Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review
Abstract
:1. Introduction
“Precision Agriculture is a management strategy that gathers, processes and analyses temporal, spatial and individual data and combines it with other information to support management decisions according to estimated variability for improved resource use efficiency, productivity, quality, profitability and sustainability of agricultural production.”
2. Remote Sensing Sensors for Spatial Variability Detection
2.1. Sensors and Technologies for Identifying the Physiological State of the Olive Tree
2.2. Sensors and Technologies for Olive Canopy Characterization
3. Monitoring Technologies That Can Be Used in Precision Olive Growing
3.1. Remote Sensing
3.1.1. Satellite
3.1.2. Aircraft
3.1.3. Unmanned Aerial Vehicles (UAV)
3.2. Proximal Sensing
4. Future Directions of Precision Oliviculture
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fountas, S.; Aggelopoulou, K.; Gemtos, T.A. Precision Agriculture: Crop Management for Improved Productivity and Reduced Environmental Impact or Improved Sustainability. In Supply Chain Management for Sustainable Food Networks; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 41–65. [Google Scholar]
- Schrijver, R.; Poppe, K.; Daheim, C. Precision Agriculture and the Future of Farming in Europe; Science and Technology Options Assessment: Brussels, Belgium, 2016; Available online: http://www.ep.europa.eu/stoa (accessed on 26 January 2022).
- Lal, R. 16 Challenges and Opportunities in Precision Agriculture. Soil-Specif. Farming Precis. Agric. 2015, 22, 391. [Google Scholar]
- López-Granados, F.; Jurado-Expósito, M.; Alamo, S.; Garcıa-Torres, L. Leaf Nutrient Spatial Variability and Site-Specific Fertilization Maps within Olive (Olea Europaea L.) Orchards. Eur. J. Agron. 2004, 21, 209–222. [Google Scholar] [CrossRef]
- Noori, O.; Panda, S.S. Site-Specific Management of Common Olive: Remote Sensing, Geospatial, and Advanced Image Processing Applications. Comput. Electron. Agric. 2016, 127, 680–689. [Google Scholar] [CrossRef]
- Van Evert, F.K.; Gaitán-Cremaschi, D.; Fountas, S.; Kempenaar, C. Can Precision Agriculture Increase the Profitability and Sustainability of the Production of Potatoes and Olives? Sustainability 2017, 9, 1863. [Google Scholar] [CrossRef] [Green Version]
- Santesteban, L.G. Precision Viticulture and Advanced Analytics. A Short Review. Food Chem. 2019, 279, 58–62. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Statistics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- De Gennaro, B.; Notarnicola, B.; Roselli, L.; Tassielli, G. Innovative Olive-Growing Models: An Environmental and Economic Assessment. J. Clean. Prod. 2012, 28, 70–80. [Google Scholar] [CrossRef]
- Baldoni, L.; Belaj, A. Olive. In Oil Crops; Springer: Cham, Switzerland, 2009; pp. 397–421. [Google Scholar]
- Rallo, L. The Olive Industry in Spain. In Proceedings of the Olivebioteq 2006, 2nd Int Sem, Recent Advances in Olive Industry, Special Seminars and Invited Lectures, Marsala, Mazara del Vallo, Italy, 5–10 November 2006; pp. 151–162. [Google Scholar]
- Tous, J.; Romero, A.; Hermoso, J. The Hedgerow System for Olive Growing. Olea FAO Olive Netw. 2007, 26, 20–26. [Google Scholar]
- Fernández-Escobar, R.; Marín, L. Nitrogen Fertilization in Olive Orchards. In Proceedings of the 3rd International Symposium on Olive Growing, Chania, Greece, 22–26 September 1997; pp. 333–336. [Google Scholar]
- Rosati, A.; Caporali, S.; Paoletti, A. Fertilization with N and K Increases Oil and Water Content in Olive (Olea Europaea L.) Fruit via Increased Proportion of Pulp. Sci. Hortic. 2015, 192, 381–386. [Google Scholar] [CrossRef]
- Bouhafa, K.; Moughli, L.; Bouchoufi, K.; Douaik, A.; Daoui, K. Nitrogen Fertilization of Olive Orchards under Rainfed Mediterranean Conditions. Am. J. Exp. Agric. 2014, 4, 890. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Use and Abuse of Nitrogen in Olive Fertilization. Acta Hortic 2011, 888, 249–257. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; Beltrán, G.; Sánchez-Zamora, M.; García-Novelo, J.; Aguilera, M.; Uceda, M. Olive Oil Quality Decreases with Nitrogen Over-Fertilization. HortScience 2006, 41, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, R.; Morales-Sillero, A.; d’Andria, R.; Fernandez, J.; Lavini, A.; Sebastiani, L.; Troncoso, A. Deficit Irrigation and Fertigation Practices in Olive Growing: Convergences and Divergences in Two Case Studies. Plant Biosyst. 2008, 142, 138–148. [Google Scholar] [CrossRef]
