Combining Citizen Science Data and Satellite Descriptors of Ecosystem Functioning to Monitor the Abundance of a Migratory Bird during the Non-Breeding Season
Abstract
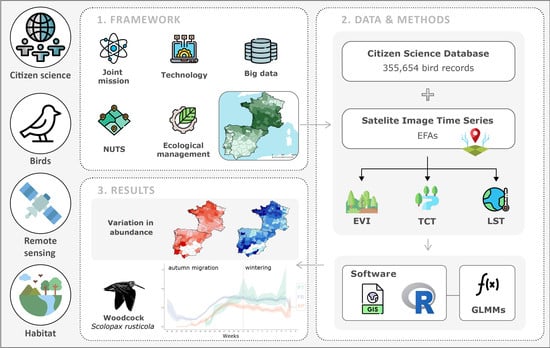
:1. Introduction
2. Materials and Methods
2.1. The Woodcock
2.2. Study Area and Hunting Data
2.3. Remote Sensed Ecosystem Functioning Variables
2.4. Variable Selection and Ranking
- 1.
- Autumnal migration, from early September (first week of the month, week 35) to mid-December (second week of the month, week 50).
- 2.
- Wintering season, from mid-December (week 51) to late February (week 9).
3. Results
3.1. Patterns of Spatiotemporal Variation in Woodcock Abundance
3.2. Predictors of Spatiotemporal Variation in Woodcock Abundance
3.2.1. Autumnal Migration
3.2.2. Wintering
4. Discussion
4.1. Ecosystem Functioning Attributes and Woodcock Abundance
4.2. Advantages, Limitations, and Future Perspectives
4.3. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Band | Red | Near-IR | Blue | Green | M-IR | M-IR | M-IR |
|---|---|---|---|---|---|---|---|
| MODIS band wavelength (nm) | 620–670 | 841–876 | 459–479 | 545–565 | 1230–1250 | 1628–1652 | 2105–2155 |
| Brightness | 0.3956 | 0.4718 | 0.3354 | 0.3834 | 0.3946 | 0.3434 | 0.2964 |
| Greenness | −0.3399 | 0.5952 | −0.2129 | −0.2222 | 0.4617 | −0.1037 | −0.4600 |
| Wetness | 0.1084 | 0.0912 | 0.5065 | 0.4040 | −0.2410 | −0.4658 | −0.5306 |
| Fourth | 0.4527 | 0.4480 | −0.3869 | −0.1277 | −0.3164 | −0.4993 | 0.2829 |
| Fifth | 0.6478 | −0.2448 | −0.3705 | 0.0068 | 0.1385 | 0.2564 | −0.5461 |
| Sixth | −0.2332 | 0.3348 | −0.2764 | 0.3516 | −0.5986 | 0.5032 | −0.1515 |
| Seventh | −0.1930 | −0.2052 | −0.4725 | 0.7049 | 0.3107 | −0.2935 | 0.1334 |
| Country | NUTS-III | Designation | N° of Journeys |
|---|---|---|---|
| Portugal | PT111 | Alto Minho | 1342 |
| Portugal | PT112 | Cávado | 63 |
| Portugal | PT119 | Ave | 39 |
| Portugal | PT11A | Área Metropolitana do Porto | 20 |
| Portugal | PT11B | Alto Tâmega | 319 |
| Portugal | PT11C | Tâmega e Sousa | 10 |
| Portugal | PT11D | Douro | 71 |
| Portugal | PT11E | Terras de Trás-os-Montes | 11 |
| Portugal | PT150 | Algarve | 10 |
| Portugal | PT16B | Oeste | 61 |
| Portugal | PT16D | Região de Aveiro | 82 |
| Portugal | PT16E | Região de Coimbra | 119 |
| Portugal | PT16F | Região de Leiria | 72 |
| Portugal | PT16G | Viseu Dão-Lafões | 32 |
| Portugal | PT16H | Beira Baixa | 17 |
| Portugal | PT16I | Médio Tejo | 201 |
| Portugal | PT16J | Beiras e Serra da Estrela | 15 |
| Portugal | PT170 | Área Metropolitana de Lisboa | 53 |
| Portugal | PT181 | Alentejo Litoral | 67 |
| Portugal | PT184 | Baixo Alentejo | 45 |
| Portugal | PT185 | Lezíria do Tejo | 51 |
| Portugal | PT186 | Alto Alentejo | 115 |
| Portugal | PT187 | Alentejo Central | 574 |
| Spain | ES111 | A Coruña | 320 |
| Spain | ES112 | Lugo | 551 |
| Spain | ES113 | Ourense | 11 |
| Spain | ES114 | Pontevedra | 430 |
| Spain | ES120 | Asturias | 1082 |
| Spain | ES130 | Cantabria | 4065 |
| Spain | ES211 | Álava/Araba | 2337 |
| Spain | ES212 | Guipúzcoa/Gipuzkoa | 814 |
| Spain | ES213 | Vizcaya/Bizkaia | 1228 |
| Spain | ES220 | Navarre | 2786 |
| Spain | ES230 | La Rioja | 23 |
| Spain | ES241 | Huesca | 392 |
| Spain | ES242 | Teruel | 173 |
| Spain | ES243 | Zaragoza | 264 |
| Spain | ES300 | Madrid | 101 |
| Spain | ES411 | Ávila | 20 |
| Spain | ES412 | Burgos | 2543 |
| Spain | ES413 | León | 983 |
| Spain | ES414 | Palencia | 859 |
| Spain | ES415 | Salamanca | 295 |
| Spain | ES416 | Segovia | 26 |
| Spain | ES417 | Soria | 428 |
| Spain | ES418 | Valladolid | 101 |
| Spain | ES419 | Zamora | 91 |
| Spain | ES423 | Cuenca | 1 |
| Spain | ES424 | Guadalajara | 65 |
| Spain | ES425 | Toledo | 21 |
| Spain | ES431 | Badajoz | 2 |
| Spain | ES432 | Cáceres | 11 |
