Evaluating the Performance of Sentinel-3A OLCI Products in the Subarctic Northeast Pacific
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Datasets
2.3. In Situ Data
2.3.1. Phytoplankton Pigment Analysis
2.3.2. In Situ Remote Sensing Reflectance
2.4. Sentinel-3A OLCI Data and Atmospheric Correction
2.5. Matchup Analysis: OLCI Rrs and Chla
3. Results
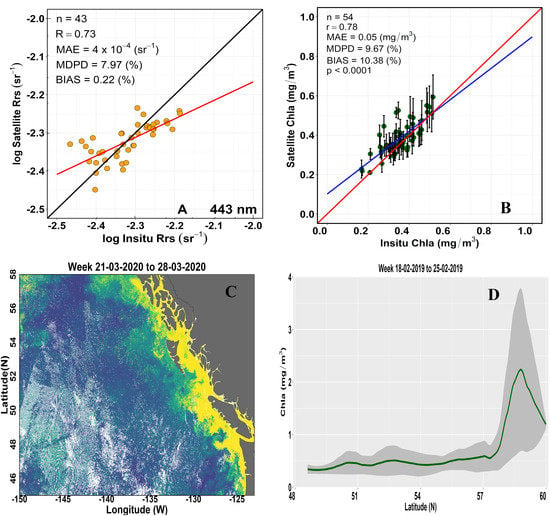
3.1. Remote Sensing Reflectance Validation
3.2. Chlorophyll-a Matchup Analysis
3.3. Spatial Variability of Sentinel-3A OLCI-Derived Chlorophyll-a
4. Discussion
4.1. Evaluation of the Satellite-Derived Products
4.1.1. Remote Sensing Reflectance
4.1.2. Chlorophyll-a Concentration
4.2. Spatial–Temporal Dynamics of Phytoplankton Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary Production of the Biosphere: Integrating Terrestrial and Oceanic Components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Le Quere, C.; Harrison, S.P.; Prentice, I.C.; Buitenhuis, E.T.; Aumont, O.; Bopp, L.; Claustre, H.; Da Cunha, L.C.; Geinder, R.; Giraud, X.; et al. Ecosystem Dynamics Based on Plankton Functional Types for Global Ocean Biogeochemistry Models. Glob. Chang. Biol. 2005, 11, 2016–2040. [Google Scholar] [CrossRef]
- Falkowski, P. Ocean Science: The Power of Plankton. Nature 2012, 483, S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Bouman, H.A.; Ulloa, O.; Scanlan, D.J.; Zwirglmaier, K.; Li, W.K.W.; Platt, T.; Stuart, V.; Barlow, R.; Leth, O.; Clementson, L.; et al. Oceanographic Basis of the Global Surface Distribution of Prochlorococcus Ecotypes. Science 2006, 312, 918–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.B.; Frost, B.W.; Wheeler, P.A.; Landry, M.R.; Welschmeyer, N.; Powell, T.M.; Limnology, S.; Phytoplankton, W.C.; Miller, C.B.; Frost, B.W.; et al. Ecological Dynamics in the Subarctic Pacific, A Possibly Iron-Limited Ecosystem. Limnol. Oceanogr. 1991, 36, 1600–1615. [Google Scholar] [CrossRef] [Green Version]
- Brickley, P.J.; Thomas, A.C. Satellite-Measured Seasonal and Inter-Annual Chlorophyll Variability in the Northeast Pacific and Coastal Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 229–245. [Google Scholar] [CrossRef]
- Strom, S.L.; Fredrickson, K.A.; Bright, K.J. Spring Phytoplankton in the Eastern Coastal Gulf of Alaska: Photosynthesis and Production during High and Low Bloom Years. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 132, 107–121. [Google Scholar] [CrossRef]
- Evans, G.T.; Parslow, J.S. A Model of Annual Plankton Cycles. Biol. Oceanogr. 1985, 3, 327–347. [Google Scholar] [CrossRef]
- McClain, C.R. A Decade of Satellite Ocean Color Observations. Annu. Rev. Mar. Sci. 2009, 1, 19–42. [Google Scholar] [CrossRef] [Green Version]
