OC4-SO: A New Chlorophyll-a Algorithm for the Western Antarctic Peninsula Using Multi-Sensor Satellite Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Satellite Chl-a: OC-CCI
2.2. Satellite Chl-a: Regional Algorithms
2.3. In-Situ Chl-a Dataset
2.4. Match-Up Identification and Comparison of Satellite Algorithms
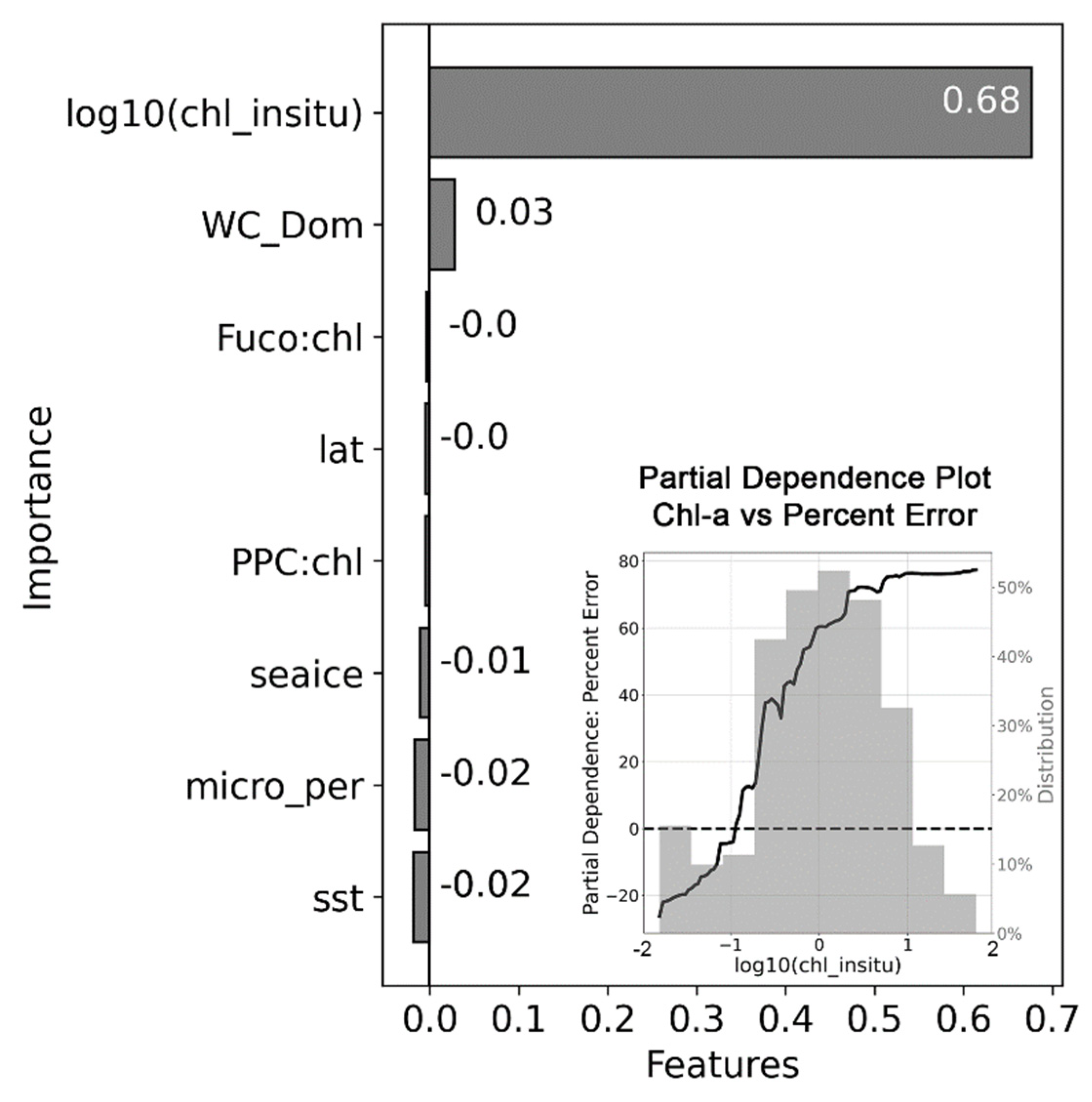
2.5. Evaluating Potential Drivers of Chl-a Underestimation
2.5.1. Pigment Packaging
2.5.2. Adjacent Sea Ice
2.5.3. Random Forest Model
3. Results
3.1. Identification of Match-Ups
3.2. Satellite Chl-a Performance
3.3. Evaluating the Causes of Chl-a Underestimation
3.4. OC4-SO: Design and Implementation
3.5. Testing of the OC4-SO Algorithm
4. Discussion
4.1. OC4-SO Corrects Previous Satellite-Underestimated Retrievals of Chl-a
4.2. Understanding the Causes of Chl-a Underestimation
4.3. The Role of Satellite Ocean Colour in the Southern Ocean
5. Final Considerations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gruber, N.; Gloor, M.; Fletcher, S.E.M.; Doney, S.C.; Dutkiewicz, S.; Follows, M.J.; Gerber, M.; Jacobson, A.R.; Joos, F.; Lindsay, K.; et al. Oceanic sources, sinks, and transport of atmospheric CO2. Glob. Biogeochem. Cycles 2009, 23, GB1005. [Google Scholar] [CrossRef] [Green Version]
- Frölicher, T.L.; Sarmiento, J.L.; Paynter, D.J.; Dunne, J.P.; Krasting, J.P.; Winton, M. Dominance of the Southern Ocean in anthropogenic carbon and heat uptake in CMIP5 models. J. Clim. 2015, 28, 862–886. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Cambridge University Press: Cambridge, UK, 2019; 755p, in press. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Pope, A.; Wagner, P.; Johnson, R.; Shutler, J.D.; Baeseman, J.; Newman, L. Community review of Southern Ocean satellite needs. Antarct. Sci. 2017, 29, 97–138. [Google Scholar] [CrossRef] [Green Version]
- Szeto, M.; Werdell, P.J.; Moore, T.S.; Campbell, J.W. Are the world’s oceans optically different? J. Geophys. Res. Ocean. 2011, 116. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.M.; Huot, Y.; Schuback, N.; Ryan-Keogh, T.J.; Thomalla, S.J.; Antoine, D. High latitude Southern Ocean phytoplankton have distinctive bio-optical properties. Opt. Express 2021, 29, 21084–21112. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Smith, R.C. Bio-optical properties and remote sensing ocean color algorithms for Antarctic Peninsula waters. J. Geophys. Res. Ocean. 2000, 105, 26301–26312. [Google Scholar] [CrossRef]
- Gregg, W.W.; Casey, N.W. Global and regional evaluation of the SeaWiFS chlorophyll data set. Remote Sens. Environ. 2004, 93, 463–479. [Google Scholar] [CrossRef]
- Jena, B. The effect of phytoplankton pigment composition and packaging on the retrieval of chlorophyll-a concentration from satellite observation in the Southern Ocean. Int. J. Remote Sens. 2017, 38, 3763–3784. [Google Scholar] [CrossRef]
