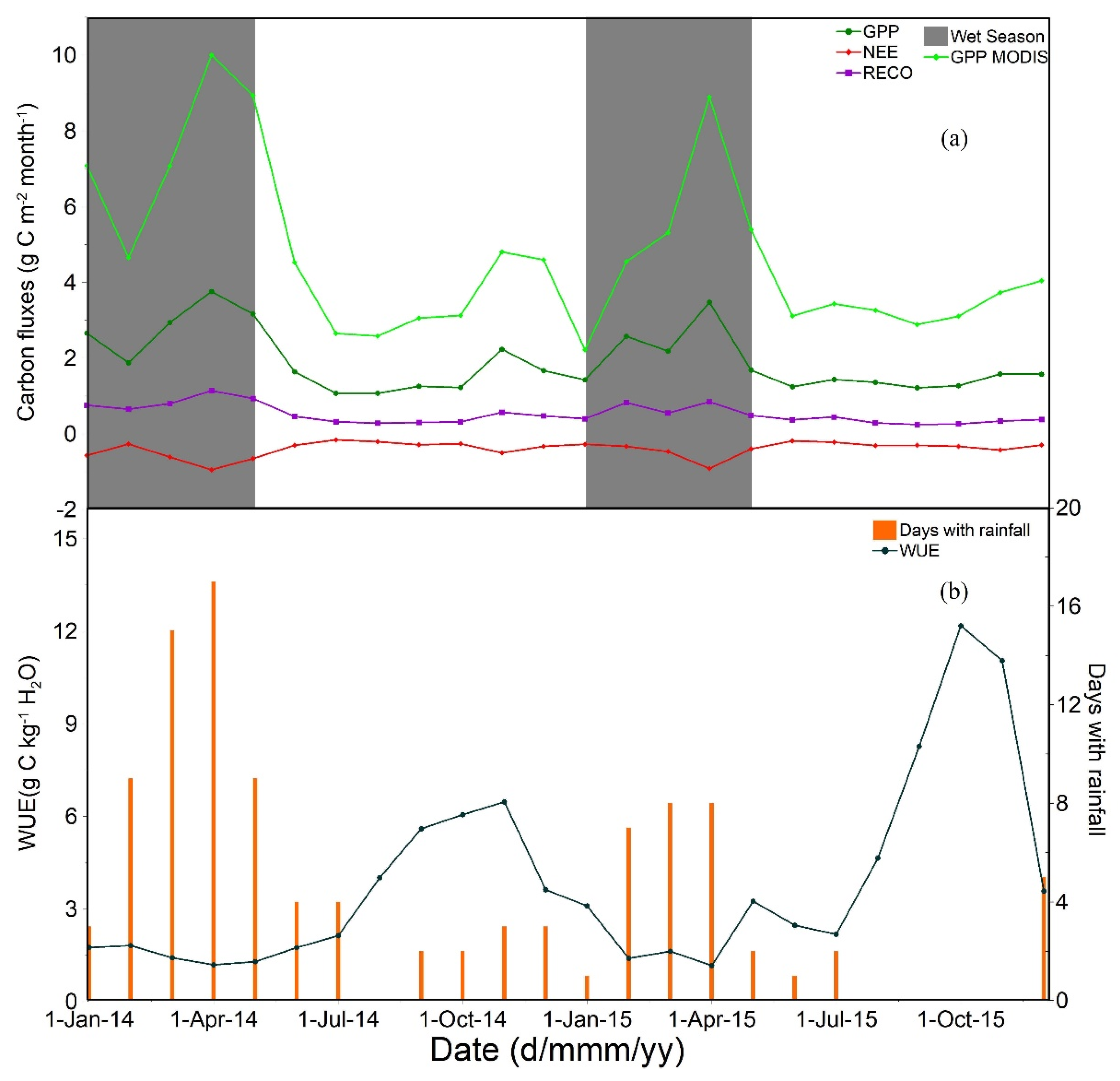
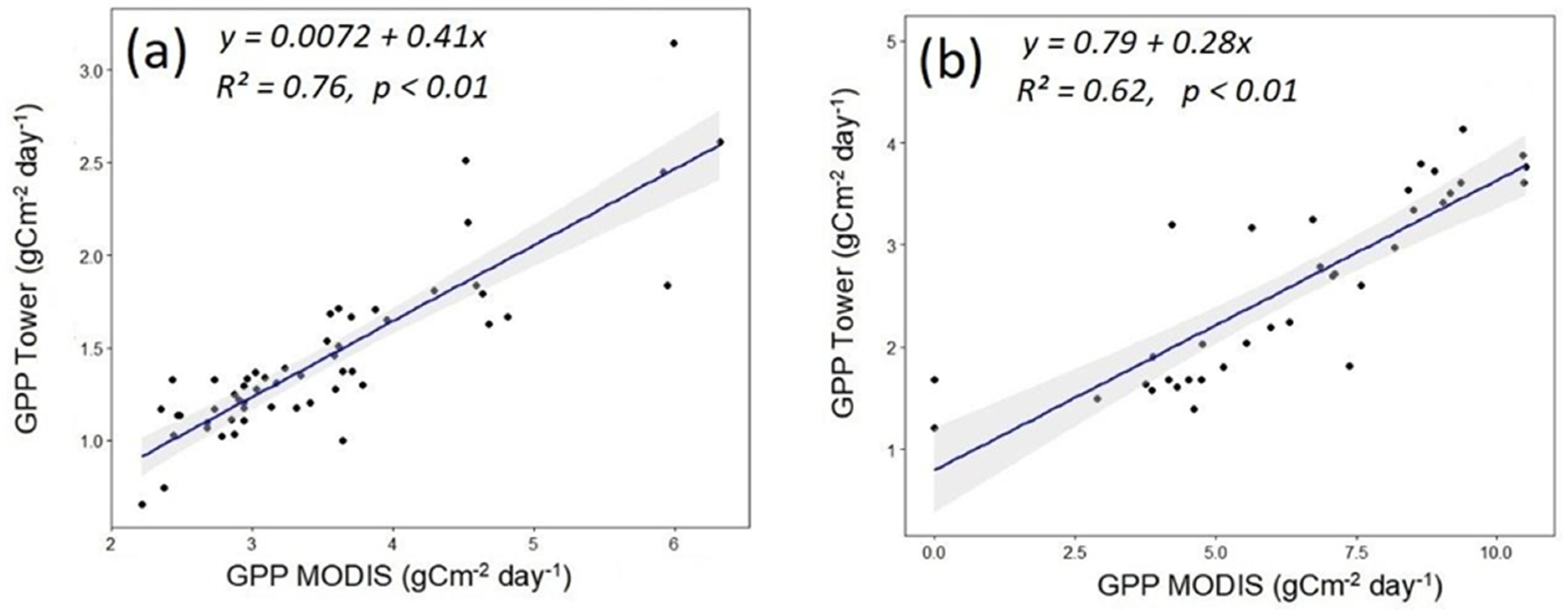
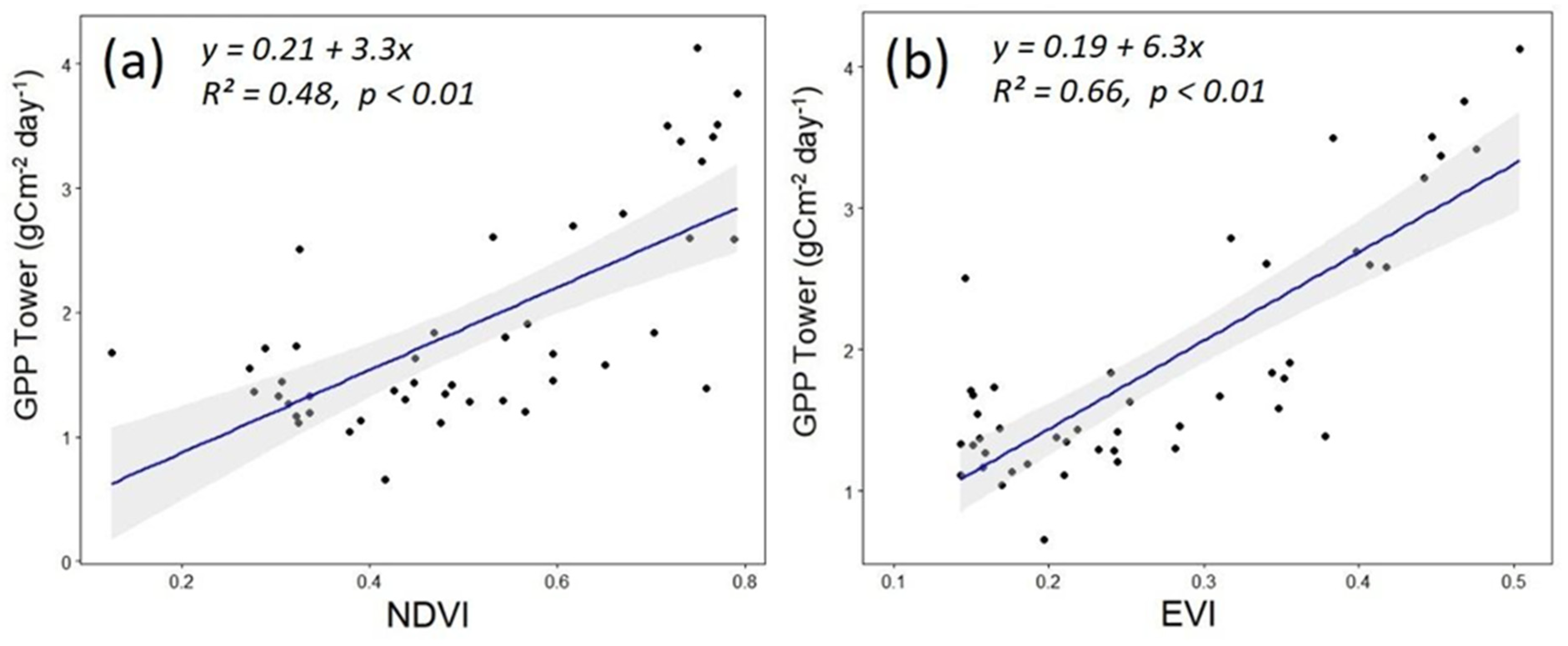
3.2. Seasonal Dynamics of the Carbon Cycle, WUE, Tower-Measured GPP, and Remotely Sensed GPP
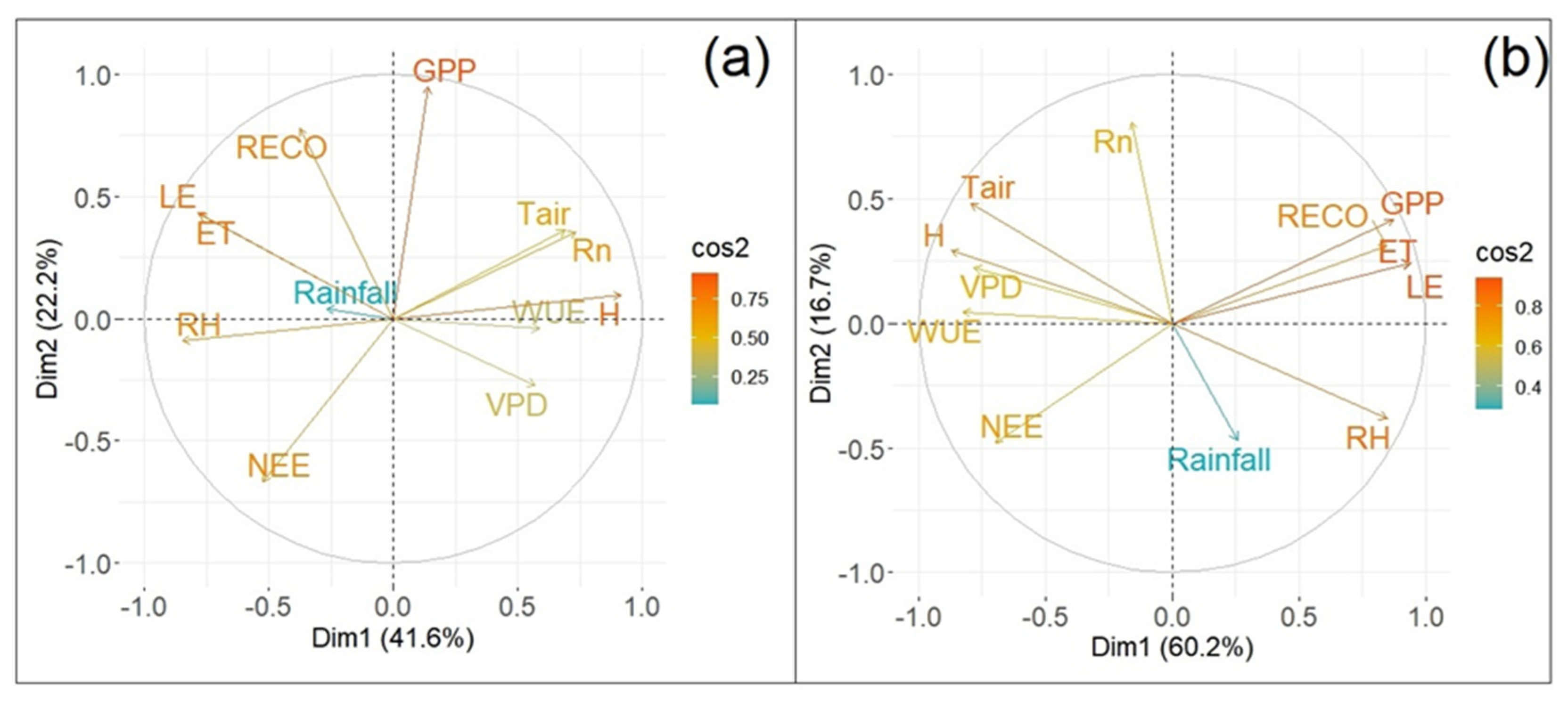
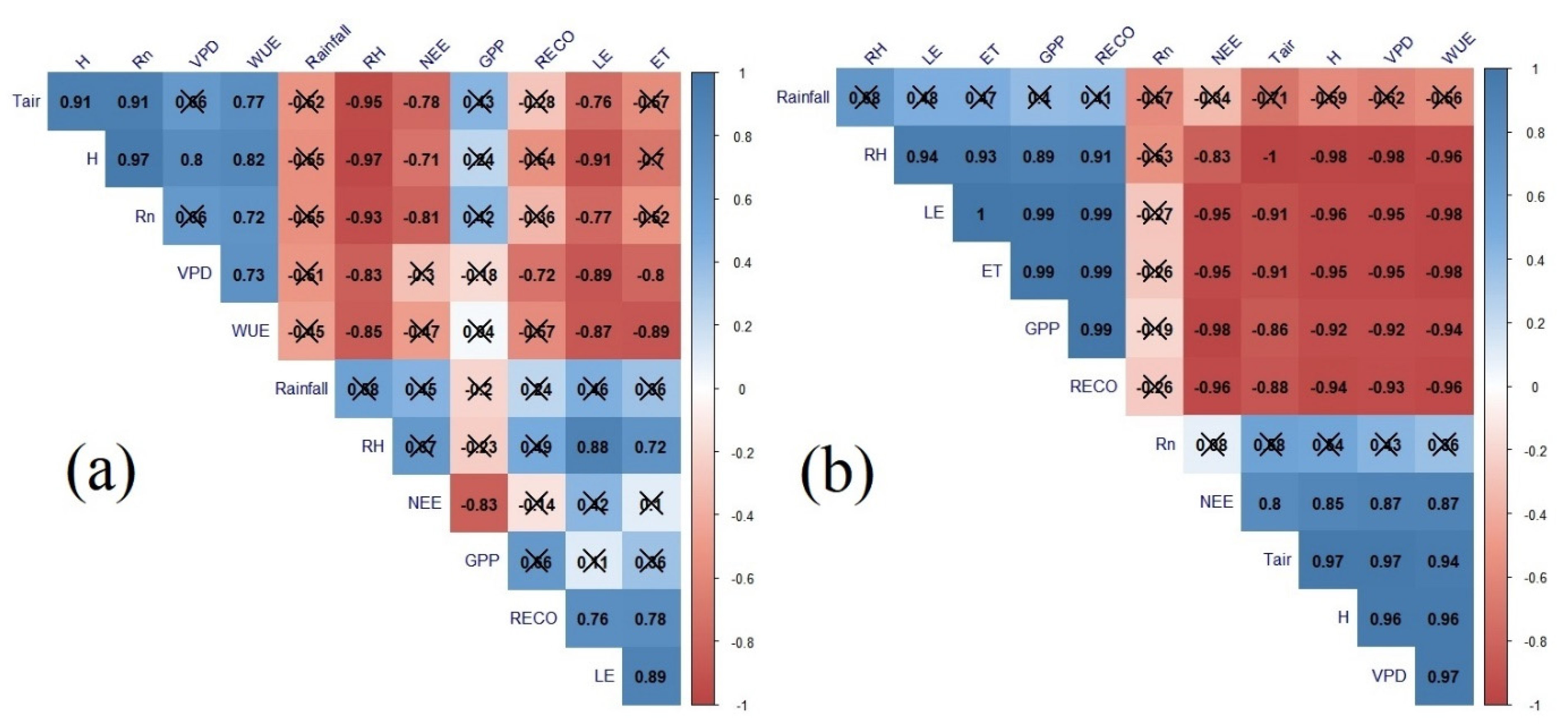
3.3. Principal Component Analysis and GPP Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rocha, H.R.; Freitas, H.C.; Rosolem, R.; Juarez, R.I.N.; Tannus, R.N.; Ligo, M.; Cabral, O.M.R.; Dias, M.A.F.S. Measurements of CO2 fluxes in a Cerrado Sensu stricto in southeastern Brazil. Neotrop. Biota 2002, 2, 1. [Google Scholar] [CrossRef]
- Campos, S.; Mendes, K.R.; da Silva, L.L.; Mutti, P.R.; Medeiros, S.S.; Amorim, L.B.; dos Santos, C.A.; Perez-Marin, A.M.; Ramos, T.M.; Marques, T.V.; et al. Closure and partitioning of the energy balance in a preserved area of a Brazilian seasonally dry tropical forest. Agric. For. Meteorol. 2019, 271, 398–412. [Google Scholar] [CrossRef]
- Marques, T.V.; Mendes, K.; Mutti, P.; Medeiros, S.; Silva, L.; Perez-Marin, A.M.; Campos, S.; Lúcio, P.S.; Lima, K.; dos Reis, J.; et al. Environmental and biophysical controls of evapotranspiration from Seasonally Dry Tropical Forests (Caatinga) in the Brazilian Semiarid. Agric. For. Meteorol. 2020, 287, 107957. [Google Scholar] [CrossRef]
- Silva, A.C.; Mendes, K.R.; e Silva, C.M.S.; Rodrigues, D.T.; Costa, G.B.; da Silva, D.T.C.; Mutti, P.R.; Ferreira, R.R.; Bezerra, B.G. Energy Balance, CO2 Balance, and Meteorological Aspects of Desertification Hotspots in Northeast Brazil. Water 2021, 13, 2962. [Google Scholar] [CrossRef]
- Saad, S.I.; Da Rocha, H.R.; Dias, M.A.F.S.; Rosolem, R. Can the Deforestation Breeze Change the Rainfall in Amazonia? A Case Study for the BR-163 Highway Region. Earth Interactions 2010, 14, 1. [Google Scholar] [CrossRef]
