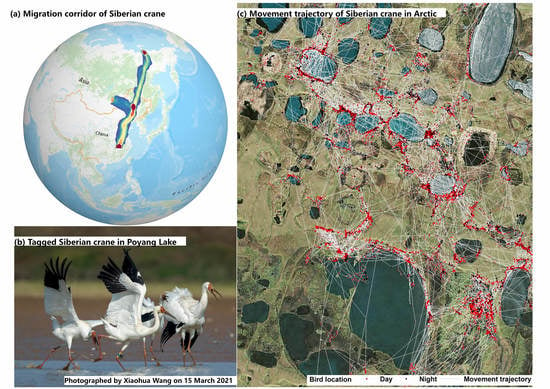
Using Tracking Data to Identify Gaps in Knowledge and Conservation of the Critically Endangered Siberian Crane (Leucogeranus leucogeranus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Tracking Cranes Marked with GPS/GSM Transmitters
2.2. Data Processing
2.3. Defining Migration Parameters
2.4. Land Use and Conservation Status of Key Sites
3. Results
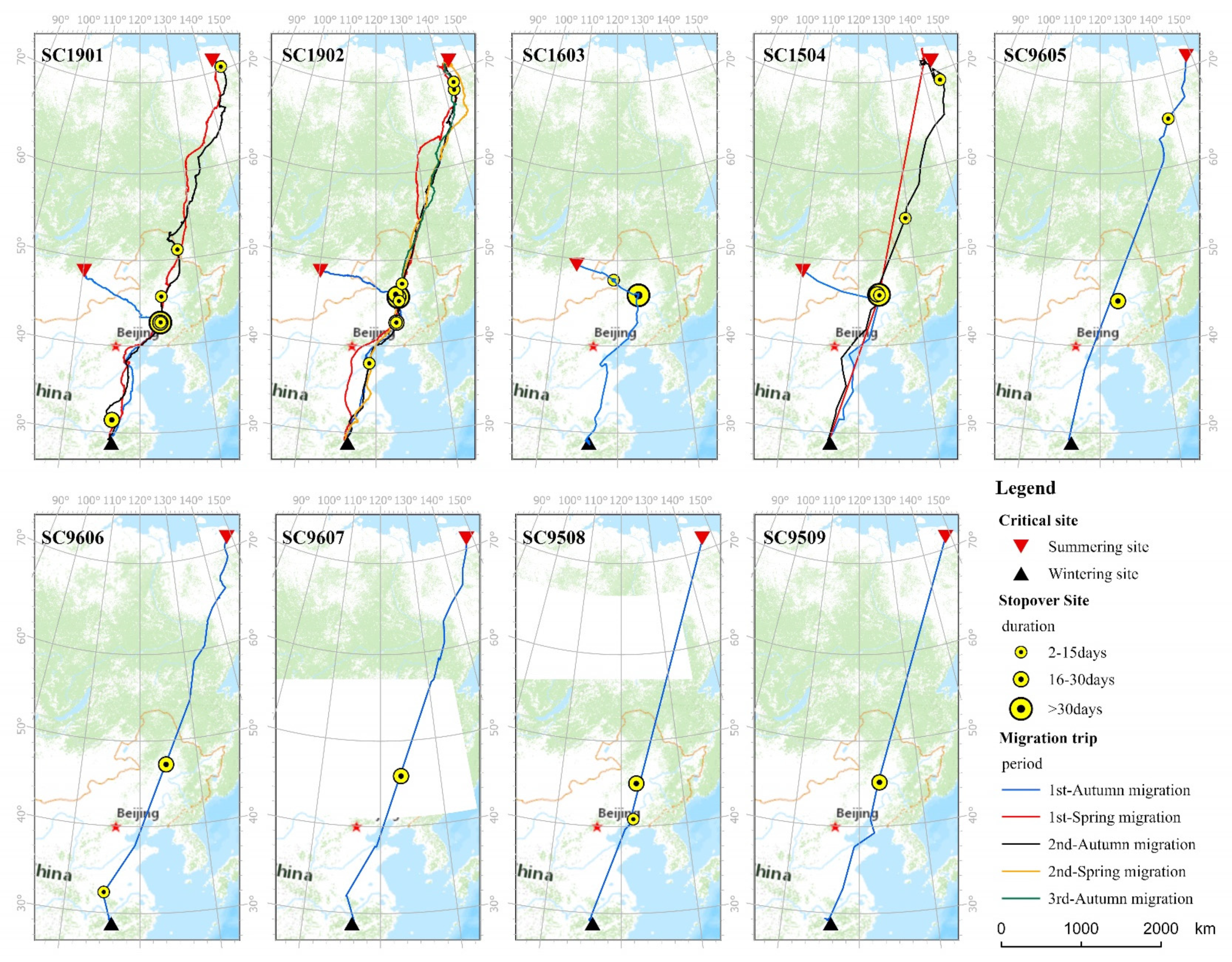
3.1. Round-Trip Time Budgets and Key Migration Parameters
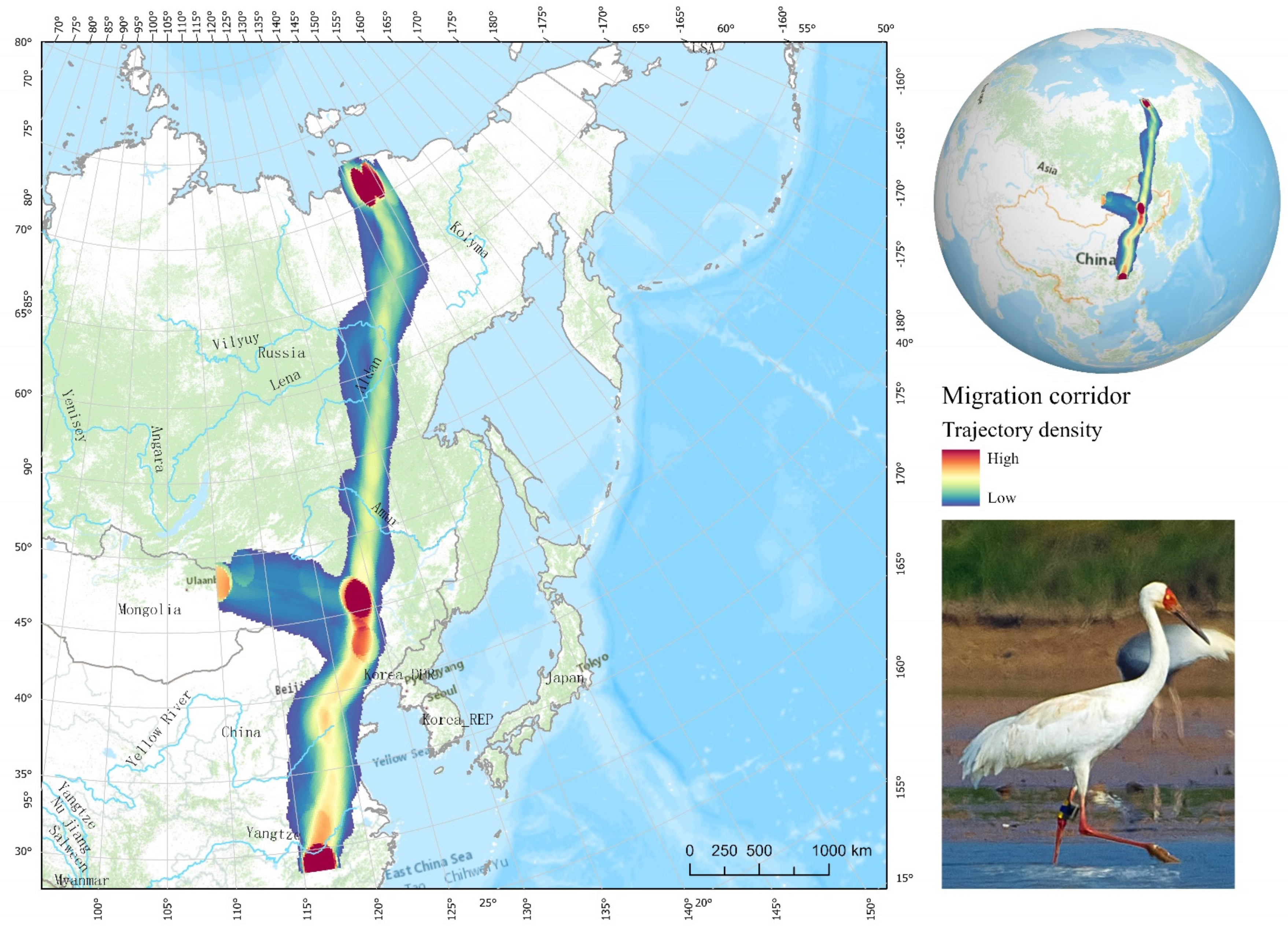
3.2. Extent of Used Areas and Habitat Types of Tagged Cranes
4. Discussion
4.1. Migration Patterns and Habitat Use
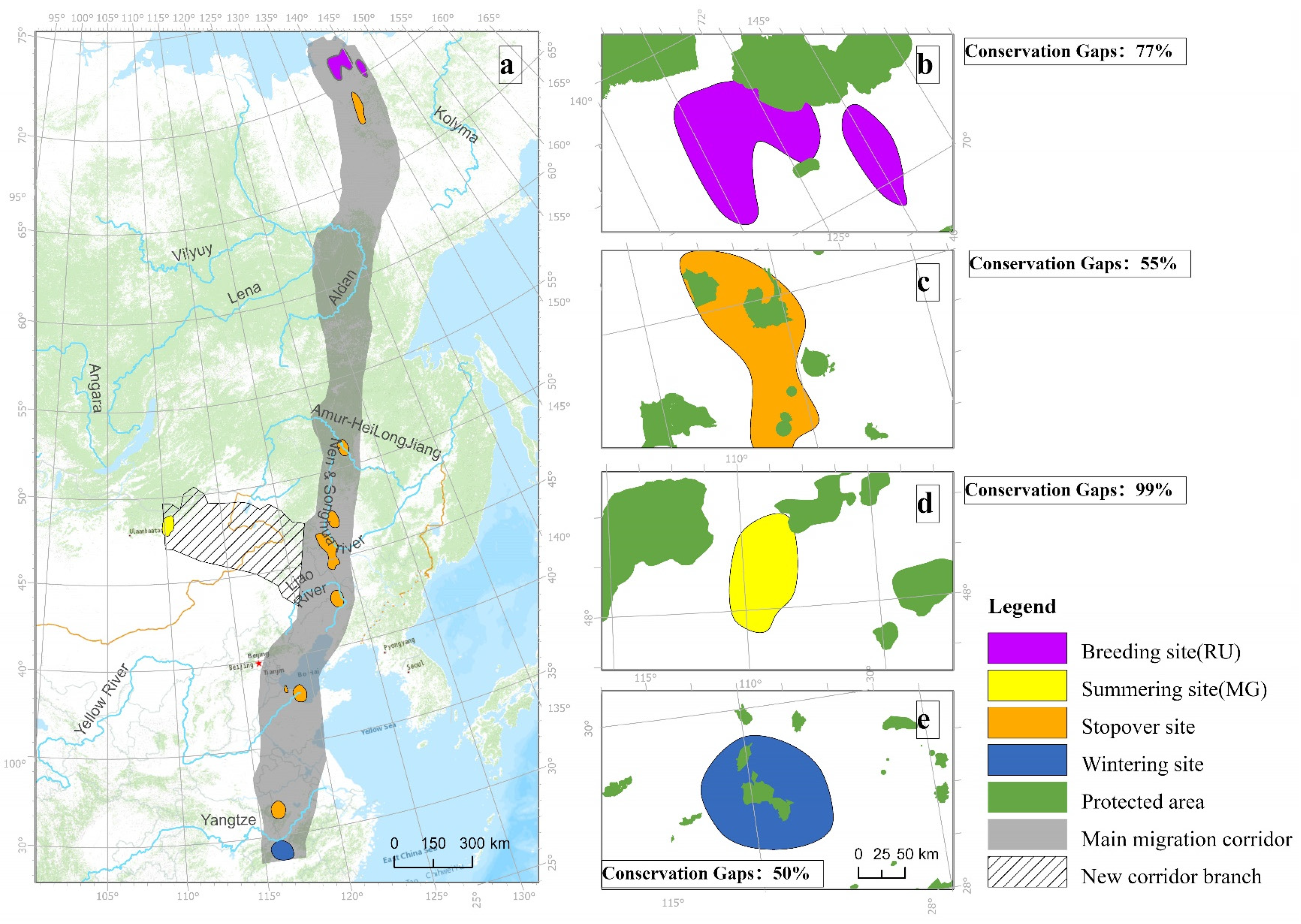
4.2. Conservation Priorities and Gaps in Large River Basins
4.3. High Fidelity and Conservation in Poyang Lake