- Dag, A.; Ben-David, E.; Kerem, Z.; Ben-Gal, A.; Erel, R.; Basheer, L.; Yermiyahu, U. Olive Oil Composition as a Function of Nitrogen, Phosphorus and Potassium Plant Nutrition. J. Sci. Food Agric. 2009, 89, 1871–1878. [Google Scholar] [CrossRef]
- Fernández-Escobar, R.; García Novelo, J.; Sánchez Zamora, M.; Uceda, M.; Beltrán, G.; Aguilera, M. Efecto del Abonado Nitrogenado en la Producción y la Calidad del Aceite de Oliva; Dirección General de Investigación y Formación Agraria y Pesquera, Ed.; Jornadas de Investigación y Transferencia de Tecnologıa al Sector Oleıcola: Córdoba, Spain, 2002; pp. 299–302. [Google Scholar]
- Fernández, J.E.; Diaz-Espejo, A.; Romero, R.; Hernandez-Santana, V.; García, J.M.; Padilla-Díaz, C.M.; Cuevas, M.V. Precision Irrigation in Olive (Olea Europaea L.) Tree Orchards. In Water Scarcity and Sustainable Agriculture in Semiarid Environment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 179–217. [Google Scholar]
- Tognetti, R.; Giovannelli, A.; Lavini, A.; Morelli, G.; Fragnito, F.; d’Andria, R. Assessing Environmental Controls over Conductances through the Soil–Plant–Atmosphere Continuum in an Experimental Olive Tree Plantation of Southern Italy. Agric. For. Meteorol. 2009, 149, 1229–1243. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, M.; Wang, N. Precision Agriculture—A Worldwide Overview. Comput. Electron. Agric. 2002, 36, 113–132. [Google Scholar] [CrossRef]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-Based Multispectral Remote Sensing for Precision Agriculture: A Comparison between Different Cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Rubio-Delgado, J.; Pérez, C.J.; Vega-Rodríguez, M.A. Predicting Leaf Nitrogen Content in Olive Trees Using Hyperspectral Data for Precision Agriculture. Precis. Agric. 2021, 22, 1–21. [Google Scholar] [CrossRef]
- Liang, S. Quantitative Remote Sensing of Land Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 30, ISBN 0-471-72371-1. [Google Scholar]
- Tanriverdi, C. A Review of Remote Sensing and Vegetation Indices in Precision Farming. J. Sci. Eng. 2006, 9, 69–76. [Google Scholar]
- Lee, W.-S.; Alchanatis, V.; Yang, C.; Hirafuji, M.; Moshou, D.; Li, C. Sensing Technologies for Precision Specialty Crop Production. Comput. Electron. Agric. 2010, 74, 2–33. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef] [Green Version]
- Hansen, P.; Schjoerring, J. Reflectance Measurement of Canopy Biomass and Nitrogen Status in Wheat Crops Using Normalized Difference Vegetation Indices and Partial Least Squares Regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar]
- Ali, M.; Al-Ani, A.; Eamus, D.; Tan, D.K. Leaf Nitrogen Determination Using Non-Destructive Techniques–A Review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar]
- Barranco Navero, D.; Fernandez Escobar, R.; Rallo Romero, L. El Cultivo del Olivo, 7th ed.; Mundi-Prensa Libros: Madrid, Spain, 2017; ISBN 84-8476-714-0. [Google Scholar]
- Rotundo, A.; Lombardo, N.; Marone, E.; Fiorino, P. La Nutrizione Minerale e Le Concimazioni. 2003. Available online: https://www.scirp.org/%28S%28lz5mqp453edsnp55rrgjct55%29%29/reference/referencespapers.aspx?referenceid=2134774 (accessed on 26 January 2022).
- Zarco-Tejada, P.J.; Miller, J.R.; Morales, A.; Berjón, A.; Agüera, J. Hyperspectral Indices and Model Simulation for Chlorophyll Estimation in Open-Canopy Tree Crops. Remote Sens. Environ. 2004, 90, 463–476. [Google Scholar]
- Rallo, G.; Minacapilli, M.; Ciraolo, G.; Provenzano, G. Detecting Crop Water Status in Mature Olive Groves Using Vegetation Spectral Measurements. Biosyst. Eng. 2014, 128, 52–68. [Google Scholar]
- Jurado, J.M.; Ortega, L.; Cubillas, J.J.; Feito, F. Multispectral Mapping on 3D Models and Multi-Temporal Monitoring for Individual Characterization of Olive Trees. Remote Sens. 2020, 12, 1106. [Google Scholar]
- Jorge, J.; Vallbé, M.; Soler, J.A. Detection of Irrigation Inhomogeneities in an Olive Grove Using the NDRE Vegetation Index Obtained from UAV Images. Eur. J. Remote Sens. 2019, 52, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Casero, M.T.; López-Granados, F.; Pena-Barragán, J.M.; Jurado-Expósito, M.; García-Torres, L.; Fernández-Escobar, R. Assessing Nitrogen and Potassium Deficiencies in Olive Orchards through Discriminant Analysis of Hyperspectral Data. J. Am. Soc. Hortic. Sci. 2007, 132, 611–618. [Google Scholar]