- Bai, Y.; Li, X.; Zhou, S.; Yang, X.; Yu, K.; Wang, M.; Liu, S.; Wang, P.; Wu, X.; Wang, X.; et al. Quantifying plant transpiration and canopy conductance using eddy flux data: An underlying water use efficiency method. Agric. For. Meteorol. 2019, 271, 375–384. [Google Scholar] [CrossRef]
- Mendes, K.R.; Campos, S.; Mutti, P.R.; Ferreira, R.R.; Ramos, T.M.; Marques, T.V.; Dos Reis, J.S.; Vieira, M.M.D.L.; Silva, A.C.N.; Marques, A.M.S.; et al. Assessment of SITE for CO2 and Energy Fluxes Simulations in a Seasonally Dry Tropical Forest (Caatinga Ecosystem). Forests 2021, 12, 86. [Google Scholar] [CrossRef]
- Ruhoff, A.L.; Paz, A.R.; Collischonn, W.; Aragao, L.E.; Rocha, H.R.; Malhi, Y.S. A MODIS-Based Energy Balance to Estimate Evapotranspiration for Clear-Sky Days in Brazilian Tropical Savannas. Remote Sens. 2012, 4, 703–725. [Google Scholar] [CrossRef]
- Fonseca, L.D.M.; Dalagnol, R.; Malhi, Y.; Rifai, S.W.; Costa, G.B.; Silva, T.S.F.; Da Rocha, H.R.; Tavares, I.B.; Borma, L.S. Phenology and Seasonal Ecosystem Productivity in an Amazonian Floodplain Forest. Remote Sens. 2019, 11, 1530. [Google Scholar] [CrossRef]
- Moreira, A.A.; Ruhoff, A.L.; Roberti, D.R.; Souza, V.D.A.; da Rocha, H.R.; de Paiva, R.C.D. Assessment of terrestrial water balance using remote sensing data in South America. J. Hydrol. 2019, 575, 131–147. [Google Scholar] [CrossRef]
- Laipelt, L.; Ruhoff, A.L.; Fleischmann, A.S.; Kayser, R.H.B.; Kich, E.D.M.; da Rocha, H.R.; Neale, C.M.U. Assessment of an Automated Calibration of the SEBAL Algorithm to Estimate Dry-Season Surface-Energy Partitioning in a Forest–Savanna Transition in Brazil. Remote Sens. 2020, 12, 1108. [Google Scholar] [CrossRef]