- Hamme, R.C.; Webley, P.W.; Crawford, W.R.; Whitney, F.A.; Degrandpre, M.D.; Emerson, S.R.; Eriksen, C.C.; Giesbrecht, K.E.; Gower, J.F.R.; Kavanaugh, M.T.; et al. Volcanic Ash Fuels Anomalous Plankton Bloom in Subarctic Northeast Pacific. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Frost, B.W. The Role of Grazing in Nutrient-rich Areas of the Open Sea. Limnol. Oceanogr. 1991, 36, 1616–1630. [Google Scholar] [CrossRef]
- Banse, K.; English, D.C. Comparing Phytoplankton Seasonality in the Eastern and Western Subarctic Pacific and the Western Bering Sea. Prog. Oceanogr. 1999, 43, 235–288. [Google Scholar] [CrossRef]
- Harrison, P.J.; Boyd, P.W.; Varela, D.E.; Takeda, S.; Shiomoto, A.; Odate, T. Comparison of Factors Controlling Phytoplankton Productivity in the NE and NW Subarctic Pacific Gyres. Prog. Oceanogr. 1999, 43, 205–234. [Google Scholar] [CrossRef]
- Bishop, J.K.B.; Davis, R.E.; Sherman, J.T. Robotic Observations of Dust Storm Enhancement of Carbon Biomass in the North Pacific. Science 2002, 298, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.L.; Varela, D.E.; Whitney, F.W.; Harrison, P.J. Nutrient and Phytoplankton Dynamics off the West Coast of Vancouver Island during the 1997/98 ENSO Event. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 2487–2502. [Google Scholar] [CrossRef]
- Marchetti, A.; Juneau, P.; Whitney, F.A.; Wong, C.S.; Harrison, P.J. Phytoplankton Processes during a Mesoscale Iron Enrichment in the NE Subarctic Pacific: Part II-Nutrient Utilization. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 2114–2130. [Google Scholar] [CrossRef]
- Beamish, R.J. What the Past Tells Us about the Future of Pacific Salmon Research. Fish Fish. 2017, 18, 1161–1175. [Google Scholar] [CrossRef]
- Siddon, E.C.; De Forest, L.G.; Blood, D.M.; Doyle, M.J.; Matarese, A.C. Early Life History Ecology for Five Commercially and Ecologically Important Fish Species in the Eastern and Western Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 7–25. [Google Scholar] [CrossRef]
- Womble, J.N.; Sigler, M.F. Seasonal Availability of Abundant, Energy-Rich Prey Influences the Abundance and Diet of a Marine Predator, the Steller Sea Lion Eumetopias Jubatus. Mar. Ecol. Prog. Ser. 2006, 325, 281–293. [Google Scholar] [CrossRef]
- Womble, J.N.; Sigler, M.F.; Willson, M.F. Linking Seasonal Distribution Patterns with Prey Availability in a Central-Place Forager, the Steller Sea Lion. J. Biogeogr. 2009, 36, 439–451. [Google Scholar] [CrossRef]
- Johnson, S.W.; Neff, A.D.; Thedinga, J.F.; Lindeberg, M.R.; Maselko, J.M. Atlas of Nearshore Fishes of Alaska: A Synthesis of Marine Surveys from 1998 to 2011; NOAA Technical Memorandum NMFS-AFSC-239; U.S. Department of Commerce: Washington, DC, USA, 2012; 261p.