- Haëntjens, N.; Boss, E.; Talley, L.D. Revisiting ocean color algorithms for chlorophyll a and particulate organic carbon in the Southern Ocean using biochemical floats. J. Geophys. Res. Ocean. 2017, 122, 6583–6593. [Google Scholar] [CrossRef] [Green Version]
- Mourier, W.; Thomalla, S.J.; Bernard, S.; Wind, G.; Ryan-Keogh, T.J.; Smith, M.E. Evaluation of chlorophyll-a and POC Modis Aqua products in the Southern Ocean. Remote Sens. 2019, 11, 1793. [Google Scholar] [CrossRef] [Green Version]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
- Bélanger, S.; Ehn, J.K.; Babin, M. Impact of sea ice on the retrieval of water-leaving reflectance, chlorophyll a concentration and inherent optical properties from satellite ocean color data. Remote Sens. Environ. 2007, 111, 51–68. [Google Scholar] [CrossRef]
- Zeng, C.; Xu, H.; Fischer, A.M. Chlorophyll-a estimation around the Antarctica Peninsula using satellite algorithms: Hints from field water leaving reflectance. Sensors 2016, 16, 2075. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Zeng, T.; Fischer, A.M.; Xu, H. Fluorescence-based approach to estimate the chlorophyll-a concentration of a phytoplankton bloom in Ardley Cove (Antarctica). Remote Sens. 2017, 9, 210. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Ciotti, Á.M.; Mendes, C.R.B.; Uitz, J.; Bricaud, A. Phytoplankton light absorption and the package effect in relation to photosynthetic and photoprotective pigments in the northern tip of Antarctic Peninsula. J. Geophys. Res. Ocean. 2017, 122, 7344–7363. [Google Scholar] [CrossRef]
- Ferreira, A.; Ciotti, Á.M.; Garcia, C.A.E. Bio-optical characterization of the northern Antarctic Peninsula waters: Absorption budget and insights on particulate backscattering. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 138–149. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Kahru, M. Bio-optical algorithms for ADEOS-2 GLI. J. Remote Sens. Soc. Jpn. 2009, 29, 80–85. [Google Scholar]
- Johnson, R.; Strutton, P.G.; Wright, S.W.; McMinn, A.; Meiners, K.M. Three improved satellite chlorophyll algorithms for the Southern Ocean. J. Geophys. Res. Ocean. 2013, 118, 3694–3703. [Google Scholar] [CrossRef]
- Pereira, E.S.; Garcia, C.A. Evaluation of satellite-derived MODIS chlorophyll algorithms in the northern Antarctic Peninsula. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 124–137. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Brewin, R.J.W.; Brockmann, C.; Brotas, V.; Calton, B.; Chuprin, A.; Cipollini, P.; Couto, A.B.; Dingle, J.; Doerffer, R.; et al. An Ocean-Colour Time Series for Use in Climate Studies: The Experience of the Ocean-Colour Climate Change Initiative (OC-CCI). Sensors 2019, 19, 4285. [Google Scholar] [CrossRef] [Green Version]