- Mendes, K.R.; Batista-Silva, W.; Dias-Pereira, J.; Pereira, M.P.S.; Souza, E.V.; Serrão, J.E.; Granja, J.A.A.; Pereira, E.C.; Gallacher, D.J.; Mutti, P.R.; et al. Leaf plasticity across wet and dry seasons in Croton blanchetianus (Euphorbiaceae) at a tropical dry forest. Sci. Rep. 2022, 12, 954. [Google Scholar] [CrossRef]
- Tang, X.; Carvalhais, N.; Moura, C.; Ahrens, B.; Koirala, S.; Fan, S.; Reichstein, M. Global variability of carbon use efficiency in terrestrial ecosystems. Biogeosciences Discuss 2019, 1–19. [Google Scholar] [CrossRef]
- Da Rocha, H.R.; Goulden, M.; Miller, S.D.; Menton, M.; Pinto, L.D.V.O.; De Freitas, H.C.; Figueira, A.M.E.S. Seasonality of water and heat fluxes over a tropical forest in eastern amazonia. Ecol. Appl. 2004, 14, 22–32. [Google Scholar] [CrossRef]
- Espírito-Santo, F.; Gloor, M.; Keller, M.; Malhi, Y.; Saatchi, S.; Nelson, B.; Junior, R.C.O.; Pereira, C.; Lloyd, J.; Frolking, S.; et al. Size and frequency of natural forest disturbances and the Amazon forest carbon balance. Nat. Commun. 2014, 5, 3434. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Frankenberg, C.; van der Tol, C.; Berry, J.A.; Guanter, L.; Boyce, C.K.; Fisher, J.; Morrow, E.; Worden, J.R.; Asefi, S.; et al. Forest productivity and water stress in Amazonia: Observations from GOSAT chlorophyll fluorescence. Proc. R. Soc. 2013, 280, 20130171. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y.; Pegoraro, E.; Nobre, A.D.; Pereira, M.G.P.; Grace, J.; Culf, A.D.; Clement, R. Energy and water dynamics of a central Amazonian rain forest. J. Geophys. Res. Earth Surf. 2002, 107, LBA 45-1–LBA 45-17. [Google Scholar] [CrossRef]