4.4. Climate Changes and Human Activities Impact on Cranes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalby, L.; McGill, B.J.; Fox, A.D.; Svenning, J.C. Seasonality drives global-scale diversity patterns in waterfowl (A nseriformes) via temporal niche exploitation. Global. Ecol. Biogeogr. 2014, 23, 550–562. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Q.; Zhang, J.; Kolzsch, A.; Solovyeva, D.; Bysykatova-Harmey, I.; Xu, Z.; Kruckenberg, H.; Cao, L.; Fox, A.D. Contrasting habitat use and conservation status of Chinese-wintering and other Eurasian Greater White-fronted Goose (Anser albifrons) populations. Avian Res. 2021, 12, 71. [Google Scholar] [CrossRef]
- Damba, I.; Zhang, J.; Yi, K.; Dou, H.; Batbayar, N.; Natsagdorj, T.; Davaasuren, B.; Cao, L.; Fox, A.D. Seasonal and regional differences in migration patterns and conservation status of Swan Geese (Anser cygnoides) in the East Asian Flyway. Avian Res. 2021, 12, 73. [Google Scholar] [CrossRef]
- Donnelly, J.P.; King, S.L.; Knetter, J.; Gammonley, J.H.; Dreitz, V.J.; Grisham, B.A.; Nowak, M.C.; Collins, D.P. Migration efficiency sustains connectivity across agroecological networks supporting sandhill crane migration. Ecosphere 2021, 12, e03543. [Google Scholar] [CrossRef]
- Lopez-Lopez, P. Individual-Based Tracking Systems in Ornithology: Welcome to the Era of Big Data. Ardeola-Int. J. Ornithol. 2016, 63, 103–136. [Google Scholar] [CrossRef]
- Harcourt, R.; Sequeira, A.M.M.; Zhang, X.; Roquet, F.; Komatsu, K.; Heupel, M.; McMahon, C.; Whoriskey, F.; Meekan, M.; Carroll, G.; et al. Animal-Borne Telemetry: An Integral Component of the Ocean Observing Toolkit. Front. Mar. Sci. 2019, 6, 326. [Google Scholar] [CrossRef]
- Westley, P.A.H.; Berdahl, A.M.; Torney, C.J.; Biro, D. Collective movement in ecology: From emerging technologies to conservation and management. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018, 373, 20170004. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Possingham, H.P. Commentary: Linking Movement Ecology with Wildlife Management and Conservation. Front. Ecol. Evol. 2016, 4, 30. [Google Scholar] [CrossRef][Green Version]
- Bysykatova, I.P.; Krapu, G.L.; Germogenov, N.I.; Buhl, D.A. Distribution, densities, and ecology of Siberian cranes in the Khroma River region of northern Yakutia in northeastern Russia. In Proceedings of the North American Crane Workshop, Lafayette, Louisiana, 14–17 April 2014; pp. 50–64. Available online: https://digitalcommons.unl.edu/nacwgproc/381 (accessed on 18 April 2019).