- Stateras, D.; Kalivas, D. Assessment of Olive Tree Canopy Characteristics and Yield Forecast Model Using High Resolution UAV Imagery. Agriculture 2020, 10, 385. [Google Scholar] [CrossRef]
- Noguera, M.; Millán, B.; Pérez-Paredes, J.J.; Ponce, J.M.; Aquino, A.; Andújar, J.M. A New Low-Cost Device Based on Thermal Infrared Sensors for Olive Tree Canopy Temperature Measurement and Water Status Monitoring. Remote Sens. 2020, 12, 723. [Google Scholar]
- Rotbart, N.; Schmilovitch, Z.; Cohen, Y.; Alchanatis, V.; Erel, R.; Ignat, T.; Shenderey, C.; Dag, A.; Yermiyahu, U. Estimating Olive Leaf Nitrogen Concentration Using Visible and Near-Infrared Spectral Reflectance. Biosyst. Eng. 2013, 114, 426–434. [Google Scholar]
- Cohen, Y.; Alchanatis, V.; Meron, M.; Saranga, Y.; Tsipris, J. Estimation of Leaf Water Potential by Thermal Imagery and Spatial Analysis. J. Exp. Bot. 2005, 56, 1843–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agam, N.; Segal, E.; Peeters, A.; Levi, A.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. Spatial Distribution of Water Status in Irrigated Olive Orchards by Thermal Imaging. Precis. Agric. 2014, 15, 346–359. [Google Scholar] [CrossRef]
- Elsayed, S.; Mistele, B.; Schmidhalter, U. Can Changes in Leaf Water Potential Be Assessed Spectrally? Funct. Plant Biol. 2011, 38, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Idso, S.; Jackson, R.; Pinter, P., Jr.; Reginato, R.; Hatfield, J. Normalizing the Stress-Degree-Day Parameter for Environmental Variability. Agric. Meteorol. 1981, 24, 45–55. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.; Reginato, R.; Pinter, P., Jr. Canopy Temperature as a Crop Water Stress Indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of Thermal and Visible Imagery for Estimating Crop Water Status of Irrigated Grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Ben-Gal, A.; Agam, N.; Alchanatis, V.; Cohen, Y.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Sprintsin, M.; Dag, A. Evaluating Water Stress in Irrigated Olives: Correlation of Soil Water Status, Tree Water Status, and Thermal Imagery. Irrig. Sci. 2009, 27, 367–376. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a Crop Water Stress Index Derived from Aerial Thermal Imaging and Infrared Thermometry in Super-High Density Olive Orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.; Sobrino, J.; Jiménez-Muñoz, J.; Villalobos, F. Spatial Variability of Crop Water Stress in an Olive Grove with High-Spatial Thermal Remote Sensing Imagery. Proc. Precis. Agric 2005, 267–272. [Google Scholar]
- Veysi, S.; Naseri, A.A.; Hamzeh, S.; Bartholomeus, H. A Satellite Based Crop Water Stress Index for Irrigation Scheduling in Sugarcane Fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Berni, J.A.; Zarco-Tejada, P.J.; Suárez, L.; Fereres, E. Thermal and Narrowband Multispectral Remote Sensing for Vegetation Monitoring from an Unmanned Aerial Vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Tognetti, R.; d’Andria, R.; Lavini, A.; Morelli, G. The Effect of Deficit Irrigation on Crop Yield and Vegetative Development of Olea Europaea L.(Cvs. Frantoio and Leccino). Eur. J. Agron. 2006, 25, 356–364. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Gonzalez-Dugo, V.; Fereres, E.; Ustin, S.L.; Zarco-Tejada, P.J. Airborne Thermal Imagery to Detect the Seasonal Evolution of Crop Water Status in Peach, Nectarine and Saturn Peach Orchards. Remote Sens. 2016, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G. Use of Infrared Thermometry for Estimation of Stomatal Conductance as a Possible Aid to Irrigation Scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Irmak, S.; Haman, D.Z.; Bastug, R. Determination of Crop Water Stress Index for Irrigation Timing and Yield Estimation of Corn. Agron. J. 2000, 92, 1221–1227. [Google Scholar] [CrossRef]
- Meron, M.; Tsipris, J.; Charitt, D. Remote Mapping of Crop Water Status to Assess Spatial Variability of Crop Stress; Wageningen Academic Publishers: Wageningen, The Netherlands, 2003; pp. 405–410. [Google Scholar]
- Agam, N.; Cohen, Y.; Berni, J.; Alchanatis, V.; Kool, D.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. An Insight to the Performance of Crop Water Stress Index for Olive Trees. Agric. Water Manag. 2013, 118, 79–86. [Google Scholar] [CrossRef]