- Saleska, S.R.; da Rocha, H.R.; Kruijt, B.; Nobre, A.D. Ecosystem carbon fluxes and Amazonian forest metabolism. In Amazonia and Global Change; American Geophysical Union: Washington, DC, USA, 2009; pp. 389–407. [Google Scholar]
- Mendes, K.R.; Campos, S.; Da Silva, L.L.; Mutti, P.R.; Ferreira, R.R.; Medeiros, S.S.; Perez-Marin, A.M.; Marques, T.V.; Ramos, T.M.; Vieira, M.M.D.L.; et al. Seasonal variation in net ecosystem CO2 exchange of a Brazilian seasonally dry tropical forest. Sci. Rep. 2020, 10, 9454. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Mutti, P.; Mendes, K.R.; Campos, S.; Marques, T.V.; Oliveira, C.P.; Gonçalves, W.; Mota, J.; Difante, G.; Urbano, S.A.; et al. An assessment of the MOD17A2 gross primary production product in the Caatinga biome, Brazil. Int. J. Remote Sens. 2020, 42, 1275–1291. [Google Scholar] [CrossRef]
- Junttila, S.; Näsi, R.; Koivumäki, N.; Imangholiloo, M.; Saarinen, N.; Raisio, J.; Holopainen, M.; Hyyppä, H.; Hyyppä, J.; Lyytikäinen-Saarenmaa, P.; et al. Multispectral Imagery Provides Benefits for Mapping Spruce Tree Decline Due to Bark Beetle Infestation When Acquired Late in the Season. Remote Sens. 2022, 14, 909. [Google Scholar] [CrossRef]
- Maselli, F.; Papale, D.; Puletti, N.; Chirici, G.; Corona, P. Combining remote sensing and ancillary data to monitor the gross productivity of water-limited forest ecosystems. Remote Sens. Environ. 2009, 113, 657–667. [Google Scholar] [CrossRef]
- Morales, P.; Sykes, M.T.; Prentice, I.C.; Smith, P.; Smith, B.; Bugmann, H.; Zierl, B.; Friedlingstein, P.; Viovy, N.; Sabaté, S.; et al. Comparing and evaluating process-based ecosystem model predictions of carbon and water fluxes in major European forest biomes. Glob. Chang. Biol. 2005, 11, 2211–2233. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, J.; Ju, W.; Zhou, Y.; Wang, S.; Wu, X. Water use efficiency of China’s terrestrial ecosystems and responses to drought. Sci. Rep. 2015, 5, 13799. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Albertson, J.D. Canopy scale measurements of CO2 and water vapor exchange along a precipitation gradient in southern Africa. Glob. Chang. Biol. 2004, 10, 329–341. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, J.; Meng, P.; Li, J.; Zheng, N. Ecosystem water use efficiency in a warm-temperate mixed plantation in the NorthChina. J. Hydrol. 2014, 512, 221–228. [Google Scholar] [CrossRef]
- Song, Q.-H.; Fei, X.-H.; Zhang, Y.-P.; Sha, L.-Q.; Liu, Y.-T.; Zhou, W.-J.; Wu, C.-S.; Lu, Z.-Y.; Luo, K.; Gao, J.-B.; et al. Water use efficiency in a primary subtropical evergreen forest in Southwest China. Sci. Rep. 2017, 7, 43031. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Li, R.; Long, F.; Zhang, L.; Zhang, Q.; Li, J. Water-use efficiency of an old-growth forest in lower subtropical China. Sci. Rep. 2017, 7, 42761. [Google Scholar] [CrossRef]
- Marengo, J.A.; Torres, R.R.; Alves, L.M. Drought in Northeast Brazil—past, present, and future. Theor. Appl. Climatol. 2017, 129, 1189–1200. [Google Scholar] [CrossRef]
- Helman, D. Land surface phenology: What do we really ‘see’ from space? Sci. Total Environ. 2018, 618, 665–673. [Google Scholar] [CrossRef]
- Tavares-Damasceno, J.P.; de Souza Silveira, J.L.G.; Câmara, T.; de Castro Stedile, P.; Macario, P.; Toledo-Lima, G.S.; Pichorim, M. Effect of drought on demography of Pileated Finch (Coryphospingus pileatus: Thraupidae) in northeastern Brazil. J. Arid Environ. 2017, 147, 63–79. [Google Scholar] [CrossRef]