- Garnesson, P.; Mangin, A.; d’Andon, O.F.; Demaria, J.; Bretagnon, M. The CMEMS GlobColour chlorophyll a product based on satellite observation: Multi-sensor merging and flagging strategies. Ocean Sci. 2019, 15, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Concha, J.A.; Bracaglia, M.; Brando, V.E. Assessing the influence of different validation protocols on ocean colour match-up analyses. Remote Sens. Environ. 2021, 259, 112415. [Google Scholar] [CrossRef]
- Montes-Hugo, M.; Doney, S.C.; Ducklow, H.W.; Fraser, W.; Martinson, D.; Stammerjohn, S.E.; Schofield, O. Recent changes in phytoplankton communities associated with rapid regional climate change along the Western Antarctic Peninsula. Science 2009, 323, 1470–1473. [Google Scholar] [CrossRef] [PubMed]
- Schofield, O.; Saba, G.; Coleman, K.; Carvalho, F.; Couto, N.; Ducklow, H.; Finkel, Z.; Irwin, A.; Kahl, A.; Miles, T.; et al. Decadal variability in coastal phytoplankton community composition in a changing West Antarctic Peninsula. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 124, 42–54. [Google Scholar] [CrossRef]
- Mendes, C.R.B.; Tavano, V.M.; Dotto, T.S.; Kerr, R.; de Souza, M.S.; Garcia, C.A.E.; Secchi, E.R. New insights on the dominance of cryptophytes in Antarctic coastal waters: A case study in Gerlache Strait. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 149, 161–170. [Google Scholar] [CrossRef]
- Ferreira, A.; Costa, R.R.; Dotto, T.S.; Kerr, R.; Tavano, V.M.; Brito, A.C.; Brotas, V.; Secchi, E.R.; Mendes, C.R.B. Changes in phytoplankton communities along the Northern Antarctic Peninsula: Causes, impacts and research priorities. Front. Mar. Sci. 2020, 7, 576254. [Google Scholar] [CrossRef]
- Jackson, T.; Sathyendranath, S.; Mélin, F. An improved optical classification scheme for the Ocean Colour Essential Climate Variable and its applications. Remote Sens. Environ. 2017, 203, 152–161. [Google Scholar] [CrossRef]
- Valente, A.; Sathyendranath, S.; Brotas, V.; Groom, S.; Grant, M.; Taberner, M.; Antoine, D.; Arnone, R.; Balch, W.M.; Barker, K.; et al. A compilation of global bio-optical in situ data for ocean-colour satellite applications—version two. Earth Syst. Sci. Data 2019, 11, 1037–1068. [Google Scholar] [CrossRef] [Green Version]
- Mendes, C.R.B.; Tavano, V.M.; Leal, M.C.; de Souza, M.S.; Brotas, V.; Garcia, C.A.E. Shifts in dominance between diatoms and cryptophytes during three late summers in the Bransfield Strait (Antarctic Peninsula). Polar Biol. 2013, 36, 537–547. [Google Scholar] [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Mendes, C.R.; Cartaxana, P.; Brotas, V. HPLC determination of phytoplankton and microphytobenthos pigments: Comparing resolution and sensitivity of a C18 and a C8 method. Limnol. Oceanogr. Methods 2007, 5, 363–370. [Google Scholar] [CrossRef]