- Wang, Y.; Damba, I.; Zhao, Q.; Xie, Y.; Deng, X.; Ga, R.; Liu, G.; Xu, Z.; Li, Y.; Gao, D.; et al. Organising a juvenile ratio monitoring programme for 10 key waterbird species in the Yangtze River floodplain: Analysis and proposals. Avian Res. 2021, 12, 72. [Google Scholar] [CrossRef]
- Wang, Y. Study on Population Dynamics of 10 Waterbirds in Wetlands of Middle and Lower Reaches of Yangtze River. Master’s Thesis, University of Science and Technology of China, HeFei, China, 2021. [Google Scholar]
- Kanai, Y.; Nagendran, M.; Ueta, M.; Markin, Y.; Rinne, J.; Sorokin, A.G.; Higuchi, H.; Archibald, G.W. Discovery of breeding grounds of a Siberian Crane Grus leucogeranus flock that winters in Iran, via satellite telemetry. Bird Conserv. Int. 2002, 12, 327–333. [Google Scholar] [CrossRef]
- Kanai, Y.; Ueta, M.; Germogenov, N.; Nagendran, M.; Mita, N.; Higuchi, H. Migration routes and important resting areas of Siberian cranes (Grus leucogeranus) between northeastern Siberia and China as revealed by satellite tracking. Biol. Conserv. 2002, 106, 339–346. [Google Scholar] [CrossRef]
- Xue, L.; Xu, J.; Li, X.; Wu, A.; Qian, F. The difference of migration routes and movement patterns between the rescued and the wild Siberian Crane. Acta Ecol. Sin. 2020, 40, 2367–2375. [Google Scholar]
- Li, M.; Qian, F.; Wu, A.; Pan, K.; Wu, T. Identification of Important Stopover Sites for Siberian Cranes and Changes of Their Land Use Patterns. Wetl. Sci. 2021, 19, 304–316. [Google Scholar]
- Wang, X.; Cao, L.; Bysykatova, I.; Xu, Z.; Rozenfeld, S.; Jeong, W.; Vangeluwe, D.; Zhao, Y.; Xie, T.; Yi, K.; et al. The Far East taiga forest: Unrecognized inhospitable terrain for migrating Arctic-nesting waterbirds? Peerj 2018, 6, e4353. [Google Scholar] [CrossRef]
- Koelzsch, A.; Mueskens, G.J.D.M.; Kruckenberg, H.; Glazov, P.; Weinzierl, R.; Nolet, B.A.; Wikelski, M. Towards a new understanding of migration timing: Slower spring than autumn migration in geese reflects different decision rules for stopover use and departure. Oikos 2016, 125, 1496–1507. [Google Scholar] [CrossRef]
- van Wijk, R.E.; Kolzsch, A.; Kruckenberg, H.; Ebbinge, B.S.; Muskens, G.; Nolet, B.A. Individually tracked geese follow peaks of temperature acceleration during spring migration. Oikos 2012, 121, 655–664. [Google Scholar] [CrossRef]
- Deng, X.; Zhao, Q.; Fang, L.; Xu, Z.; Wang, X.; He, H.; Cao, L.; Fox, A.D. Spring migration duration exceeds that of autumn migration in Far East Asian Greater White-fronted Geese (Anser albifrons). Avian Res. 2019, 10, 19. [Google Scholar] [CrossRef]
- Esri. Sentinel-2 10m Land Use/Land Cover Time Series. Available online: https://www.arcgis.com/home/item.html?id=d3da5dd386d140cf93fc9ecbf8da5e31 (accessed on 10 February 2022).