- Jackson, R.D.; Kustas, W.P.; Choudhury, B.J. A Reexamination of the Crop Water Stress Index. Irrig. Sci. 1988, 9, 309–317. [Google Scholar] [CrossRef]
- Berni, J.; Zarco-Tejada, P.; Sepulcre-Cantó, G.; Fereres, E.; Villalobos, F. Mapping Canopy Conductance and CWSI in Olive Orchards Using High Resolution Thermal Remote Sensing Imagery. Remote Sens. Environ. 2009, 113, 2380–2388. [Google Scholar] [CrossRef]
- Moriana, A.; Villalobos, F.; Fereres, E. Stomatal and Photosynthetic Responses of Olive (Olea Europaea L.) Leaves to Water Deficits. Plant Cell Environ. 2002, 25, 395–405. [Google Scholar] [CrossRef]
- Testi, L.; Orgaz, F.; Villalobos, F.J. Variations in Bulk Canopy Conductance of an Irrigated Olive (Olea Europaea L.) Orchard. Environ. Exp. Bot. 2006, 55, 15–28. [Google Scholar] [CrossRef]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Jiménez-Muñoz, J.; Sobrino, J.; De Miguel, E.; Villalobos, F.J. Detection of Water Stress in an Olive Orchard with Thermal Remote Sensing Imagery. Agric. For. Meteorol. 2006, 136, 31–44. [Google Scholar] [CrossRef]
- Bastiaanssen, W.G.; Menenti, M.; Feddes, R.; Holtslag, A. A Remote Sensing Surface Energy Balance Algorithm for Land (SEBAL). 1. Formulation. J. Hydrol. 1998, 212, 198–212. [Google Scholar] [CrossRef]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.; Jiménez-Muñoz, J.; Sobrino, J.; de Miguel, E.; Villalobos, F. Within-Field Thermal Variability Detection as Function of Water Stress in Olea Europaea L. Orchards with High Spatial Remote Sensing Imagery. Agric. For. Meteorol. 2006, 136, 31–44. [Google Scholar]
- Sepulcre-Cantó, G.; Zarco-Tejada, P.J.; Jiménez-Muñoz, J.; Sobrino, J.; Soriano, M.; Fereres, E.; Vega, V.; Pastor, M. Monitoring Yield and Fruit Quality Parameters in Open-Canopy Tree Crops under Water Stress. Implications for ASTER. Remote Sens. Environ. 2007, 107, 455–470. [Google Scholar] [CrossRef]
- Fuentes-Peñailillo, F.; Ortega-Farías, S.; Acevedo-Opazo, C.; Fonseca-Luengo, D. Implementation of a Two-Source Model for Estimating the Spatial Variability of Olive Evapotranspiration Using Satellite Images and Ground-Based Climate Data. Water 2018, 10, 339. [Google Scholar] [CrossRef] [Green Version]
- Shuttleworth, W.J.; Wallace, J. Evaporation from Sparse Crops—An Energy Combination Theory. Q. J. R. Meteorol. Soc. 1985, 111, 839–855. [Google Scholar] [CrossRef]
- Caruso, G.; Zarco-Tejada, P.J.; González-Dugo, V.; Moriondo, M.; Tozzini, L.; Palai, G.; Rallo, G.; Hornero, A.; Primicerio, J.; Gucci, R. High-Resolution Imagery Acquired from an Unmanned Platform to Estimate Biophysical and Geometrical Parameters of Olive Trees under Different Irrigation Regimes. PLoS ONE 2019, 14, e0210804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, E.; Arnó, J.; Llorens, J.; Sanz, R.; Llop, J.; Rosell-Polo, J.R.; Gallart, M. Advanced Technologies for the Improvement of Spray Application Techniques in Spanish Viticulture: An Overview. Sensors 2014, 14, 691–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufat, J.; Romero-Aroca, A.J.; Arbonés, A.; Villar, J.M.; Hermoso, J.F.; Pascual, M. Mechanical Harvesting and Irrigation Strategy Responses on ‘Arbequina’Olive Oil Quality. HortTechnology 2018, 28, 607–614. [Google Scholar] [CrossRef] [Green Version]
- Sola-Guirado, R.R.; Castillo-Ruiz, F.J.; Jiménez-Jiménez, F.; Blanco-Roldan, G.L.; Castro-Garcia, S.; Gil-Ribes, J.A. Olive Actual “on Year” Yield Forecast Tool Based on the Tree Canopy Geometry Using UAS Imagery. Sensors 2017, 17, 1743. [Google Scholar] [CrossRef] [Green Version]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of Methods for in Situ Leaf Area Index Determination: Part I. Theories, Sensors and Hemispherical Photography. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Villalobos, F.; Testi, L.; Hidalgo, J.; Pastor, M.; Orgaz, F. Modelling Potential Growth and Yield of Olive (Olea Europaea L.) Canopies. Eur. J. Agron. 2006, 24, 296–303. [Google Scholar] [CrossRef]
- Zaman, Q.; Schumann, A.W. Performance of an Ultrasonic Tree Volume Measurement System in Commercial Citrus Groves. Precis. Agric. 2005, 6, 467–480. [Google Scholar] [CrossRef]
- Hernández-Clemente, R.; Navarro-Cerrillo, R.M.; Ramírez, F.J.R.; Hornero, A.; Zarco-Tejada, P.J. A Novel Methodology to Estimate Single-Tree Biophysical Parameters from 3D Digital Imagery Compared to Aerial Laser Scanner Data. Remote Sens. 2014, 6, 11627–11648. [Google Scholar] [CrossRef] [Green Version]