- Hooker, S.B.; Clementson, L.; Thomas, C.S.; Schlüter, L.; Allerup, M.; Ras, J.; Claustre, H.; Normandeau, C.; Cullen, J.; Kienast, M.; et al. The Fifth SeaWiFS HPLC Analysis Round-Robin Experiment (SeaHARRE-5): NASA Technical Memorandum 2012–217503; NASA Goddard Space Flight Center: Greenbelt, MA, USA, 2012. [Google Scholar]
- Evers-King, H.; Martinez-Vicente, V.; Brewin, R.J.; Dall’Olmo, G.; Hickman, A.E.; Jackson, T.; Kostadinov, T.S.; Krasemann, H.; Loisel, H.; Röttgers, R.; et al. Validation and intercomparison of ocean color algorithms for estimating particulate organic carbon in the oceans. Front. Mar. Sci. 2017, 4, 251. [Google Scholar] [CrossRef]
- Bailey, S.W.; Werdell, P.J. A multi-sensor approach for the on-orbit validation of ocean color satellite data products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Chai, T.; Draxler, R.R. Root mean square error (RMSE) or mean absolute error (MAE)? Geosci. Model Dev. Discuss. 2014, 7, 1525–1534. [Google Scholar]
- Seegers, B.N.; Stumpf, R.P.; Schaeffer, B.A.; Loftin, K.A.; Werdell, P.J. Performance metrics for the assessment of satellite data products: An ocean color case study. Opt. Express. 2018, 26, 7404–7422. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shi, W. Detection of ice and mixed ice-water pixels for MODIS ocean color data processing. IEEE Trans. Geosci. Remote Sens. 2009, 47, 2510–2518. [Google Scholar] [CrossRef]
- Reynolds, R.A.; Stramski, D.; Mitchell, B.G. A chlorophyll-dependent semianalytical reflectance model derived from field measurements of absorption and backscattering coefficients within the Southern Ocean. J. Geophys. Res. Ocean. 2001, 106, 7125–7138. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Holm-Hansen, O. Bio-optical properties of Antarctic Peninsula waters: Differentiation from temperate ocean models. Deep Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, 1009–1028. [Google Scholar] [CrossRef]
- Brewin, R.J.W.; Sathyendranath, S.; Jackson, T.; Barlow, R.; Brotas, V.; Airs, R.; Lamont, T. Influence of light in the mixed-layer on the parameters of a three-component model of phytoplankton size class. Remote Sens. Environ. 2015, 168, 437–450. [Google Scholar] [CrossRef]
- Vidussi, F.; Claustre, H.; Manca, B.B.; Luchetta, A.; Marty, J.-C. Phytoplankton pigment distribution in relation to upper thermocline circulation in the eastern Mediterranean Sea during winter. J. Geophys. Res. 2001, 106, 19939–19956. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Ocean. 2006, 111, C08005. [Google Scholar] [CrossRef]
- Stuart, V.; Sathyendranath, S.; Platt, T.; Maass, H.; Irwin, B. Pigments and species composition of natural phytoplankton populations: Effect on the absorption spectra. J. Plankton. Res. 1998, 20, 187–217. [Google Scholar] [CrossRef] [Green Version]