- Pagotto, M.; Roig, F.A.; Ribeiro, A.; Lisi, C. Influence of regional rainfall and Atlantic sea surface temperature on tree-ring growth of Poincianella pyramidalis, semiarid forest from Brazil. Dendrochronologia 2015, 35, 14–23. [Google Scholar] [CrossRef]
- Jensen, R.; Herbst, M.; Friborg, T. Direct and indirect controls of the interannual variability in atmospheric CO2 exchange of three contrasting ecosystems in Denmark. Agric. For. Meteorol. 2017, 269–270, 136–144. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Lasslop, G.; Reichstein, M.; Papale, D.; Richardson, A.D.; Arneth, A.; Barr, A.; Stoy, P.; Wohlfahrt, G. Separation of net ecosystem exchange into assimilation and respiration using a light response curve approach: Critical issues and global evaluation. Glob. Chang. Biol. 2009, 16, 187–208. [Google Scholar] [CrossRef]
- Didan, K. MOD13A2 MODIS/Terra Vegetation Indices 16-Day L3 Global 1km SIN Grid V006. 2015, Distributed by NASA EOSDIS Land Processes DAAC. Available online: https://lpdaac.usgs.gov/products/mod13a2v006/ (accessed on 19 October 2021). [CrossRef]
- Running, S.W.; Nemani, R.R.; Heinsch, F.A.; Zhao, M.; Reeves, M.; Hashimoto, H. A Ontinuous Satellite–derived Measure of Global Terrestrial Primary Production. Bioscience 2004, 54, 547–560. [Google Scholar] [CrossRef]
- Monteith, J.L. Solar Radiation and Productivity in Tropical Ecosystems. J. Appl. Ecol. 1972, 9, 747. [Google Scholar] [CrossRef]
- Myneni, R.; Knyazikhin, Y.; Park, T. MOD15A2H MODIS/Terra Leaf Area Index/FPAR 8-Day L4 Global 500m SIN Grid V006 [Data Set]. NASA EOSDIS Land Processes DAAC. 2015. Available online: https://lpdaac.usgs.gov/products/mod15a2hv006/ (accessed on 12 February 2022).