| Spain | ES511 | Barcelona | 2782 |
| Spain | ES512 | Girona | 2099 |
| Spain | ES513 | Lleida | 289 |
| Spain | ES514 | Tarragona | 82 |
| Spain | ES521 | Alicante | 63 |
| Spain | ES522 | Castellón/Castelló | 93 |
| Spain | ES612 | Cádiz | 20 |
| Spain | ES614 | Granada | 1 |
| Spain | ES615 | Huelva | 61 |
| Spain | ES616 | Jaén | 1 |
| Spain | ES617 | Málaga | 159 |
| Spain | ES620 | Murcia | 73 |
| France | FR102 | Seine-et-Marne | 66 |
| France | FR103 | Yvelines | 23 |
| France | FR104 | Essonne | 79 |
| France | FR108 | Val-d’Oise | 10 |
| France | FRB01 | Cher | 1641 |
| France | FRB02 | Eure-et-Loir | 596 |
| France | FRB03 | Indre | 1913 |
| France | FRB04 | Indre-et-Loire | 2213 |
| France | FRB05 | Loir-et-Cher | 437 |
| France | FRB06 | Loiret | 435 |
| France | FRC11 | Côte-d’Or | 1512 |
| France | FRC12 | Nièvre | 1592 |
| France | FRC13 | Saône-et-Loire | 1608 |
| France | FRC14 | Yonne | 553 |
| France | FRC21 | Doubs | 3514 |
| France | FRC22 | Jura | 5954 |
| France | FRC23 | Haute-Saône | 3437 |
| France | FRC24 | Territoire de Belfort | 139 |
| France | FRD11 | Calvados | 700 |
| France | FRD12 | Manche | 3203 |
| France | FRD13 | Orne | 703 |
| France | FRD21 | Eure | 403 |
| France | FRD22 | Seine-Maritime | 248 |
| France | FRE11 | Nord | 90 |
| France | FRE12 | Pas-de-Calais | 1361 |
| France | FRE21 | Aisne | 350 |
| France | FRE22 | Oise | 478 |
| France | FRE23 | Somme | 377 |
| France | FRF12 | Haut-Rhin | 2 |
| France | FRF21 | Ardennes | 174 |
| France | FRF22 | Aube | 158 |
| France | FRF23 | Marne | 25 |
| France | FRF24 | Haute-Marne | 675 |
| France | FRF31 | Meurthe-et-Moselle | 4 |
| France | FRF33 | Moselle | 25 |
| France | FRF34 | Vosges | 6 |
| France | FRG01 | Loire-Atlantique | 1116 |
| France | FRG02 | Maine-et-Loire | 3148 |
| France | FRG03 | Mayenne | 745 |
| France | FRG04 | Sarthe | 1156 |
| France | FRG05 | Vendée | 2640 |
| France | FRH01 | Côtes-d’Armor | 6553 |
| France | FRH02 | Finistère | 5842 |
| France | FRH03 | Ille-et-Vilaine | 1421 |
| France | FRH04 | Morbihan | 5698 |
| France | FRI11 | Dordogne | 13,413 |
| France | FRI12 | Gironde | 15,319 |
| France | FRI13 | Landes | 19,028 |
| France | FRI14 | Lot-et-Garonne | 3640 |
| France | FRI15 | Pyrénées-Atlantiques | 11,162 |
| France | FRI21 | Corrèze | 9949 |
| France | FRI22 | Creuse | 5263 |
| France | FRI23 | Haute-Vienne | 7420 |
| France | FRI31 | Charente | 6615 |
| France | FRI32 | Charente-Maritime | 18,732 |
| France | FRI33 | Deux-Sèvres | 1687 |
| France | FRI34 | Vienne | 4161 |
| France | FRJ11 | Aude | 2166 |
| France | FRJ12 | Gard | 12,795 |
| France | FRJ13 | Hérault | 10,565 |
| France | FRJ14 | Lozère | 4667 |
| France | FRJ15 | Pyrénées-Orientales | 2030 |
| France | FRJ21 | Ariège | 3181 |
| France | FRJ22 | Aveyron | 3804 |
| France | FRJ23 | Haute-Garonne | 2385 |
| France | FRJ24 | Gers | 5468 |
| France | FRJ25 | Lot | 8634 |
| France | FRJ26 | Hautes-Pyrénées | 3786 |
| France | FRJ27 | Tarn | 4454 |
| France | FRJ28 | Tarn-et-Garonne | 5205 |
| France | FRK11 | Allier | 1424 |
| France | FRK12 | Cantal | 1923 |
| France | FRK13 | Haute-Loire | 5946 |
| France | FRK14 | Puy-de-Dôme | 5035 |
| France | FRK21 | Ain | 2024 |
| France | FRK22 | Ardèche | 6359 |
| France | FRK23 | Drôme | 13,397 |
| France | FRK24 | Isère | 8457 |
| France | FRK25 | Loire | 2115 |
| France | FRK26 | Rhône | 562 |
| France | FRK27 | Savoie | 3556 |
| France | FRK28 | Haute-Savoie | 1595 |
| France | FRL01 | Alpes-de-Haute-Provence | 6768 |
| France | FRL02 | Hautes-Alpes | 556 |
| France | FRL03 | Alpes-Maritimes | 3906 |
| France | FRL04 | Bouches-du-Rhône | 4159 |
| France | FRL05 | Var | 7229 |
| France | FRL06 | Vaucluse | 2956 |
| Statistical Parameter | Index Name | Code |
|---|---|---|
| Minimum | EVI | evi_min |