- Merchant, C.J.; Embury, O.; Bulgin, C.E.; Block, T.; Corlett, G.K.; Fiedler, E.; Good, S.A.; Mittaz, J.; Rayner, N.A.; Berry, D.; et al. Satelite-based time-series of sea-surface temperature since 1981 for climate applications. Sci. Data 2019, 6, 223. [Google Scholar] [CrossRef] [Green Version]
- Parkinson, C.L.; Cavalieri, D.J.; Gloersen, P.; Zwally, H.J.; Comiso, J.C. Arctic sea ice extents, areas, and trends, 1978–1996. J. Geophys. Res. Ocean. 1999, 104, 20837–20856. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Hu, C.; Barnes, B.B.; Xie, Y.; Lin, G.; Qiu, Z. Improving ocean color data coverage through machine learning. Remote Sens. Environ. 2019, 222, 286–302. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.-H.; Kim, H.-C.; Kim, B.-K.; Bae, D.; Jo, Y.-H.; Jo, N.; Lee, S.H. Reconstruction of ocean color data using machine learning techniques in polar regions: Focusing on off Cape Hallett, Ross Sea. Remote Sens. 2019, 11, 1366. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.; Brotas, V.; Palma, C.; Borges, C.; Brito, A.C. Assessing phytoplakton bloom phenology in upwelling-influenced regions using ocean color remote sensing. Remote Sens. 2021, 13, 675. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C., Jr.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef]
- Scornet, E.; Biau, G.; Vert, J.-P. Consistency of random forests. Ann. Stat. 2015, 43, 1716–1741. [Google Scholar] [CrossRef]
- Genuer, R.; Poggi, J.-M.; Tuleau-Malot, C.; Villa-Vialaneix, N. Random forests for big data. Big Data Res. 2017, 9, 28–46. [Google Scholar] [CrossRef]
- Hu, S.; Liu, H.; Zhao, W.; Shi, T.; Hu, Z.; Li, Q.; Wu, G. Comparison of machine learning techniques in inferring phytoplankton size classes. Remote Sens. 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.L.; Fargion, G.S.; McClain, C.R. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 4, Volume III: Radiometric Measurements and Data Analysis Protocols; NASA Goddard Space Flight Center: Greenbelt, MA, USA, 2003; 78p. [Google Scholar]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll algorithms for ocean color sensors—OC4, OC5 & OC6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar] [PubMed]
- O’Reilly, J.E.; Maritorena, D.; Siegel, M.C.; O’brien, D.; Toole, B.G.; Mitchell, M.; Kahru, F.P.; Chavez, P.; Strutton, G.; Cota, S.B.; et al. Culver. In Ocean Color Chlorophyll a Algorithms for SeaWiFS, OC2, and OC4: Version 4; Hooker, S.B., Firestone, E.R., Eds.; SeaWiFSPostlaunch Technical Report Series, SeaWiFS Post-launch Calibration and Validation Analyses; NASA Goddard Space Flight Center: Greenbelt, MA, USA, 2000; Volume 11, Part 3; pp. 9–23. [Google Scholar]
- Bailey, S.W.; McClain, C.R.; Werdell, P.J.; Schieber, B.D. Normalized water-leaving radiance and chlorophyll a match-up analyses. NASA Tech. Memo. 2000, 206892, 45–52. [Google Scholar]