- McGowan, D.W.; Horne, J.K.; Parker-Stetter, S.L. Variability in Species Composition and Distribution of Forage Fish in the Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 165, 221–237. [Google Scholar] [CrossRef]
- Budge, S.M.; Wang, S.W.; Ormseth, O.A.; Rand, K.M. Foraging Ecology of Nearshore Fishes in the Gulf of Alaska. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2022, 195, 105013. [Google Scholar] [CrossRef]
- Dragoo, D.E.; Renner, H.M.; Kaler, R.S.A. Breeding Status, Population Trends and Diets of Seabirds in Alaska, 2016; U.S. Fish and Wildlife Service Report AMNWR 2017/06; Alaska Maritime National Wildlife Refuge: Homer, Alaska, 2017.
- Sambrotto, R.N.; Lorenzen, C.J. The Gulf of Alaska: Physical Environment and Biological Resources; Hood, D.W., Zimmerman, S.T., Eds.; Ocean Assessments Division, NOAA. U.S. Department of Commerce: Washington, DC, USA, 1987; pp. 249–282. ISBN 0080432077.
- Childers, A.R.; Whitledge, T.E.; Stockwell, D.A. Seasonal and Interannual Variability in the Distribution of Nutrients and Chlorophyll a across the Gulf of Alaska Shelf: 1998–2000. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 193–216. [Google Scholar] [CrossRef]
- Waite, J.N.; Mueter, F.J. Spatial and Temporal Variability of Chlorophyll-a Concentrations in the Coastal Gulf of Alaska, 1998–2011, Using Cloud-Free Reconstructions of SeaWiFS and MODIS-Aqua Data. Prog. Oceanogr. 2013, 116, 179–192. [Google Scholar] [CrossRef]
- Stabeno, P.J.; Bond, N.A.; Hermann, A.J.; Kachel, N.B.; Mordy, C.W.; Overland, J.E. Meteorology and Oceanography of the Northern Gulf of Alaska. Cont. Shelf Res. 2004, 24, 859–897. [Google Scholar] [CrossRef]
- Olson, R.J.; Sosik, H.M. A Submersible Imaging-in-Flow Instrument to Analyze Nano-and Microplankton: Imaging FlowCytobot. Limnol. Oceanogr. Methods 2007, 5, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Lombard, F.; Boss, E.; Waite, A.M.; Uitz, J.; Stemmann, L.; Sosik, H.M.; Schulz, J.; Romagnan, J.-B.; Picheral, M.; Pearlman, J.; et al. Globally Consistent Quantitative Observations of Planktonic Ecosystems. Front. Mar. Sci. 2019, 6, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Harrison, P.J.; Whitney, F.A.; Tsuda, A.; Saito, H.; Tadokoro, K. Nutrient and Plankton Dynamics in the NE and NW Gyres of the Subarctic Pacific Ocean. J. Oceanogr. 2004, 60, 93–117. [Google Scholar] [CrossRef]
- Booth, B.C.; Lewin, J.; Postel, J.R. Temporal Variation in the Structure of Autotrophic and Heterotrophic Communities in the Subarctic Pacific. Prog. Oceanogr. 1993, 32, 57–99. [Google Scholar] [CrossRef]
- Peterson, T.D.; Harrison, P.J. Diatom Dynamics in a Long-Lived Mesoscale Eddy in the Northeast Subarctic Pacific Ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2012, 65, 157–170. [Google Scholar] [CrossRef]
- Obayashi, Y.; Tanoue, E.; Suzuki, K.; Handa, N.; Nojiri, Y.; Wong, C.S. Spatial and Temporal Variabilities of Phytoplankton Community Structure in the Northern North Pacific as Determined by Phytoplankton Pigments. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2001, 48, 439–469. [Google Scholar] [CrossRef]
- Suzuki, K.; Minami, C.; Liu, H.; Saino, T. Temporal and Spatial Patterns of Chemotaxonomic Algal Pigments in the Subarctic Pacific and the Bering Sea during the Early Summer of 1999. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5685–5704. [Google Scholar] [CrossRef]