| TCTwet | wetness_min | |
| LST | lst_min | |
| Average | EVI | evi_avg * |
| TCTwet | wetness_avg * | |
| LST | lst_avg * | |
| Maximum | EVI | evi_max * |
| TCTwet | wetness_max | |
| LST | lst_max * | |
| Range | EVI | evi_range * |
| TCTwet | wetness_range | |
| LST | lst_range * |



References
- Bairlein, F. Migratory birds under threat. Science 2016, 354, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.-C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef] [PubMed]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49). In Monographs in Population Biology; Princeton University Press: Princeton, NJ, USA, 2011; Volume 49. [Google Scholar] [CrossRef]
- Franklin, J. Species distribution models in conservation biogeography: Developments and challenges. Divers. Distrib. 2013, 19, 1217–1223. [Google Scholar] [CrossRef]
- Pettorelli, N.; Laurance, W.F.; O’Brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite remote sensing for applied ecologists: Opportunities and challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- Sousa-Silva, R.; Alves, P.; Honrado, J.; Lomba, A. Improving the assessment and reporting on rare and endangered species through species distribution models. Glob. Ecol. Conserv. 2014, 2, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Kaliontzopoulou, A.; Brito, J.C.; Carretero, M.A.; Larbes, S.; Harris, D.J. Modelling the partially unknown distribution of wall lizards (Podarcis) in North Africa: Ecological affinities, potential areas of occurrence, and methodological constraints. Can. J. Zool. 2008, 86, 992–1001. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Gonçalves, J.; Alves, P.; Alcaraz-Segura, D.; Honrado, J.P. Assessing the multi-scale predictive ability of ecosystem functional attributes for species distribution modelling. PLoS ONE 2018, 13, e0199292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, K.S.; Bradley, B.A.; Cord, A.F.; Rocchini, D.; Tuanmu, M.-N.; Schmidtlein, S.; Turner, W.; Wegmann, M.; Pettorelli, N. Will remote sensing shape the next generation of species distribution models? Remote Sens. Ecol. Conserv. 2015, 1, 4–18. [Google Scholar] [CrossRef] [Green Version]
- Joseph, L.N.; Field, S.A.; Wilcox, C.; Possingham, H.P. Presence–absence versus abundance data for monitoring threatened species. Conserv. Biol. 2006, 20, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Kissling, W.D.; Ahumada, J.A.; Bowser, A.; Fernandez, M.; Fernández, N.; García, E.A.; Guralnick, R.P.; Isaac, N.J.B.; Kelling, S.; Los, W.; et al. Building essential biodiversity variables (EBV s) of species distribution and abundance at a global scale. Biol. Rev. 2018, 93, 600–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, C.; Stephens, P.A.; Pearce-Higgins, J.W.; Gregory, R.D.; Willis, S.G. Improving species distribution models: The value of data on abundance. Methods Ecol. Evol. 2014, 5, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Arenas-Castro, S.; Regos, A.; Gonçalves, J.F.; Alcaraz-Segura, D.; Honrado, J. Remotely sensed variables of ecosystem functioning support robust predictions of abundance patterns for rare species. Remote Sens. 2019, 11, 2086. [Google Scholar] [CrossRef] [Green Version]
- McPherson, J.M.; Jetz, W.; Rogers, D.J. Using coarse-grained occurrence data to predict species distributions at finer spatial resolutions—possibilities and limitations. Ecol. Model. 2006, 192, 499–522. [Google Scholar] [CrossRef]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Cabello, J.; Fernández, N.; Alcaraz-Segura, D.; Oyonarte, C.; Pineiro, G.; Altesor, A.; Delibes, M.; Paruelo, J.M. The ecosystem functioning dimension in conservation: Insights from remote sensing. Biodivers. Conserv. 2012, 21, 3287–3305. [Google Scholar] [CrossRef] [Green Version]