- Church, M.J.; DeLong, E.F.; Ducklow, H.W.; Karner, M.B.; Preston, C.M.; Karl, D.M. Abundance and distribution of planktonic Archae and Bacteria in waters west of the Antarctic Peninsula. Limnol. Oceanogr. 2003, 48, 1893–1902. [Google Scholar] [CrossRef]
- Stramski, D.; Boss, E.; Bogucki, D.; Voss, K.J. The role of seawater constituents in light backscattering in the ocean. Prog. Oceanogr. 2004, 61, 27–56. [Google Scholar] [CrossRef]
- Caldeira, K.; Duffy, P.B. The role of the Southern Ocean in uptake and storage of anthropogenic carbon dioxide. Science 2009, 287, 620–622. [Google Scholar] [CrossRef] [Green Version]
- Hauck, J.; Völker, C.; Wolf-Gladrow, D.A.; Lauftkötter, C.; Vogt, M.; Aumont, O.; Bopp, L.; Buitenhuis, E.T.; Doney, S.C.; Dunne, J.; et al. On the Southern Ocean CO2 uptake and the role of the biological carbon pump in the 21st century. Glob. Biogeochem. Cycles 2015, 29, 1451–1470. [Google Scholar] [CrossRef]
- Groom, S.; Sathyendranath, S.; Ban, Y.; Bernard, S.; Brewin, R.; Brotas, V.; Brockmann, C.; Chauhan, P.; Choi, J.-k.; Chuprin, A.; et al. Satellite ocean colour: Current status and future perspective. Front. Mar. Sci. 2019, 6, 485. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, C.W.; McClain, C.R.; Comiso, J.C.; Smith, W.O., Jr. Phytoplankton standing crops within an Antarctic ice edge assessed by satellite remote sensing. J. Geophys. Res. 1988, 93, 12487–12498. [Google Scholar] [CrossRef]
- Sullivan, C.W.; Arrigo, K.R.; McClain, C.R.; Comiso, J.C.; Firestone, J. Distributions of phytoplankton blooms in the Southern Ocean. Science 1993, 262, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, C.E.; Signorini, S.R.; Karaköylü, E.M.; Rivero-Calle, S. Is the Southern Ocean getting greener? Geophys. Res. Lett. 2019, 46, 6034–6040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahru, M.; Brotas, V.; Manzano-Sarabia, M.; Mitchell, B.G. Are phytoplankton blooms occurring earlier in the Arctic? Glob. Chang. Biol. 2011, 17, 1733–1739. [Google Scholar] [CrossRef]
- Oziel, L.; Baudena, A.; Ardyna, M.; Massicotte, P.; Randelhoff, A.; Sallée, J.-B.; Ingvaldsen, R.B.; Devred, E.; Babin, M. Faster Atlantic currents drive poleward expansion of temperate phytoplankton in the Arctic Ocean. Nat. Commun. 2020, 11, 1705. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K.M.; van Dijken, G.L.; Arrigo, K.R. Changes in phytoplankton concentration now drive increased Arctic Ocean primary production. Science 2020, 369, 198–202. [Google Scholar] [CrossRef]
- Schofield, O.; Ducklow, H.W.; Martinson, D.G.; Meredith, M.P.; Moline, M.A.; Fraser, W.R. How do polar marine ecosystems respond to rapid climate change. Science 2010, 328, 1520–1523. [Google Scholar] [CrossRef] [Green Version]
- Newman, L.; Heil, P.; Trebilco, R.; Katsumata, K.; Constable, A.; van Wijk, E.; Assman, K.; Beja, J.; Bricher, P.; Coleman, R.; et al. Delivering sustained, coordinated, and integrated observations of the Southern Ocean for global impact. Front. Mar. Sci. 2019, 6, 433. [Google Scholar] [CrossRef] [Green Version]