- Rosell, J.R.; Llorens, J.; Sanz, R.; Arno, J.; Ribes-Dasi, M.; Masip, J.; Escolà, A.; Camp, F.; Solanelles, F.; Gràcia, F. Obtaining the Three-Dimensional Structure of Tree Orchards from Remote 2D Terrestrial LIDAR Scanning. Agric. For. Meteorol. 2009, 149, 1505–1515. [Google Scholar] [CrossRef] [Green Version]
- Sola-Guirado, R.R.; Bayano-Tejero, S.; Rodríguez-Lizana, A.; Gil-Ribes, J.A.; Miranda-Fuentes, A. Assessment of the Accuracy of a Multi-Beam LED Scanner Sensor for Measuring Olive Canopies. Sensors 2018, 18, 4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovira-Más, F.; Zhang, Q.; Reid, J. Creation of Three-Dimensional Crop Maps Based on Aerial Stereoimages. Biosyst. Eng. 2005, 90, 251–259. [Google Scholar] [CrossRef]
- Castillo-Ruiz, F.J.; Castro-Garcia, S.; Blanco-Roldan, G.L.; Sola-Guirado, R.R.; Gil-Ribes, J.A. Olive Crown Porosity Measurement Based on Radiation Transmittance: An Assessment of Pruning Effect. Sensors 2016, 16, 723. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, R.; Magnanini, E.; Fragassa, C.; Nerozzi, F. Ground Monitoring the Light–Shadow Windows of a Tree Canopy to Yield Canopy Light Interception and Morphological Traits. Plant Cell Environ. 2000, 23, 783–796. [Google Scholar] [CrossRef]
- Bongers, F. Methods to Assess Tropical Rain Forest Canopy Structure: An Overview. Trop. For. Canopies Ecol. Manag. 2001, 69, 263–277. [Google Scholar]
- Stuppy, W.H.; Maisano, J.A.; Colbert, M.W.; Rudall, P.J.; Rowe, T.B. Three-Dimensional Analysis of Plant Structure Using High-Resolution X-ray Computed Tomography. Trends Plant Sci. 2003, 8, 2–6. [Google Scholar] [CrossRef]
- Rosell, J.; Sanz, R. A Review of Methods and Applications of the Geometric Characterization of Tree Crops in Agricultural Activities. Comput. Electron. Agric. 2012, 81, 124–141. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Fuentes, A.; Llorens, J.; Gamarra-Diezma, J.L.; Gil-Ribes, J.A.; Gil, E. Towards an Optimized Method of Olive Tree Crown Volume Measurement. Sensors 2015, 15, 3671–3687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, J.; Zarco-Tejada, P.; García-Morillo, J.; Gama, J.; Soriano, M. Determining Biophysical Parameters for Olive Trees Using CASI-Airborne and Quickbird-Satellite Imagery. Agron. J. 2011, 103, 644–654. [Google Scholar] [CrossRef] [Green Version]
- Torres-Sánchez, J.; López-Granados, F.; Serrano, N.; Arquero, O.; Peña, J.M. High-Throughput 3-D Monitoring of Agricultural-Tree Plantations with Unmanned Aerial Vehicle (UAV) Technology. PLoS ONE 2015, 10, e0130479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarco-Tejada, P.J.; Diaz-Varela, R.; Angileri, V.; Loudjani, P. Tree Height Quantification Using Very High Resolution Imagery Acquired from an Unmanned Aerial Vehicle (UAV) and Automatic 3D Photo-Reconstruction Methods. Eur. J. Agron. 2014, 55, 89–99. [Google Scholar] [CrossRef]
- Gamarra-Diezma, J.L.; Miranda-Fuentes, A.; Llorens, J.; Cuenca, A.; Blanco-Roldán, G.L.; Rodríguez-Lizana, A. Testing Accuracy of Long-Range Ultrasonic Sensors for Olive Tree Canopy Measurements. Sensors 2015, 15, 2902–2919. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Casasnovas, J.A.; Rufat, J.; Arnó, J.; Arbonés, A.; Sebé, F.; Pascual, M.; Gregorio, E.; Rosell-Polo, J.R. Mobile Terrestrial Laser Scanner Applications in Precision Fruticulture/Horticulture and Tools to Extract Information from Canopy Point Clouds. Precis. Agric. 2017, 18, 111–132. [Google Scholar]
- Tumbo, S.; Salyani, M.; Whitney, J.D.; Wheaton, T.; Miller, W. Investigation of Laser and Ultrasonic Ranging Sensors for Measurements of Citrus Canopy Volume. Appl. Eng. Agric. 2002, 18, 367. [Google Scholar] [CrossRef]
- Anifantis, A.S.; Camposeo, S.; Vivaldi, G.A.; Santoro, F.; Pascuzzi, S. Comparison of UAV Photogrammetry and 3D Modeling Techniques with Other Currently Used Methods for Estimation of the Tree Row Volume of a Super-High-Density Olive Orchard. Agriculture 2019, 9, 233. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Varela, R.A.; De la Rosa, R.; León, L.; Zarco-Tejada, P.J. High-Resolution Airborne UAV Imagery to Assess Olive Tree Crown Parameters Using 3D Photo Reconstruction: Application in Breeding Trials. Remote Sens. 2015, 7, 4213–4232. [Google Scholar] [CrossRef] [Green Version]