- Leitão, P.J.; Santos, M.J. Improving models of species ecological niches: A remote sensing overview. Front. Ecol. Evol. 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Alcaraz-Segura, D.; Lomba, A.; Sousa-Silva, R.; Nieto-Lugilde, D.; Alves, P.; Georges, D.; Vicente, J.R.; Honrado, J.P. Potential of satellite-derived ecosystem functional attributes to anticipate species range shifts. Int. J. Appl. Earth Obs. 2017, 57, 86–92. [Google Scholar] [CrossRef]
- Regos, A.; Gómez-Rodríguez, P.; Arenas-Castro, S.; Tapia, L.; Vidal, M.; Domínguez, J. Model-Assisted Bird Monitoring Based on Remotely Sensed Ecosystem Functioning and Atlas Data. Remote Sens. 2020, 12, 2549. [Google Scholar] [CrossRef]
- Wiegand, T.; Naves, J.; Garbulsky, M.F.; Fernández, N. Animal habitat quality and ecosystem functioning: Exploring seasonal patterns using NDVI. Ecol. Monogr. 2008, 78, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Cramp, S.; Simmons, K.E.L. Handbook of the Birds of Europe, the Middle East and North Africa: The Birds of the Western Palearctic; Oxford University Press: Oxford, UK, 1983; Volume 3: Waders to Gulls, ISBN 978-0-19-857506-1. [Google Scholar]
- Piersma, T.; van Gils, J.; Wiersma, P. Family Scolopacidae (sandpipers, snipes and phalaropes). In Handbook of the Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Eds.; Lynx Edicions: Barcelona, Spain, 1996; Volume 3: Hoatzin to Auks, pp. 444–533. [Google Scholar]
- Tavecchia, G.; Pradel, R.; Gossmann, F.; Bastat, C.; Ferrand, Y.; Lebreton, J.D. Temporal variation in annual survival probability of the Eurasian woodcock Scolopax rusticola wintering in France. Wildl. Biol. 2002, 8, 21–30. [Google Scholar] [CrossRef]
- Guzmán, J.L.; Ferrand, Y.; Arroyo, B. Origin and migration of woodcock Scolopax rusticola wintering in Spain. Eur. J. Wildl. Res. 2011, 57, 647–655. [Google Scholar] [CrossRef]
- Le Rest, K.; Hoodless, A.; Heward, C.; Cazenave, J.L.; Ferrand, Y. Effect of weather conditions on the spring migration of Eurasian Woodcock and consequences for breeding. Ibis 2019, 161, 559–572. [Google Scholar] [CrossRef]
- Braña, F.; Prieto, L.; González-Quirós, P. Habitat change and timing of dusk flight in the Eurasian woodcock: A trade-off between feeding and predator avoidance? Ann. Zool. Fenn. 2010, 47, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Duriez, O.; Ferrand, Y.; Binet, F.; Corda, E.; Gossmann, F.; Fritz, H. Habitat selection of the Eurasian woodcock in winter in relation to earthworms availability. Biol. Conserv. 2005, 122, 479–490. [Google Scholar] [CrossRef]
- Birtsas, P.; Sokos, C.; Papaspyropoulos, K.G.; Batselas, T.; Valiakos, G.; Billinis, C. Abiotic factors and autumn migration phenology of Woodcock (Scolopax rusticola Linnaeus, 1758, Charadriiformes: Scolopacidae) in a Mediterranean area. Ital. J. Zool. 2013, 80, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Duriez, O. Individual Wintering Strategies in the Eurasian Woodcock Scolopax rusticola: Energetic Trade-Offs for Habitat Selection. Ph.D. Thesis, Université de Paris VI, Paris, France, 2003. [Google Scholar]
- Hoodless, A.N.; Lang, D.; Aebischer, N.J.; Fuller, R.J.; Ewald, J.A. Densities and population estimates of breeding Eurasian Woodcock Scolopax rusticola in Britain in 2003. Bird Study 2009, 56, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Heward, C.J.; Hoodless, A.N.; Conway, G.J.; Aebischer, N.J.; Gillings, S.; Fuller, R.J. Current status and recent trend of the Eurasian Woodcock Scolopax rusticola as a breeding bird in Britain. Bird Study 2015, 62, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Granval, P. Régime alimentaire diurne de la Bécasse des bois (Scolopax rusticola) en hivernage: Approche quantitative. Gibier Faune Sauvag. 1987, 4, 125–147. [Google Scholar]