- Fujiki, T.; Matsumoto, K.; Honda, M.C.; Kawakami, H.; Watanabe, S. Phytoplankton Composition in the Subarctic North Pacific during Autumn 2005. J. Plankton Res. 2009, 31, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Peterson, T.D.; Crawford, D.W.; Harrison, P.J. Evolution of the Phytoplankton Assemblage in a Long-Lived Mesoscale Eddy in the Eastern Gulf of Alaska. Mar. Ecol. Prog. Ser. 2011, 424, 53–73. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Emerson, S.R.; Penã, M.A. The Effect of the 2013–2016 High Temperature Anomaly in the Subarctic Northeast Pacific (the “Blob”) on Net Community Production. Biogeosciences 2018, 15, 6747–6759. [Google Scholar] [CrossRef] [Green Version]
- Peña, M.A.; Nemcek, N.; Robert, M. Phytoplankton Responses to the 2014–2016 Warming Anomaly in the Northeast Subarctic Pacific Ocean. Limnol. Oceanogr. 2019, 64, 515–525. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Xiu, P.; Chai, F. Modeling the Seasonal Variability of Phytoplankton in the Subarctic Northeast Pacific Ocean. Mar. Ecol. Prog. Ser. 2021, 680, 33–50. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Aiken, J.; Alvain, S.; Barlow, R.; Bouman, H.; Bracher, A.; Brewin, R.J.W.; Bricaud, A.; Brown, C.W.; Ciotti, A.M.; et al. Phytoplankton Functional Types from Space; Report of the International Ocean-Colour Coordinating Group (IOCCG), 15; International Ocean-Colour Coordinating Group: Dartmouth, NS, Canada, 2014. [Google Scholar]
- Vinogradov, M.E.; Shushkina, E.A.; Vedernikov, V.I.; Nezlin, N.P.; Gagarin, V.I. Primary Production and Plankton Stocks in the Pacific Ocean and Their Seasonal Variation According to Remote Sensing and Field Observations. Deep. Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1979–2001. [Google Scholar] [CrossRef]
- EUMETSAT. Sentinel-3 OLCI Marine User Handbook 2018; EUMETSAT: Darmstadt, Germany, 2018; ISBN 4961518077. [Google Scholar]
- Weingartner, T. The Physical Environment of the Gulf of Alaska, 1st ed.; Spies, R.B., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780444529602. [Google Scholar]
- Weingartner, T.; Eisner, L.; Eckert, G.L.; Danielson, S. Southeast Alaska: Oceanographic Habitats and Linkages. J. Biogeogr. 2009, 36, 387–400. [Google Scholar] [CrossRef]
- Peña, M.A.; Varela, D.E. Seasonal and Interannual Variability in Phytoplankton and Nutrient Dynamics along Line P in the NE Subarctic Pacific. Prog. Oceanogr. 2007, 75, 200–222. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wang, Y.; Xiu, P.; Qi, Y.; Chai, F. Roles of Iron Limitation in Phytoplankton Dynamics in the Western and Eastern Subarctic Pacific. Front. Mar. Sci. 2021, 8, 735826. [Google Scholar] [CrossRef]
- Welschmeyer, N.A.; Strom, S.; Goericke, R.; DiTullio, G.; Belvin, M.; Petersen, W. Primary Production in the Subarctic Pacific Ocean: Project SUPER. Prog. Oceanogr. 1993, 32, 101–135. [Google Scholar] [CrossRef]
- Westberry, T.K.; Schultz, P.; Behrenfeld, M.J.; Dunne, J.P.; Hiscock, M.R.; Maritorena, S.; Sarmiento, J.L.; Siegel, D.A. Annual Cycles of Phytoplankton Biomass in the Subarctic Atlantic and Pacific Ocean. Glob. Biogeochem. Cycles 2016, 30, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Marchese, C.; Hunt, B.P.V.; Giannini, F.; Ehrler, M.; Costa, M. Bioregionalization of the Coastal and Open Oceans of British Columbia and Southeast Alaska Based on Sentinel-3A Satellite-Derived Phytoplankton Seasonality. Front. Mar. Sci. 2022, 9, 968470. [Google Scholar] [CrossRef]