- Küng, O.; Strecha, C.; Beyeler, A.; Zufferey, J.-C.; Floreano, D.; Fua, P.; Gervaix, F. The Accuracy of Automatic Photogrammetric Techniques on Ultra-Light UAV Imagery. In Proceedings of the UAV-g 2011—Unmanned Aerial Vehicle in Geomatics, Zurich, Switzerland, 14–16 September 2011. [Google Scholar]
- Blaschke, T.; Hay, G.J.; Kelly, M.; Lang, S.; Hofmann, P.; Addink, E.; Feitosa, R.Q.; Van der Meer, F.; Van der Werff, H.; Van Coillie, F. Geographic Object-Based Image Analysis–towards a New Paradigm. ISPRS J. Photogramm. Remote Sens. 2014, 87, 180–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karydas, C.; Gewehr, S.; Iatrou, M.; Iatrou, G.; Mourelatos, S. Olive Plantation Mapping on a Sub-Tree Scale with Object-Based Image Analysis of Multispectral UAV Data; Operational Potential in Tree Stress Monitoring. J. Imaging 2017, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Boussadia, O.; Steppe, K.; Zgallai, H.; El Hadj, S.B.; Braham, M.; Lemeur, R.; Van Labeke, M.-C. Effects of Nitrogen Deficiency on Leaf Photosynthesis, Carbohydrate Status and Biomass Production in Two Olive Cultivars ‘Meski’ and ‘Koroneiki’. Sci. Hortic. 2010, 123, 336–342. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of Different Irrigation Volumes during Fruit Development on Quality of Virgin Olive Oil of Cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Jiménez-Brenes, F.M.; López-Granados, F.; De Castro, A.; Torres-Sánchez, J.; Serrano, N.; Peña, J. Quantifying Pruning Impacts on Olive Tree Architecture and Annual Canopy Growth by Using UAV-Based 3D Modelling. Plant Methods 2017, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Moorthy, I.; Miller, J.R.; Berni, J.A.J.; Zarco-Tejada, P.; Hu, B.; Chen, J. Field Characterization of Olive (Olea Europaea L.) Tree Crown Architecture Using Terrestrial Laser Scanning Data. Agric. For. Meteorol. 2011, 151, 204–214. [Google Scholar] [CrossRef]
- Moorthy, I.; Miller, J.R.; Hu, B.; Chen, J.; Li, Q. Retrieving Crown Leaf Area Index from an Individual Tree Using Ground-Based Lidar Data. Can. J. Remote Sens. 2008, 34, 320–332. [Google Scholar]
- Senay, G.B.; Ward, A.D.; Lyon, J.G.; Fausey, N.R.; Nokes, S.E. Manipulation of High Spatial Resolution Aircraft Remote Sensing Data for Use in Site-Specific Farming. Trans. ASAE 1998, 41, 489. [Google Scholar] [CrossRef]
- Fountas, S.; Aggelopoulou, K.; Bouloulis, C.; Nanos, G.; Wulfsohn, D.; Gemtos, T.; Paraskevopoulos, A.; Galanis, M. Site-Specific Management in an Olive Tree Plantation. Precis. Agric. 2011, 12, 179–195. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F. Technology in Precision Viticulture: A State of the Art Review. Int. J. Wine Res. 2015, 7, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Apan, A.; Young, F.R.; Phinn, S.; Held, A.; Favier, J. Mapping Olive Varieties and Within-Field Spatial Variability Using High Resolution QuickBird Imagery; Spatial Sciences Institute: Los Angeles, CA, USA, 2004. [Google Scholar]
- Cruz-Ramírez, M.; Hervás-Martínez, C.; Jurado-Expósito, M.; López-Granados, F. A Multi-Objective Neural Network Based Method for Cover Crop Identification from Remote Sensed Data. Expert Syst. Appl. 2012, 39, 10038–10048. [Google Scholar] [CrossRef] [Green Version]
- Ferwerda, J.G.; Skidmore, A.K. Can Nutrient Status of Four Woody Plant Species Be Predicted Using Field Spectrometry? ISPRS J. Photogramm. Remote Sens. 2007, 62, 406–414. [Google Scholar] [CrossRef]
- Peña-Barragán, J.; Jurado-Expósito, M.; López-Granados, F.; Atenciano, S.; Sánchez-De la Orden, M.; Garcıa-Ferrer, A.; Garcıa-Torres, L. Assessing Land-Use in Olive Groves from Aerial Photographs. Agric. Ecosyst. Environ. 2004, 103, 117–122. [Google Scholar] [CrossRef]
- Solano, F.; Di Fazio, S.; Modica, G. A Methodology Based on GEOBIA and WorldView-3 Imagery to Derive Vegetation Indices at Tree Crown Detail in Olive Orchards. Int. J. Appl. Earth Obs. Geoinf. 2019, 83, 101912. [Google Scholar] [CrossRef]
- Zipori, I.; Erel, R.; Yermiyahu, U.; Ben-Gal, A.; Dag, A. Sustainable Management of Olive Orchard Nutrition: A Review. Agriculture 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Kovacs, J.M. The Application of Small Unmanned Aerial Systems for Precision Agriculture: A Review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Pouliot, D.; King, D.; Bell, F.; Pitt, D. Automated Tree Crown Detection and Delineation in High-Resolution Digital Camera Imagery of Coniferous Forest Regeneration. Remote Sens. Environ. 2002, 82, 322–334. [Google Scholar] [CrossRef]