- Rienecker, M.M.; Suarez, M.J.; Gelaro, R.; Todling, R.; Bacmeister, J.; Liu, E.; Bosilovich, M.G.; Schubert, S.D.; Takacs, L.; Kim, G.-K.; et al. MERRA: NASA’s Modern-era retrospective analysis for research and applications. J. Clim. 2011, 24, 3624–3648. [Google Scholar] [CrossRef]
- Pei, Y.; Dong, J.; Zhang, Y.; Yang, J.; Zhang, Y.; Jiang, C.; Xiao, X. Performance of four state-of-the-art GPP products (VPM, MOD17, BESS and PML) for grasslands in drought years. Ecol. Informatics 2020, 56, 101052. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 12 February 2022).
- Daultrey, S. Principal Components Analysis. Concepts and Techniques in Modem Geography, 8; Geo Abstracts: Norwich, UK, 1976. [Google Scholar]
- Mekonnen, Z.A.; Grant, R.; Schwalm, C. Contrasting changes in gross primary productivity of different regions of North America as affected by warming in recent decades. Agric. For. Meteorol. 2016, 218, 50–64. [Google Scholar] [CrossRef]
- Biudes, M.S.; Vourlitis, G.L.; Machado, N.G.; de Arruda, P.H.Z.; Neves, G.A.R.; Lobo, F.D.A.; Neale, C.M.U.; Nogueira, J.D.S. Patterns of energy exchange for tropical ecosystems across a climate gradient in Mato Grosso, Brazil. Agric. For. Meteorol. 2015, 202, 112–124. [Google Scholar] [CrossRef]
- Da Rocha, H.R.; Manzi, A.O.; Cabral, O.M.; Miller, S.D.; Goulden, M.; Saleska, S.R.; R.-Coupe, N.; Wofsy, S.C.; Borma, L.S.; Artaxo, P.; et al. Patterns of water and heat flux across a biome gradient from tropical forest to savanna in Brazil. J. Geophys. Res. Earth Surf. 2009, 114, 8. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Q.; Hollinger, D.; Aber, J.; Moore, I.B. Modeling seasonal dynamics of gross primary production of an evergreen needleleaf forest using MODIS images and climate data. Ecol. Appl. 2004, 15, 954–969. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Menzel, W.; Schmit, T.; Ackerman, S. Comparison between current and future environmental satellite imagers on cloud classification using MODIS. Remote Sens. Environ. 2007, 108, 311–326. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.; Lin, A.; Zou, L.; Qin, W.; Du, Q. Evaluation of the Latest MODIS GPP Products across Multiple Biomes Using Global Eddy Covariance Flux Data. Remote Sens. 2017, 9, 418. [Google Scholar] [CrossRef]
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Schwinning, S.; Sala, O. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 2004, 141, 211–220. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Martínez-Yrízar, A.; Burquez, A.; Boyle, B.; Enquist, B.J. Plant functional trait variation in tropical dry forests: A review and synthesis. In Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; Sánchez-Azofeifa, G.A., Powers, J.S., Fernandes, G.W., Eds.; Taylor & Francis Group: Abingdon, UK, 2014; ISBN 978-1-4665-1200-9. [Google Scholar]
- Reich, P.; Borchert, R. Water Stress and Tree Phenology in a Tropical Dry Forest in the Lowlands of Costa Rica. J. Ecol. 1984, 72, 61. [Google Scholar] [CrossRef]
- Singh, N.; Patel, N.; Bhattacharya, B.; Soni, P.; Parida, B.R.; Parihar, J. Analyzing the dynamics and inter-linkages of carbon and water fluxes in subtropical pine (Pinus roxburghii) ecosystem. Agric. For. Meteorol. 2014, 197, 206–218. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, L.-M.; Sun, X.-M.; Fu, Y.-L.; Wen, X.-F.; Wang, Q.-F.; Li, S.-G.; Ren, C.-Y.; Song, X.; Liu, Y.-F.; et al. Environmental controls over carbon exchange of three forest ecosystems in eastern China. Glob. Chang. Biol. 2008, 14, 2555–2571. [Google Scholar] [CrossRef]