- Péron, G.; Ferrand, Y.; Gossmann, F.; Bastat, C.; Guénézan, M.; Gimenez, O. Escape migration decisions in Eurasian Woodcocks: Insights from survival analyses using large-scale recovery data. Behav. Ecol. Sociobiol. 2011, 65, 1949–1955. [Google Scholar] [CrossRef]
- Ferrand, Y.; Gossmann, F. Elements for a woodcock (Scolopax rusticola) management plan. Game Wildl. Sci. 2001, 18, 115–139. [Google Scholar]
- Runge, C.A.; Watson, J.E.; Butchart, S.H.; Hanson, J.O.; Possingham, H.P.; Fuller, R.A. Protected areas and global conservation of migratory birds. Science 2015, 350, 1255–1258. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, W.J. Sustainable exploitation: A review of principles and methods. Wildl. Biol. 2001, 7, 131–140. [Google Scholar] [CrossRef]
- Van Gils, J.; Wiersma, P.; Kirwan, G.M. Eurasian Woodcock (Scolopax rusticola), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA; Available online: https://birdsoftheworld.org/bow/species/eurwoo/cur/introduction (accessed on 15 December 2019). [CrossRef]
- del Moral, J.C. Atlas de las Aves Reproductoras de España; Dirección General de Conservación de la Naturaleza SEO/Birdlife: Madrid, Spain, 2003; pp. 258–259. [Google Scholar]
- Hobson, K.A.; Van Wilgenburg, S.L.; Ferrand, Y.; Gossman, F.; Bastat, C. A stable isotope (δ 2 H) approach to deriving origins of harvested woodcock (Scolopax rusticola) taken in France. Eur. J. Wildl. Res. 2013, 59, 881–892. [Google Scholar] [CrossRef]
- BirdLife International and Handbook of the Birds of the World, Bird Species Distribution Maps of the World, Version 2019.1. Available online: http://datazone.birdlife.org/species/requestdis (accessed on 10 December 2019).
- European Commission, E.E. Nomenclature of Territorial Units for Statistics (2016)—Statistical Units. Data Set, E.E. European Commission, GISCO. 2018. Available online: http://data.europa.eu/88u/dataset/ESTAT-NUTS-classification (accessed on 10 January 2020).
- Clausager, I. Skovsneppen (Scolopax rusticola) som ynglefugl I Danmark. Dan. Vildtundersogelser 1972, 19, 1–39. [Google Scholar]
- Schally, G.; Katona, K.; Bleier, N.; Szemethy, L. Habitat selection of Eurasian woodcock Scolopax rusticola during the spring migration period in Hungary. In Modern Aspects of Sustainable Management of Game Population, Proceedings of the 2nd International Symposium on Hunting, Novi Sad, Serbia, 17–20 October 2013; Faculty of AgricultureUniversity of Novi Sad: Novi Sad, Serbia, 2013. [Google Scholar]
- Hirons, G.; Johnson, T. A quantitative analysis of habitat preferences of Woodcock Scolopax rusticola in the breeding season. Ibis 1987, 129, 371–381. [Google Scholar] [CrossRef]
- Hoodless, A.N.; Hirons, G.J. Habitat selection and foraging behaviour of breeding Eurasian Woodcock Scolopax rusticola: A comparison between contrasting landscapes. Ibis 2007, 149, 234–249. [Google Scholar] [CrossRef]
- Gossmann, F.; Ferrand, Y.; Loidon, Y.; Sardet, G. Méthodes et résultats de baguages des bécasses des bois (Scolopax rusticola) en Bretagne. In Proceedings of the Third European Woodcock and Snipe Workshop, Paris, France, 14–16 October 1988; pp. 34–41. [Google Scholar]
- Hirons, G.; Bickford-Smith, P. The diet and behaviour of Eurasian woodcock wintering in Cornwall. In Second European Woodcock and Snipe Workshop; International Waterfowl Research Bureau: Fordingbridge, UK, 1983; pp. 11–17. [Google Scholar]
- Nagy, S.; Flink, S.; Langendoen, T. Report on the conservation status of migratory waterbirds in the agreement area. In Proceedings of the 6th Meeting of the Parties under the Agreement on the Conservation of African-Eurasian Migratory Waterbirds, Bonn, Germany, 9–14 November 2015. [Google Scholar]
- Wetlands International. Waterbird Population Estimates. Available online: http://wpe.wetlands.org/ (accessed on 20 March 2020).