- Haberman, K.L.; Ross, R.M.; Quetin, L.B. Diet of the Antarctic krill (Euphausia superba Dana): II. Selective grazing in mixed phytoplankton assemblages. J. Exp. Mar. Bio. Ecol. 2003, 283, 97–113. [Google Scholar] [CrossRef]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P. Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature 2004, 432, 100–103. [Google Scholar] [CrossRef]









| Algorithm | N | In-Situ Data * | a0 | a1 | a2 | a3 | a4 | Reference |
|---|---|---|---|---|---|---|---|---|
| OC4Sze | 400 | F | 0.6728 | −2.3832 | −0.3546 | 2.2753 | −2.2788 | [6] |
| OC4Jo | 345 | H | 0.6736 | −2.0714 | −0.4939 | 0.4756 | [20] | |
| GLOJo | 577 | H | 0.3205 | −2.9139 | 8.7428 | −16.1811 | 9.0051 | [20] |
| OC3M/FURG-SO | 198 | F + H | 0.3078 | −2.2309 | 1.6349 | −1.5566 | −0.6904 | [21] |
| Match-Up Identification Method | N | R2 | RMSE | MAE | Bias |
|---|---|---|---|---|---|
| Within 4 km radius | 316 | 0.51 | 1.84 | 2.77 | 0.41 |
| 3 × 3 box + valid centre pixel | 275 | 0.48 | 1.83 | 2.74 | 0.42 |
| 3 × 3 box + 50% valid | 269 | 0.46 | 1.81 | 2.73 | 0.43 |
| 5 × 5 box + 50% + filtered mean + CV < 0.15 | 196 | 0.42 | 1.89 | 2.87 | 0.39 |
| Algorithm | N | R2 | RMSE | MAE | Bias | Slope | Intercept |
|---|---|---|---|---|---|---|---|
| CCI 4.2 [22] | 316 | 0.51 | 3.27 | 2.77 | 0.41 | 0.42 | −0.44 |
| OC4Sz [6] | 316 | 0.50 | 2.25 | 1.94 | 0.83 | 0.54 | −0.12 |
| GLOJo [20] | 316 | 0.50 | 2.37 | 2.02 | 0.74 | 0.37 | −0.20 |
| OC4Jo [20] | 316 | 0.50 | 2.2 | 1.89 | 0.93 | 0.53 | −0.08 |
| OC3M/FURG-SO [21] | 316 | 0.50 | 2.84 | 2.42 | 0.50 | 0.48 | −0.35 |
| MBR | a0 | a1 | a2 | a3 | a4 | |
|---|---|---|---|---|---|---|
| OC4-SO | <3 | 0.60159 | −3.20362 | 11.17268 | −26.78898 | 18.64112 |
| 3–5 | Linearly weighted mean | |||||
| >5 | 0.63668 | −1.94561 | 0.15707 | −0.5716 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.; Brito, A.C.; Mendes, C.R.B.; Brotas, V.; Costa, R.R.; Guerreiro, C.V.; Sá, C.; Jackson, T. OC4-SO: A New Chlorophyll-a Algorithm for the Western Antarctic Peninsula Using Multi-Sensor Satellite Data. Remote Sens. 2022, 14, 1052. https://doi.org/10.3390/rs14051052
Ferreira A, Brito AC, Mendes CRB, Brotas V, Costa RR, Guerreiro CV, Sá C, Jackson T. OC4-SO: A New Chlorophyll-a Algorithm for the Western Antarctic Peninsula Using Multi-Sensor Satellite Data. Remote Sensing. 2022; 14(5):1052. https://doi.org/10.3390/rs14051052
Chicago/Turabian StyleFerreira, Afonso, Ana C. Brito, Carlos R. B. Mendes, Vanda Brotas, Raul R. Costa, Catarina V. Guerreiro, Carolina Sá, and Thomas Jackson. 2022. "OC4-SO: A New Chlorophyll-a Algorithm for the Western Antarctic Peninsula Using Multi-Sensor Satellite Data" Remote Sensing 14, no. 5: 1052. https://doi.org/10.3390/rs14051052