- Li, H.; Fang, L.; Wang, X.; Yi, K.; Cao, L.; Fox, A.D. Does snowmelt constrain spring migration progression in sympatric wintering Arctic-nesting geese? Results from a Far East Asia telemetry study. Ibis 2020, 162, 548–555. [Google Scholar] [CrossRef]
- Aharon-Rotman, Y.; McEvoy, J.; Zheng, Z.; Yu, H.; Wang, X.; Si, Y.; Xu, Z.; Yuan, Z.; Jeong, W.; Cao, L.; et al. Water level affects availability of optimal feeding habitats for threatened migratory waterbirds. Ecol. Evol. 2017, 7, 10440–10450. [Google Scholar] [CrossRef]
- Zhang, Y.; Fox, A.D.; Cao, L.; Jia, Q.; Lu, C.; Prins, H.H.T.; de Boer, W.F. Effects of ecological and anthropogenic factors on waterbird abundance at a Ramsar Site in the Yangtze River Floodplain. Ambio 2019, 48, 293–303. [Google Scholar] [CrossRef]
- Cao, L.; Meng, F.; Zhao, Q. Understanding Effects of Large-scale Development on Bird Migration and Habitats Through Cutting Edge Avian Monitoring Techniques. Bull. Chin. Acad. Sci. 2021, 36, 436–447. [Google Scholar]
- Cao, L.; Meng, F.; Zhang, J.; Deng, X.; Sawa, Y.; Fox, A.D. Moving forward: How best to use the results of waterbird monitoring and telemetry studies to safeguard the future of Far East Asian Anatidae species. Wildfowl 2020, 6, 293–319. [Google Scholar]
- Jia, Y.; Guan, L.; Wang, Y.; Liu, G.; Lei, G.; Wen, L. Combining Population Growth Model and Generalized Additive Model to Determine Optimal Water Level FOR Waterbird Conservation: A Case Study of Siberian Crane (Leucogeranus Leucogeranus) in Lake Poyang, China. River Res. Appl. 2016, 32, 100–109. [Google Scholar] [CrossRef]
- Jia, Y.F.; Jiao, S.W.; Zhang, Y.M.; Zhou, Y.; Lei, G.C.; Liu, G.H. Diet Shift and Its Impact on Foraging Behavior of Siberian Crane (Grus Leucogeranus) in Poyang Lake. PLoS ONE 2013, 8, e65843. [Google Scholar] [CrossRef]
- Bayartogtokh, B.; Munkhchuluun, B.; Jargalsaikhan, P.; Bayarsaikhan, U. Khar Yamaat Nature Reserve: Biodiversity, Ecosystem Features, Threats and Conservation Management. Mong. J. Biol. Sci. 2020, 19, 3–15. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.; Flannigan, M.D. Wildfires in the Siberian taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef]
- McCarty, J.L.; Aalto, J.; Paunu, V.-V.; Arnold, S.R.; Eckhardt, S.; Klimont, Z.; Fain, J.J.; Evangeliou, N.; Venäläinen, A.; Tchebakova, N.M.; et al. Reviews and syntheses: Arctic fire regimes and emissions in the 21st century. Biogeosciences 2021, 18, 5053–5083. [Google Scholar] [CrossRef]
- Tao, S.; Fang, J.; Zhao, X.; Zhao, S.; Shen, H.; Hu, H.; Tang, Z.; Wang, Z.; Guo, Q. Rapid loss of lakes on the Mongolian Plateau. Proc. Natl. Acad. Sci. USA 2015, 112, 2281–2286. [Google Scholar] [CrossRef]
- Jiang, H.B.; Wen, Y.; Zou, L.F.; Wang, Z.Q.; He, C.G.; Zou, C.L. The effects of a wetland restoration project on the Siberian crane (Grus leucogeranus) population and stopover habitat in Momoge National Nature Reserve, China. Ecol. Eng. 2016, 96, 170–177. [Google Scholar] [CrossRef]
- Bose, R. Siberian Cranes May Skip India Forever. Available online: https://www.thegoan.net/goa-news/siberian-cranes-may-skip-india-forever/545.html (accessed on 22 August 2012).