- Fernández, J.E. Plant-Based Methods for Irrigation Scheduling of Woody Crops. Horticulturae 2017, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Ha, W.; Gowda, P.H.; Howell, T.A. A Review of Downscaling Methods for Remote Sensing-Based Irrigation Management: Part I. Irrig. Sci. 2013, 31, 831–850. [Google Scholar] [CrossRef]
- Moriondo, M.; Leolini, L.; Brilli, L.; Dibari, C.; Tognetti, R.; Giovannelli, A.; Rapi, B.; Battista, P.; Caruso, G.; Gucci, R. A Simple Model Simulating Development and Growth of an Olive Grove. Eur. J. Agron. 2019, 105, 129–145. [Google Scholar] [CrossRef]
- Martinez-Guanter, J.; Agüera, P.; Agüera, J.; Pérez-Ruiz, M. Spray and Economics Assessment of a UAV-Based Ultra-Low-Volume Application in Olive and Citrus Orchards. Precis. Agric. 2020, 21, 226–243. [Google Scholar] [CrossRef]
- Pallottino, F.; Antonucci, F.; Costa, C.; Bisaglia, C.; Figorilli, S.; Menesatti, P. Optoelectronic Proximal Sensing Vehicle-Mounted Technologies in Precision Agriculture: A Review. Comput. Electron. Agric. 2019, 162, 859–873. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Saiz-Rubio, V.; Rovira-Más, F.; Cuenca-Cuenca, A.; Alves, F. Robotics-Based Vineyard Water Potential Monitoring at High Resolution. Comput. Electron. Agric. 2021, 187, 106311. [Google Scholar] [CrossRef]
- Solanelles, F.; Planas, S. An Electronic Control System for Proportional Pesticide Application to the Canopy Volume in Tree Crops. In Proceedings of the 2005 EFITA/WCCA Joint Congress on IT in Agriculture, Vila Real, Portugal, 25–28 July 2005; pp. 25–28. [Google Scholar]
- Alcalá Jiménez, A.; Álamo Romero, S. Using GPS for Yield Mapping in Olive Orchards. In Proceedings of the First International Conference on Geospatial Information in Agriculture and Forestry, Lake Buena Vista, FL, USA, 1–3 June 1998. [Google Scholar]
- Agüera-Vega, J.; Blanco, G.; Castillo, F.; Castro-Garcia, S.; Gil-Ribes, J.; Perez-Ruiz, M. Determination of Field Capacity and Yield Mapping in Olive Harvesting Using Remote Data Acquisition. In Precision Agriculture’13; Springer: Cham, Switzerland, 2013; pp. 691–696. [Google Scholar]
- Castillo-Ruiz, F.J.; Pérez-Ruiz, M.; Blanco-Roldán, G.L.; Gil-Ribes, J.A.; Agüera, J. Development of a Telemetry and Yield-Mapping System of Olive Harvester. Sensors 2015, 15, 4001–4018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álamo, S.; Ramos, M.; Feito, F.; Cañas, A. Precision Techniques for Improving the Management of the Olive Groves of Southern Spain. Span. J. Agric. Res. 2012, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virnodkar, S.S.; Pachghare, V.K.; Patil, V.; Jha, S.K. Remote Sensing and Machine Learning for Crop Water Stress Determination in Various Crops: A Critical Review. Precis. Agric. 2020, 21, 1121–1155. [Google Scholar] [CrossRef]
- Mountrakis, G.; Im, J.; Ogole, C. Support Vector Machines in Remote Sensing: A Review. ISPRS J. Photogramm. Remote Sens. 2011, 66, 247–259. [Google Scholar] [CrossRef]
- Makhloufi, A.; Kallel, A.; Chaker, R.; Gastellu-Etchegorry, J.-P. Retrieval of Olive Tree Biophysical Properties from Sentinel-2 Time Series Based on Physical Modelling and Machine Learning Technique. Int. J. Remote Sens. 2021, 42, 8542–8571. [Google Scholar] [CrossRef]
- CEMA. European Agriculture Machinery Association Digital Farming: What Does It Really Mean; Position Paper; CEMA: Alcalá de Guadaíra, Spain, 2017. [Google Scholar]
- Sundmaeker, H.; Verdouw, C.; Wolfert, J.; Freire, L.P. Internet of Food and Farm 2020. In Digitising the Industry; River Publishers: Gistrup, Denmark, 2016; Volume 49, pp. 129–150. ISBN 87-93379-81-1. [Google Scholar]
- Vieri, M.; Sarri, D.; Rimediotti, M.; Perria, R.; Storchi, P. The New Architecture in the Vineyard System Management for Variable Rate Technologies and Traceability. In Proceedings of the 1st International Workshop on Vineyard Mechanization and Grape and Wine Quality, Piacenza, Italy, 27–29 June 2012; pp. 47–53. [Google Scholar]
- Villa-Henriksen, A.; Edwards, G.T.; Pesonen, L.A.; Green, O.; Sørensen, C.A.G. Internet of Things in Arable Farming: Implementation, Applications, Challenges and Potential. Biosyst. Eng. 2020, 191, 60–84. [Google Scholar] [CrossRef]
- Zou, Y.; Quan, L. A New Service-Oriented Grid-Based Method for AIoT Application and Implementation. Mod. Phys. Lett. B 2017, 31, 1740064. [Google Scholar] [CrossRef]