- Weingartner, T.J.; Danielson, S.L.; Royer, T.C. Freshwater Variability and Predictability in the Alaska Coastal Current. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 169–191. [Google Scholar] [CrossRef]
- Thomson, R.E.; Beamish, R.J.; Beacham, T.D.; Trudel, M.; Whitfield, P.H.; Hourston, R.A.S. Anomalous Ocean Conditions May Explain the Recent Extreme Variability in Fraser River Sockeye Salmon Production. Mar. Coast. Fish. Dyn. Manag. Ecosyst. Sci. 2012, 4, 415–437. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.M.; Thomson, R.E.; Brown, L.N.; Willis, P.G.; Borstad, G.A. Satellite Chlorophyll off the British Columbia Coast, 1997–2010. J. Geophys. Res. Ocean. 2015, 120, 4709–4728. [Google Scholar] [CrossRef]
- Peterson, T.D.; Toews, H.N.J.; Robinson, C.L.K.; Harrison, P.J. Nutrient and Phytoplankton Dynamics in the Queen Charlotte Islands (Canada) during the Summer Upwelling Seasons of 2001–2002. J. Plankton Res. 2007, 29, 219–239. [Google Scholar] [CrossRef] [Green Version]
- Mueller, J.L.; Bidigare, R.R.; Trees, C.; Balch, W.M.; Dore, J.; Drapeau, D.T.; Karl, D.M.; Van Heukelem, L.; Perl, J. Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, Revision 5: Biogeochemical and Bio-Optical Measurements and Data Analysis Protocols. Mueller, J.L., Fargion, G.S., McClain, C.R., Eds.; Goddard Space Flight Space Center: Greenbelt, MD, USA, 2003; Volume 5. [Google Scholar]
- Pinckney, J.L. The USC Method. In The Fourth SeaWiFS HPLC Analysis Round-Robin Experiment (SeaHARRE-4); NASA: City, Country, 2010. [Google Scholar]
- Mobley, C.D. Estimation of the Remote-Sensing Reflectance from above-Surface Measurements. Appl. Opt. 1999, 38, 7442–7455. [Google Scholar] [CrossRef]
- Hooker, S.B.; Morel, A. Platform and Environmental Effects on Above-Water Determinations of Water-Leaving Radiances. J. Atmos. Ocean. Technol. 2003, 20, 187–205. [Google Scholar] [CrossRef]
- Ruddick, K.; De Cauwer, V.; Van Mol, B. Use of the Near Infrared Similarity Reflectance Spectrum for the Quality Control of Remote Sensing Data. In Remote Sensing of the Coastal Oceanic Environment; Society of Photo-optical Instrumentation Engineers: Bellingham, WA, USA, 2005; p. 588501. [Google Scholar]
- Ruddick, K.G.; De Cauwer, V.; Park, Y.-J.; Moore, G. Seaborne Measurements of Near Infrared Water-Leaving Reflectance: The Similarity Spectrum for Turbid Waters. Limnol. Oceanogr. 2006, 51, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Donlon, C.; Berruti, B.; Buongiorno, A.; Ferreira, M.-H.; Féménias, P.; Frerick, J.; Goryl, P.; Klein, U.; Laur, H.; Mavrocordatos, C.; et al. The Global Monitoring for Environment and Security (GMES) Sentinel-3 mission. Remote Sens. Environ. 2012, 120, 37–57. [Google Scholar] [CrossRef]
- Giannini, F.; Hunt, B.P.V.; Jacoby, D.; Costa, M. Performance of OLCI Sentinel-3A Satellite in the Northeast Pacific Coastal Waters. Remote Sens. Environ. 2021, 256, 112317. [Google Scholar] [CrossRef]
- Steinmetz, F.; Deschamps, P.-Y.; Ramon, D. Atmospheric Correction in Presence of Sun Glint: Application to MERIS. Opt. Express 2011, 19, 9783–9800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmetz, F.; Ramon, D.; Deschamps, P.-Y. ATBD v1—Polymer Atmospheric Correction Algorithm Ref: D2.3 Date: 23/12/2016 Issue: 2.1. PML, United Kingdom 2016. Available online: https://docs.pml.space/share/s/M05k8Lw3QLeXSIiA3X87UQ (accessed on 10 April 2023).