- Gonçalves, D.A.R.; Rodrigues, T.M.; Pennacchini, P.; Lepetit, J.-P.; Taaffe, L.; Tuti, M.; Meunier, B.; Campana, J.-P.; Gregori, G.; Pellegrini, A.; et al. Survey of Wintering Eurasian Woodcock in Western Europe. In Proceedings of the Eleventh American Woodcock Symposium, Roscommon, MI, USA, 24–27 October 2017. [Google Scholar] [CrossRef] [Green Version]
- Ferrand, Y. A census method for roding Eurasian Woodcock in France. Biol. Rep. 1993, 16, 19–25. [Google Scholar]
- Fokin, S.; Blokhin, Y.; Zverev, P.; Kozlova, M.; Romanov, Y. Spring migration of the Woodcock, Scolopax rusticola, and roding in Russia in 2004. Woodcock Snipe Spec. Group Newsl. 2004, 30, 4–8. [Google Scholar]
- Ferrand, Y.; Aubry, P.; Landry, P.; Priol, P. Responses of Eurasian woodcock Scolopax rusticola to simulated hunting disturbance. Wildl. Biol. 2013, 19, 19–29. [Google Scholar] [CrossRef]
- Guzmán, J.L.; Caro, J.; Arroyo, B. Factors influencing mobility and survival of Eurasian Woodcock wintering in Spain. Avian Conserv. Ecol. 2017, 12, 21. [Google Scholar] [CrossRef]
- BirdLife International. Species Factsheet: Scolopax rusticola. Available online: http://datazone.birdlife.org/species/factsheet/eurasian-woodcock-scolopax-rusticola (accessed on 12 June 2020).
- Ferrand, Y.; Gossmann, F.; Bastat, C.; Guénézan, M. What census method for migrating and wintering Woodcock populations? In Sixth European Woodcock and Snipe Workshop, Proceedings of the an International Symposium of the Wetlands International Woodcock and Snipe Specialist Group, Nantes, France, 25–27 November 2003; International Wader Studies: Wageningen, The Netherlands, 2006; pp. 37–43. [Google Scholar]
- Ferrand, Y.; Gossmann, F.; Bastat, C.; Guénézan, M. Monitoring of the wintering and breeding Woodcock populations in France. Rev. Catalana d’Ornitologia 2008, 24, 44–52. [Google Scholar]
- Ferrand, Y.; Aubry, P.; Gossmann, F.; Bastat, C.; Guénézan, M. Monitoring of the European woodcock populations, with special reference to France. In Proceedings of the Tenth American Woodcock Symposium, Roscommon, MI, USA, 3–6 October 2006; pp. 37–44. [Google Scholar]
- Vermote, E. MOD09A1 MODIS/Terra Surface Reflectance 8-Day L3 Global 500m SIN Grid V006. Data Set, NASA EOSDIS Land Processes DAAC. 2015. Available online: https://lpdaac.usgs.gov/products/mod09a1v006/ (accessed on 15 January 2020). [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A2 MODIS/Terra Land Surface Temperature/Emissivity 8-Day L3 Global 1km SIN Grid V006. Data Set, NASA EOSDIS Land Processes DAAC. 2015. Available online: https://lpdaac.usgs.gov/products/mod11a2v006/ (accessed on 15 January 2020). [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Zhang, X.; Schaaf, C.B.; Friedl, M.A.; Strahler, A.H.; Gao, F.; Hodges, J.C. MODIS tasseled cap transformation and its utility. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; Volume 2, pp. 1063–1065. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R. Dplyr: A Grammar of Data Manipulation. R Package, Version 0.4.3. 2015. Available online: http://CRAN.R-project.org/package=dplyr (accessed on 10 September 2021).
- Wickham, H. Tidyr: Easily Tidy Data with ‘Spread()’ and ‘Gather()’ Functions. R Package, Version 0.4.1. 2016. Available online: http://CRAN.R-project.org/package=tidyr (accessed on 10 September 2021).
- Gonçalves, D.; Rodrigues, T.M. FANBPO Annual Report on Woodcock (FAROW)—2015–2016 Hunting Season. Federation of Western Palearctic National Woodcock Hunters Associations (FANBPO). 2017. Available online: https://www.fanbpo.org/uploaded/2014-2015-farow-report-v2.pdf (accessed on 20 June 2020).
- Gelman, A.; SU, Y.-S. Arm: Data Analysis Using Regression and Multilevel/Hierarchical Models. R Package, Version 1.8-6. 2015. Available online: https://cran.r-project.org/package=arm (accessed on 16 September 2021).