- Zhai, A.F.; Cheng, B.M.; Zhang, C.L.; Ding, D.T.; Liu, E.Y. Optimization of Agricultural Production Control Based on Data Processing Technology of Agricultural Internet of Things. Ital. J. Pure Appl. Math. 2017, 38, e252. [Google Scholar]
- Alahmadi, A.; Alwajeeh, T.; Mohanan, V.; Budiarto, R. Wireless Sensor Network with Always Best Connection for Internet of Farming. In Powering the Internet of Things with 5G Networks; IGI Global: Hershey, PA, USA, 2018; pp. 176–201. [Google Scholar]
- Patel, R. The Long Green Revolution. J. Peasant Stud. 2013, 40, 1–63. [Google Scholar] [CrossRef]
- Popescu, D.; Stoican, F.; Stamatescu, G.; Ichim, L.; Dragana, C. Advanced UAV–WSN System for Intelligent Monitoring in Precision Agriculture. Sensors 2020, 20, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, K.; Doshi, A.; Patel, P.; Shah, M. A Comprehensive Review on Automation in Agriculture Using Artificial Intelligence. Artif. Intell. Agric. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Catania, P.; Comparetti, A.; Febo, P.; Morello, G.; Orlando, S.; Roma, E.; Vallone, M. Positioning Accuracy Comparison of GNSS Receivers Used for Mapping and Guidance of Agricultural Machines. Agronomy 2020, 10, 924. [Google Scholar] [CrossRef]
- Catania, P.; Orlando, S.; Roma, E.; Vallone, M. Vineyard Design Supported by GPS Application. In Proceedings of the International Symposium on Precision Management of Orchards and Vineyards, Palermo, Italy, 7–11 October 2019; pp. 227–234. [Google Scholar]
- Morales, A.; Guerra, R.; Horstrand, P.; Diaz, M.; Jimenez, A.; Melian, J.; Lopez, S.; Lopez, J.F. A Multispectral Camera Development: From the Prototype Assembly until Its Use in a UAV System. Sensors 2020, 20, 6129. [Google Scholar] [CrossRef] [PubMed]
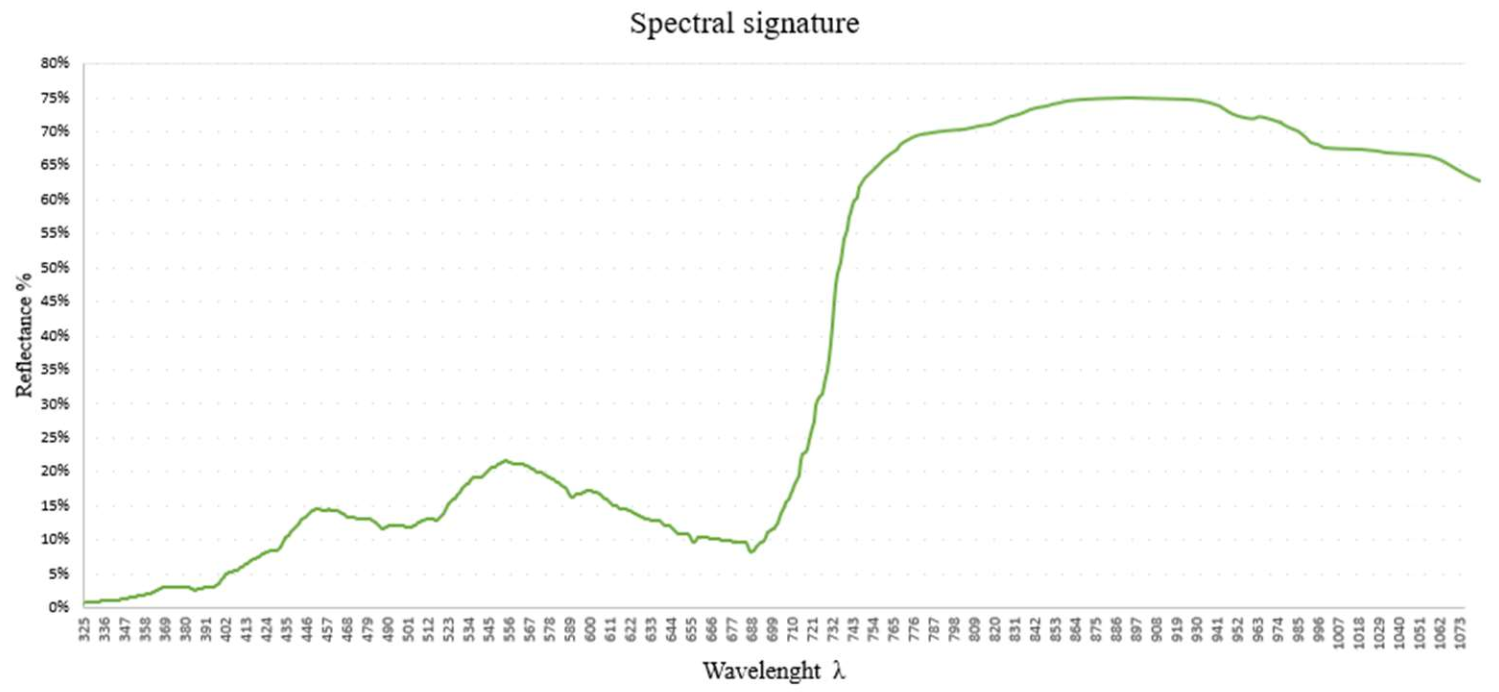
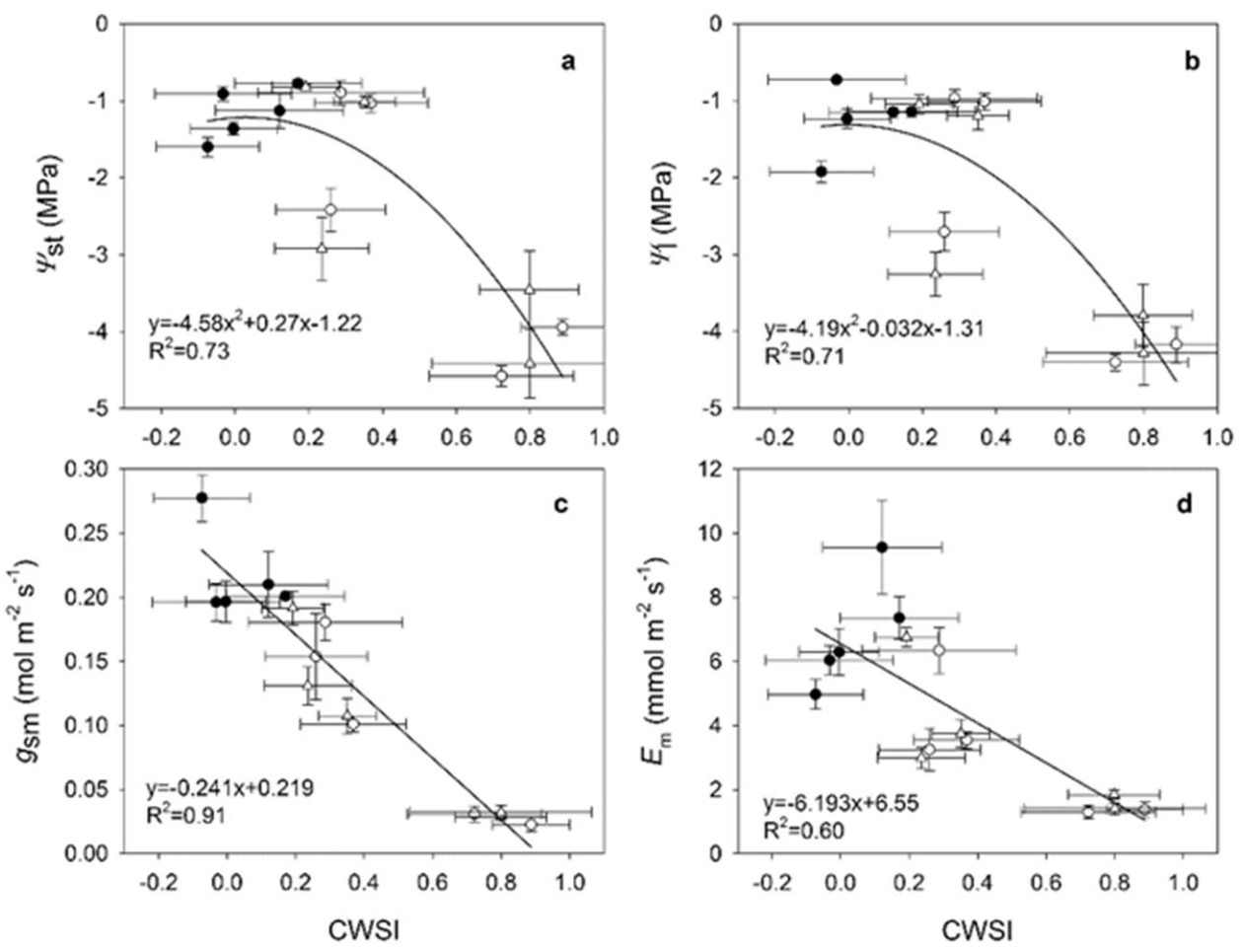

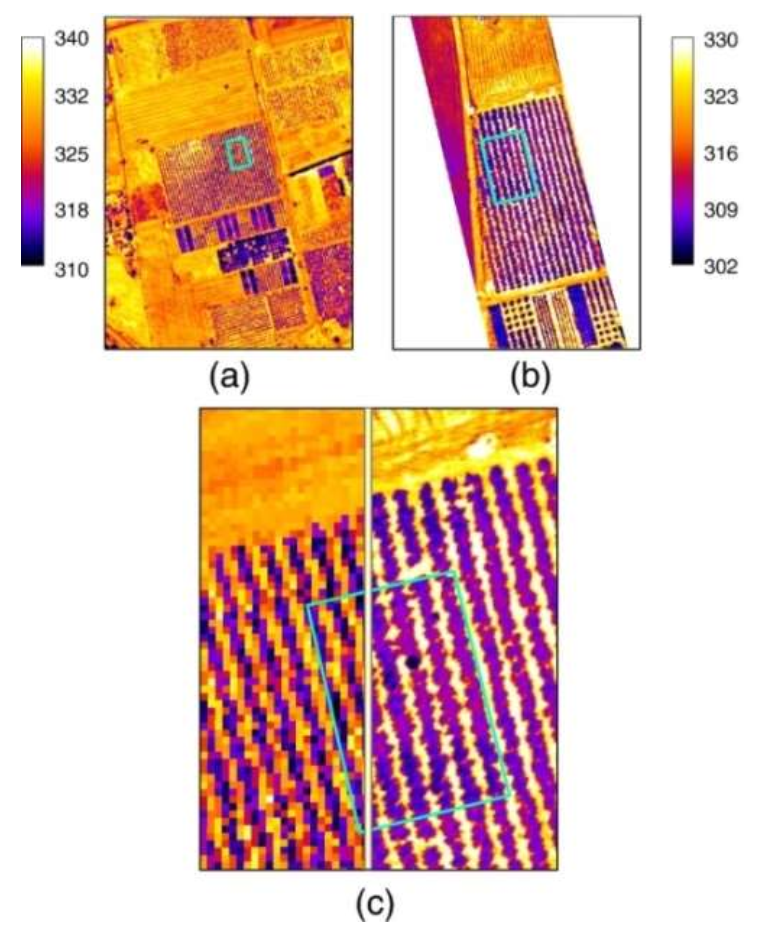
| Vegetation Index (VI) | Acronym | Equations | References | Author of Index |
|---|---|---|---|---|
| Chlorophyll Absorption in Reflectance Index | CARI | CAR*(ρ700/ρ670) | [34] | Kim et al. 1994 |
| Double-peak Canopy Nitrogen Index | DCNI | [(ρ720 − ρ700)/(ρ700 − ρ670)]/(ρ720 − ρ670 + 0.03) | [25] | Chen et al. 2010 |
| Green Index | GI | ρ 550/ρ 680 | [35] | Chamard et al. 1991 |
| Green Normalized Difference Vegetation Index | GNDVI | (ρ800 − ρ550)/(ρ800 + ρ550) | [5,34] | Gitelson and Merzlyak 1994 |
| Modified Chlorophyll Absorption in Reflectance Index | MCARI | [(ρ 700 − ρ 1510) − 0.2(ρ 700 − ρ550)] (ρ 700/ρ 1510) | [25,34] | Daughtry et al. 2000 |
| Moisture Stress Index | MSI | ρ 858/ρ 1240 | [35] | Hunt and Rock 1989 |
| Normalized Difference Greenness Vegetation Index | NDGVI | (ρ550 − ρ680)/(ρ550 + ρ680) | [35,36] | Chamard et al. 1991 |
| Normalized Difference Red-Edge Index | NDRE | (ρNir − ρ RedEdge)/(ρNir + ρRedEdge) | [36,37] | Maccioni et al. 2001 |
| Normalized Difference Vegetation Index | NDVI | (ρ800 − ρ680)/(ρ800 + ρ680) | [5,35,36,38,39] | Rouse, Haas, Deering, and Sehell 1974 |
| Normalized Difference Water Index | NDWI | (ρ858 − ρ1240)/(ρ 858 + ρ 1240) | [35] | Gao 1996 |
| Optimized Soil-Adjusted Vegetation Index | OSAVI | (ρNIR − ρR)/(ρNIR + ρR + 0.16) | [34] | Rondeaux et al. 1996 |
| Soil Adjusted Vegetation Index | SAVI | (ρNir − ρRed) (ρNir + ρRed + L) × (1 + L) | [5,37] | Huete et al. 1988 |
| Simple Ratio 550,670 | SR | ρ550/ρ670 | [38] | n.d. |
| Simple Ratio 780,550 | SR | ρ780/ρ550 | [38] | n.d. |
| Simple Ratio 780,670 | SR | ρ780/ρ670 | [36,38] | n.d. |
| Simple Ratio Water Index | SRWI | ρ680/ρ1240 | [35] | Zarco–Tejada, Rueda, and Ustin 2003 |
| Transformed Chlorophyll Absorption Ratio Index1510 | TCARI | 3[(ρ700 − ρ1510) − 0.2(ρ700 − ρ550) ( ρ700/ρ1510)] | [25,34] | Haboudane et al. 2002 |
| Water Index | WI | ρ680/ρ858 | [35] | Peñuelas et al. 1993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roma, E.; Catania, P. Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review. Remote Sens. 2022, 14, 1668. https://doi.org/10.3390/rs14071668
Roma E, Catania P. Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review. Remote Sensing. 2022; 14(7):1668. https://doi.org/10.3390/rs14071668
Chicago/Turabian StyleRoma, Eliseo, and Pietro Catania. 2022. "Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review" Remote Sensing 14, no. 7: 1668. https://doi.org/10.3390/rs14071668
APA StyleRoma, E., & Catania, P. (2022). Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review. Remote Sensing, 14(7), 1668. https://doi.org/10.3390/rs14071668