- Scott, J.P.; Werdell, P.J. Comparing Level-2 and Level-3 Satellite Ocean Color Retrieval Validation Methodologies. Opt. Express 2019, 27, 30140–30157. [Google Scholar] [CrossRef] [PubMed]
- EUMETSAT. REF: SOLVO/EUM/16/VCA/D8. ISSUE: 1.3. July 2017. In Sentinel-3 OLCI Marine User Handbook; EUMETSAT: Darmstadt, Germany, 2017. [Google Scholar]
- Antoine, D.; Morel, A. A Multiple Scattering Algorithm for Atmospheric Correction of Remotely Sensed Ocean Colour (MERIS Instrument): Principle and Implementation for Atmospheres Carrying Various Aerosols Including Absorbing Ones. Int. J. Remote Sens. 1999, 20, 1875–1916. [Google Scholar] [CrossRef]
- Moore, G.F.; Aiken, J.; Lavender, S.J. The Atmospheric Correction of Water Colour and the Quantitative Retrieval of Suspended Particulate Matter in Case II Waters: Application to MERIS. Int. J. Remote. Sens. 1999, 20, 1713–1733. [Google Scholar] [CrossRef]
- Moore, G.F.; Mazeran, C.; Huot, J.P. Case II.S Bright Pixel Atmospheric Correction. Eur. Space Agency 2017, 5. [Google Scholar]
- Tilstone, G.H.; Pardo, S.; Dall’Olmo, G.; Brewin, R.J.W.; Nencioli, F.; Dessailly, D.; Kwiatkowska, E.; Casal, T.; Donlon, C. Performance of Ocean Colour Chlorophyll a Algorithms for Sentinel-3 OLCI, MODIS-Aqua and Suomi-VIIRS in open-ocean waters of the Atlantic. Remote Sens. Environ. 2021, 260, 112444. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. 2020. Available online: https://CRAN.R-project.org/package=raster (accessed on 22 January 2022).
- Mograne, M.A.; Jamet, C.; Loisel, H.; Vantrepotte, V.; Mériaux, X.; Cauvin, A. Evaluation of Five Atmospheric Correction Algorithms over French Optically-Complex Waters for the Sentinel-3A OLCI Ocean Color Sensor. Remote Sens. 2019, 11, 668. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W.; Werdell, P.J. A Multi-Sensor Approach for the on-Orbit Validation of Ocean Color Satellite Data Products. Remote Sens. Environ. 2006, 102, 12–23. [Google Scholar] [CrossRef]
- Pramlall, S.; Jackson, J.M.; Konik, M.; Costa, M. Merged Multi-Sensor Ocean Colour Chlorophyll Product Evaluation for the British Columbia Coast. Remote Sens. 2023, 15, 687. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Lee, Z. Uncertainties of SeaWiFS and MODIS Remote Sensing Reflectance: Implications from Clear Water Measurements. Remote Sens. Environ. 2013, 133, 168–182. [Google Scholar] [CrossRef]
- Gilerson, A.; Herrera-Estrella, E.; Foster, R.; Agagliate, J.; Hu, C.; Ibrahim, A.; Franz, B. Determining the Primary Sources of Uncertainty in Retrieval of Marine Remote Sensing Reflectance From Satellite Ocean Color Sensors. Front. Remote. Sens. 2022, 3, 1–19. [Google Scholar] [CrossRef]
- Zibordi, G.; Ruddick, K.; Ansko, I.; Moore, G.; Kratzer, S.; Icely, J.; Reinart, A. In Situ Determination of the Remote Sensing Reflectance: An Inter-Comparison. Ocean Sci. 2012, 8, 567–586. [Google Scholar] [CrossRef] [Green Version]