| Parameter | Long Migration (Mean ± SD) | U-Test on Seasonal Difference | Short Migration (Mean ± SD) | U-Test on Strategy Difference | |||
|---|---|---|---|---|---|---|---|
| Spring | Autumn | W | p Value | Autumn | W | p-Value | |
| Departure date | 6 April ± 14 | 23 September ± 7 | - | - | 14 September ± 13 | 8.5 | 0.16 |
| Arrival date | 20 May ± 2 | 14 November ± 7 | - | - | 5 November ± 10 | 8 | 0.14 |
| Migration duration (day) | 45 ± 15 | 51 ± 9 | 24 | 0.41 | 52 ± 4 | 18.5 | 1.00 |
| Migration distance (Km) | 5604 ± 362 | 5265 ± 454 | 15 | 0.71 | 3323 ± 142 | 35 | <0.01 |
| Migration speed (km/day) | 134 ± 40 | 106 ± 23 | 27.5 | 0.16 | 64 ± 5 | 4 | <0.05 |
| Number of stopovers | 2 ± 1 | 2 ± 1 | 8 | 0.15 | 1 ± 0 | 0 | <0.01 |
| Stopover duration (day) | 32 ± 14 | 27 ± 10 | 10.5 | 0.28 | 44 ± 5 | 0 | <0.01 |
| Step length (km) | 2226 ± 760 | 1854 ± 642 | 9 | 0.20 | 1525 ± 297 | 27 | 0.20 |
| Travel duration (day) | 14 ± 3 | 24 ± 14 | 12 | 0.41 | 8 ± 1 | 11 | 0.33 |
| Travel speed (km/day) | 430 ± 114 | 299 ± 176 | 22 | 0.57 | 391 ± 40 | 9 | 0.16 |
| Straightness index | 0.88 ± 0.05 | 0.92 ± 0.06 | 26.5 | 0.22 | 0.66 ± 0.02 | 0 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, K.; Zhang, J.; Batbayar, N.; Higuchi, H.; Natsagdorj, T.; Bysykatova, I.P. Using Tracking Data to Identify Gaps in Knowledge and Conservation of the Critically Endangered Siberian Crane (Leucogeranus leucogeranus). Remote Sens. 2022, 14, 5101. https://doi.org/10.3390/rs14205101
Yi K, Zhang J, Batbayar N, Higuchi H, Natsagdorj T, Bysykatova IP. Using Tracking Data to Identify Gaps in Knowledge and Conservation of the Critically Endangered Siberian Crane (Leucogeranus leucogeranus). Remote Sensing. 2022; 14(20):5101. https://doi.org/10.3390/rs14205101
Chicago/Turabian StyleYi, Kunpeng, Junjian Zhang, Nyambayar Batbayar, Hiroyoshi Higuchi, Tseveenmyadag Natsagdorj, and Inga P. Bysykatova. 2022. "Using Tracking Data to Identify Gaps in Knowledge and Conservation of the Critically Endangered Siberian Crane (Leucogeranus leucogeranus)" Remote Sensing 14, no. 20: 5101. https://doi.org/10.3390/rs14205101
APA StyleYi, K., Zhang, J., Batbayar, N., Higuchi, H., Natsagdorj, T., & Bysykatova, I. P. (2022). Using Tracking Data to Identify Gaps in Knowledge and Conservation of the Critically Endangered Siberian Crane (Leucogeranus leucogeranus). Remote Sensing, 14(20), 5101. https://doi.org/10.3390/rs14205101