- Li, S.; Kang, S.; Zhang, L.; Du, T.; Tong, L.; Ding, R.; Guo, W.; Zhao, P.; Chen, X.; Xiao, H. Ecosystem water use efficiency for a sparse vineyard in arid northwest China. Agric. Water Manag. 2015, 148, 24–33. [Google Scholar] [CrossRef]
- Costa, G.B.; e Silva, C.M.S.; Mendes, K.R.; dos Santos, J.G.M.; Neves, T.T.A.T.; Silva, A.S.; Rodrigues, T.R.; Silva, J.B.; Dalmagro, H.J.; Mutti, P.R.; et al. WUE and CO2 Estimations by Eddy Covariance and Remote Sensing in Different Tropical Biomes. Remote Sens. 2022, 14, 3241. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chen, Y.N.; Li, W.H. Photosynthesis of Populus euphratica in relation to groundwater depths and high temperature in arid environment, northwest China. Photosynthetica 2010, 48, 257–268. [Google Scholar] [CrossRef]
- Ponton, S.; Flanagan, L.B.; Alstad, K.P.; Johnson, B.G.; Morgenstern, K.; Kljun, N.; Black, T.A.; Barr, A.G. Comparison of ecosystem water-use efficiency among Douglas-fir forest, aspen forest and grassland using eddy covariance and carbon isotope techniques. Glob. Chang. Biol. 2006, 12, 294–310. [Google Scholar] [CrossRef]
- Boulain, N.; Cappelaere, B.; Ramier, D.; Issoufou, H.B.A.; Halilou, O.; Seghieri, J.; Timouk, F. Towards an understanding of coupled physical and biological processes in the cultivated Sahel–2. Vegetation and carbon dynamics. J. Hydrol. 2009, 375, 190–203. [Google Scholar] [CrossRef]
- Cunha, A.P.M.A.; Zeri, M.; Leal, K.D.; Costa, L.; Cuartas, L.A.; Marengo, J.A.; Tomasella, J.; Vieira, R.M.; Barbosa, A.A.; Cunningham, C.; et al. Extreme Drought Events over Brazil from 2011 to 2019. Atmosphere 2019, 10, 642. [Google Scholar] [CrossRef]

| Variable | 2014 | 2015 |
|---|---|---|
| Tair (°C) | 27.8 ± 3.8 | 28.4 ± 4.1 |
| RH (%) | 54.8 ± 17.9 | 48.9 ± 17.1 |
| VPD (kPa) | 2.2 ± 1.18 | 2.6 ± 1.20 |
| ET (kg H2O m−2 day−1) | 1.2 ± 1.05 | 0.8 ± 0.93 |
| Rainfall (mm) | 513 | 466.5 |
| Rn (W m−2) | 161.0 ± 219.9 | 160.3 ± 220.2 |
| H (W m−2) | 83.9 ± 142.0 | 93.2 ± 152.2 |
| LE (W m−2) | 35.5 ± 66.7 | 25.5 ± 56.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, G.B.; Mendes, K.R.; Viana, L.B.; Almeida, G.V.; Mutti, P.R.; e Silva, C.M.S.; Bezerra, B.G.; Marques, T.V.; Ferreira, R.R.; Oliveira, C.P.; et al. Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data. Remote Sens. 2022, 14, 3955. https://doi.org/10.3390/rs14163955
Costa GB, Mendes KR, Viana LB, Almeida GV, Mutti PR, e Silva CMS, Bezerra BG, Marques TV, Ferreira RR, Oliveira CP, et al. Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data. Remote Sensing. 2022; 14(16):3955. https://doi.org/10.3390/rs14163955
Chicago/Turabian StyleCosta, Gabriel Brito, Keila Rêgo Mendes, Losany Branches Viana, Gabriele Vieira Almeida, Pedro Rodrigues Mutti, Cláudio Moisés Santos e Silva, Bergson Guedes Bezerra, Thiago Valentim Marques, Rosária Rodrigues Ferreira, Cristiano Prestelo Oliveira, and et al. 2022. "Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data" Remote Sensing 14, no. 16: 3955. https://doi.org/10.3390/rs14163955
APA StyleCosta, G. B., Mendes, K. R., Viana, L. B., Almeida, G. V., Mutti, P. R., e Silva, C. M. S., Bezerra, B. G., Marques, T. V., Ferreira, R. R., Oliveira, C. P., Gonçalves, W. A., Oliveira, P. E., Campos, S., Andrade, M. U. G., Antonino, A. C. D., & Menezes, R. S. C. (2022). Seasonal Ecosystem Productivity in a Seasonally Dry Tropical Forest (Caatinga) Using Flux Tower Measurements and Remote Sensing Data. Remote Sensing, 14(16), 3955. https://doi.org/10.3390/rs14163955