- Naimi, B. usdm: Uncertainty Analysis for Species Distribution Models. R Package, Version 1.1–18. 2017. Available online: https://cran.r-project.org/package=usdm (accessed on 10 September 2021).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Lawson, C.R.; Hodgson, J.A.; Wilson, R.J.; Richards, S.A. Prevalence, thresholds and the performance of presence–absence models. Methods Ecol. Evol. 2014, 5, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Hoaglin, D.C.; Iglewicz, B.; Tukey, J.W. Performance of some resistant rules for outlier labeling. J. Am. Stat. Assoc. 1986, 81, 991–999. [Google Scholar] [CrossRef]
- Barbato, G.; Barini, E.M.; Genta, G.; Levi, R. Features and performance of some outlier detection methods. J. Appl. Stat. 2011, 38, 2133–2149. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparisonand Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Barton, K.; Barton, M.K. MuMIn: Multi-Model Inference. R Package, Version 1.15.1. 2015. Available online: http://r-forge.r-project.org/projects/mumin/ (accessed on 10 September 2021).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95364-7. [Google Scholar]
- Šidák, Z. Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 1967, 62, 626–633. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Wickham, M.H. Package ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. R package, Version 2.1. 2016. Available online: https://ggplot2.tidyverse.org/ (accessed on 10 September 2021).
- Aphalo, P.J. Ggpmisc: Miscellaneous Extensions to ‘Ggplot2’. R Package, Version 0.4.3. 2021. Available online: https://cran.r-project.org/package=ggpmisc (accessed on 15 October 2021).
- Wilke, C.O.; Wilke, M.C.O. Cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2. R Package, Version 1.1.0. 2019. Available online: https://cran.r-project.org/package=cowplot (accessed on 15 October 2021).
- North, M.A. A method for implementing a statistically significant number of data classes in the Jenks algorithm. In Proceedings of the 2009 Sixth International Conference on Fuzzy Systems and Knowledge Discovery, Tianjin, China, 14–16 August 2009; IEEE: Piscataway, NJ, USA, 2009; Volume 1, pp. 35–38. [Google Scholar] [CrossRef]
- Thorup, K.; Tøttrup, A.P.; Willemoes, M.; Klaassen, R.H.; Strandberg, R.; Vega, M.L.; Dasari, H.P.; Araújo, M.B.; Wikelski, M.; Rahbek, C. Resource tracking within and across continents in long-distance bird migrants. Sci. Adv. 2017, 3, e1601360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albright, T.P.; Pidgeon, A.M.; Rittenhouse, C.D.; Clayton, M.K.; Flather, C.H.; Culbert, P.D.; Radeloff, V.C. Heat waves measured with MODIS land surface temperature data predict changes in avian community structure. Remote Sens. Environ. 2011, 115, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Regos, A.; Vidal, M.; Lorenzo, M.; Domínguez, J. Integrating intraseasonal grassland dynamics in cross-scale distribution modeling to support waterbird recovery plans. Conserv. Biol. 2020, 34, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Rounsevell, M.D.A.; Ewert, F.; Reginster, I.; Leemans, R.; Carter, T.R. Future scenarios of European agricultural land use: II. Projecting changes in cropland and grassland. Agric. Ecosyst. Environ. 2005, 107, 117–135. [Google Scholar] [CrossRef]
- Fuller, R.J.; Smith, K.W.; Grice, P.V.; Currie, F.A.; Quine, C.P. Habitat change and woodland birds in Britain: Implications for management and future research. Ibis 2007, 149, 261–268. [Google Scholar] [CrossRef]
- Knudsen, E.; Lindén, A.; Both, C.; Jonzén, N.; Pulido, F.; Saino, N.; Sutherland, W.J.; Bach, L.A.; Coppack, T.; Ergon, T.; et al. Challenging claims in the study of migratory birds and climate change. Biol. Rev. 2011, 86, 928–946. [Google Scholar] [CrossRef] [Green Version]
- Saino, N.; Ambrosini, R.; Rubolini, D.; von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O.; Lehikoinen, A.; Lehikoinen, E.; Rainio, K.; et al. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B 2011, 278, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Barbet-Massin, M.; Thuiller, W.; Jiguet, F. The fate of European breeding birds under climate, land-use and dispersal scenarios. Glob. Chang. Biol. 2012, 18, 881–890. [Google Scholar] [CrossRef]
- Gonçalves, J.; Alves, P.; Pôças, I.; Marcos, B.; Sousa-Silva, R.; Lomba, Â.; Honrado, J.P. Exploring the spatiotemporal dynamics of habitat suitability to improve conservation management of a vulnerable plant species. Biodivers. Conserv. 2016, 25, 2867–2888. [Google Scholar] [CrossRef]