- Vabson, V.; Kuusk, J.; Ansko, I.; Vendt, R.; Alikas, K.; Ruddick, K.; Ansper, A.; Bresciani, M.; Burmester, H.; Costa, M.; et al. Laboratory Intercomparison of Radiometers Used for Satellite Validation in the 400–900 nm Range. Remote Sens. 2019, 11, 1101. [Google Scholar] [CrossRef] [Green Version]
- Vabson, V.; Kuusk, J.; Ansko, I.; Vendt, R.; Alikas, K.; Ruddick, K.; Ansper, A.; Bresciani, M.; Burmester, H.; Costa, M.; et al. Field Intercomparison of Radiometers Used for Satellite Validation in the 400–900 nm Range. Remote Sens. 2019, 11, 1129. [Google Scholar] [CrossRef] [Green Version]
- Ruddick, K.G.; Voss, K.; Banks, A.C.; Boss, E.; Castagna, A.; Frouin, R.; Hieronymi, M.; Jamet, C.; Johnson, B.C.; Kuusk, J.; et al. A Review of Protocols for Fiducial Reference Measurements of Downwelling Irradiance for the Validation of Satellite Remote Sensing Data over Water. Remote Sens. 2019, 11, 1742. [Google Scholar] [CrossRef] [Green Version]
- Tilstone, G.; Dall’Olmo, G.; Hieronymi, M.; Ruddick, K.; Beck, M.; Ligi, M.; Costa, M.; D’Alimonte, D.; Vellucci, V.; Vansteenwegen, D.; et al. Field Intercomparison of Radiometer Measurements for Ocean Colour Validation. Remote Sens. 2020, 12, 1587. [Google Scholar] [CrossRef]
- Lin, J.; Dall’Olmo, G.; Tilstone, G.H.; Brewin, R.J.W.; Vabson, V.; Ansko, I.; Evers-King, H.; Casal, T.; Donlon, C. Derivation of Uncertainty Budgets for Continuous Above-Water Radiometric Measurements along an Atlantic Meridional Transect. Opt. Express 2022, 30, 45648–45675. [Google Scholar] [CrossRef]
- Morel, A.; Gentili, B. Diffuse Reflectance of Oceanic Waters III Implication of Bidirectionality for the Remote-Sensing Problem. Appl. Opt. 1996, 35, 4850–4862. [Google Scholar] [CrossRef] [PubMed]
- Sá, C.; D’Alimonte, D.; Brito, A.C.; Kajiyama, T.; Mendes, C.R.; Vitorino, J.; Oliveira, P.B.; da Silva, J.C.B.; Brotas, V. Validation of Standard and Alternative Satellite Ocean-Color Chlorophyll Products off Western Iberia. Remote Sens. Environ. 2015, 168, 403–419. [Google Scholar] [CrossRef]
- Meyer, M.G.; Gong, W.; Kafrissen, S.M.; Torano, O.; Varela, D.E.; Santoro, A.E.; Cassar, N.; Gifford, S.; Niebergall, A.K.; Sharpe, G.; et al. Phytoplankton Size-Class Contributions to New and Regenerated Production during the EXPORTS Northeast Pacific Ocean Field Deployment. Elem. Sci. Anthr. 2022, 10, 00068. [Google Scholar] [CrossRef]
- Bracher, A.; Bouman, H.A.; Brewin, R.J.W.; Bricaud, A.; Brotas, V.; Ciotti, A.M.; Clementson, L.; Devred, E.; Di Cicco, A.; Dutkiewicz, S.; et al. Obtaining Phytoplankton Diversity from Ocean Color: A Scientific Roadmap for Future Development. Front. Mar. Sci. 2017, 4, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Van Heukelem, L.; Hooker, S.B. The Importance of a Quality Assurance Plan for Method Validation and Minimizing Uncertainties in the HPLC Analysis of Phytoplankton Pigments. In Phytoplankton pigments: Characterization, Chemotaxonomy, and Applications in Oceanography; Suzanne, R., Llewellyn, C.A., Egeland, E.S., Ohnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 195–242. ISBN 978-1-107-00066-7. [Google Scholar]