- Regos, A.; Gagne, L.; Alcaraz-Segura, D.; Honrado, J.P.; Domínguez, J. Effects of species traits and environmental predictors on performance and transferability of ecological niche models. Sci. Rep. 2019, 9, 4221. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, J.L.; Arroyo, B. Predicting winter abundance of woodcock Scolopax rusticola using weather data: Implications for hunting management. Eur. J. Wildl. Res. 2015, 61, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Middleton, A. Periodic fluctuations in British game populations. J. Anim. Ecol. 1934, 3, 231–249. [Google Scholar] [CrossRef]
- Cattadori, I.M.; Haydon, D.T.; Thirgood, S.J.; Hudson, P.J. Are indirect measures of abundance a useful index of population density? The case of red grouse harvesting. Oikos 2003, 100, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Antolí, A.B.; Martínez-Pérez, J.E.; Peiro, V.; Román, E.S.; Arques, J. Main landscape metrics affecting abundance and diversity of game species in a semi-arid agroecosystem in the Mediterranean region. Span. J. Agric. Res. 2011, 9, 1197–1212. [Google Scholar] [CrossRef] [Green Version]
- Mahdianpari, M.; Salehi, B.; Mohammadimanesh, F.; Homayouni, S.; Gill, E. The first wetland inventory map of Newfoundland at a spatial resolution of 10 m using sentinel-1 and sentinel-2 data on the google earth engine cloud computing platform. Remote Sens. 2019, 11, 43. [Google Scholar] [CrossRef] [Green Version]




| Components of Ecosystem Functioning | Ecosystem Functioning Attributes—EFAs | MODIS Product(s)/Pixel Size and Time Frequency | Zonal Statistical Parameters (NUTS-III) |
|---|---|---|---|
| Carbon cycle (primary productivity) | EVI—Enhanced vegetation index | MOD09A1 (v006)—Surface reflectance 500 m (8-day composite) | Minimum (min.) Average (avg.) Maximum (max.) Range (range) |
| Water cycle (water in soil/vegetation) | TCTwet—Tasseled cap transformation—water component | ||
| Energy balance | LST—Land surface temperature | MOD11A2 (v006)—Surface temperature and emissivity 1000 m (8-day composite) |
| Variable | Autumn Migration | Wintering | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Error | Z Value | Probability (>|z|) | Number of Models Contained | Estimate | Error | Z Value | Probability (>|z|) | Number of Models Contained | |
| evi_range | 0.072 | 0.008 | 9.541 | <0.001 | 2 | 0.069 | 0.009 | 7.723 | <0.001 | 4 |
| evi_max | −0.104 | 0.010 | −10.512 | <0.001 | 2 | −0.107 | 0.010 | −10.774 | <0.001 | 4 |
| evi_avg | 0.073 | 0.008 | 8.982 | <0.001 | 2 | 0.128 | 0.011 | 11.692 | <0.001 | 4 |
| lst_range | −0.010 | 0.008 | −1.138 | 0.255 | 1 | 0.014 | 0.001 | 1.350 | 0.177 | 2 |
| lst_max | 0.044 | 0.009 | 4.659 | <0.001 | 2 | 0.056 | 0.011 | 5.130 | <0.001 | 4 |
| lst_avg | −0.209 | 0.015 | −13.766 | <0.001 | 2 | −0.095 | 0.018 | −5.277 | <0.001 | 4 |
| wetness_avg | −0.080 | 0.010 | −8.220 | <0.001 | 2 | −0.021 | 0.010 | −2.129 | 0.0333 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, F.S.; Regos, A.; Gonçalves, J.F.; Rodrigues, T.M.; Verde, A.; Pagès, M.; Pérez, J.A.; Meunier, B.; Lepetit, J.-P.; Honrado, J.P.; et al. Combining Citizen Science Data and Satellite Descriptors of Ecosystem Functioning to Monitor the Abundance of a Migratory Bird during the Non-Breeding Season. Remote Sens. 2022, 14, 463. https://doi.org/10.3390/rs14030463
Moreira FS, Regos A, Gonçalves JF, Rodrigues TM, Verde A, Pagès M, Pérez JA, Meunier B, Lepetit J-P, Honrado JP, et al. Combining Citizen Science Data and Satellite Descriptors of Ecosystem Functioning to Monitor the Abundance of a Migratory Bird during the Non-Breeding Season. Remote Sensing. 2022; 14(3):463. https://doi.org/10.3390/rs14030463
Chicago/Turabian StyleMoreira, Francisco S., Adrián Regos, João F. Gonçalves, Tiago M. Rodrigues, André Verde, Marc Pagès, José A. Pérez, Bruno Meunier, Jean-Pierre Lepetit, João P. Honrado, and et al. 2022. "Combining Citizen Science Data and Satellite Descriptors of Ecosystem Functioning to Monitor the Abundance of a Migratory Bird during the Non-Breeding Season" Remote Sensing 14, no. 3: 463. https://doi.org/10.3390/rs14030463
APA StyleMoreira, F. S., Regos, A., Gonçalves, J. F., Rodrigues, T. M., Verde, A., Pagès, M., Pérez, J. A., Meunier, B., Lepetit, J. -P., Honrado, J. P., & Gonçalves, D. (2022). Combining Citizen Science Data and Satellite Descriptors of Ecosystem Functioning to Monitor the Abundance of a Migratory Bird during the Non-Breeding Season. Remote Sensing, 14(3), 463. https://doi.org/10.3390/rs14030463