- Claustre, H.; Hooker, S.B.; Van Heukelem, L.; Berthon, J.-F.; Barlow, R.; Ras, J.; Sessions, H.; Targa, C.; Thomas, C.S.; Van Der Linde, D.; et al. An Intercomparison of HPLC Phytoplankton Pigment Methods Using in Situ Samples: Application to Remote Sensing and Database Activities. Mar. Chem. 2004, 85, 41–61. [Google Scholar] [CrossRef]
- Ribalet, F.; Marchetti, A.; Hubbard, K.A.; Brown, K.; Durkin, C.A.; Morales, R.; Robert, M.; Swalwell, J.E.; Tortell, P.D.; Armbrust, E.V. Unveiling a Phytoplankton Hotspot at a Narrow Boundary between Coastal and Offshore Waters. Proc. Natl. Acad. Sci. USA 2010, 107, 16571–16576. [Google Scholar] [CrossRef] [Green Version]
- Landry, M.R.; Monger, B.C.; Selph, K.E. Time-Dependency of Microzooplankton Grazing and Phytoplankton Growth in the Subarctic Pacific. Prog. Oceanogr. 1993, 32, 205–222. [Google Scholar] [CrossRef]
- Suchy, K.D.; Le Baron, N.; Hilborn, A.; Perry, R.I.; Costa, M. Influence of Environmental Drivers on Spatio-Temporal Dynamics of Satellite-Derived Chlorophyll a in the Strait of Georgia. Prog. Oceanogr. 2019, 176, 102134. [Google Scholar] [CrossRef]
- Strom, S.L.; Olson, M.B.; Macri, E.L.; Mordy, C.W. Cross-Shelf Gradients in Phytoplankton Community Structure, Nutrient Utilization, and Growth Rate in the Coastal Gulf of Alaska. Mar. Ecol. Prog. Ser. 2006, 328, 75–92. [Google Scholar] [CrossRef] [Green Version]
- Henson, S.A. Water Column Stability and Spring Bloom Dynamics in the Gulf of Alaska. J. Mar. Res. 2007, 65, 715–736. [Google Scholar] [CrossRef]
- Malick, M.J.; Cox, S.P.; Mueter, F.J.; Peterman, R.M. Linking Phytoplankton Phenology to Salmon Productivity along a North-South Gradient in the Northeast Pacific Ocean. Can. J. Fish. Aquat. Sci. 2015, 72, 697–708. [Google Scholar] [CrossRef]
- Suchy, K.D.; Young, K.; Galbraith, M.; Perry, R.I.; Costa, M. Match/Mismatch Between Phytoplankton and Crustacean Zooplankton Phenology in the Strait of Georgia, Canada. Front. Mar. Sci. 2022, 9, 1–21. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vishnu, P.S.; Costa, M. Evaluating the Performance of Sentinel-3A OLCI Products in the Subarctic Northeast Pacific. Remote Sens. 2023, 15, 3244. https://doi.org/10.3390/rs15133244
Vishnu PS, Costa M. Evaluating the Performance of Sentinel-3A OLCI Products in the Subarctic Northeast Pacific. Remote Sensing. 2023; 15(13):3244. https://doi.org/10.3390/rs15133244
Chicago/Turabian StyleVishnu, Perumthuruthil Suseelan, and Maycira Costa. 2023. "Evaluating the Performance of Sentinel-3A OLCI Products in the Subarctic Northeast Pacific" Remote Sensing 15, no. 13: 3244. https://doi.org/10.3390/rs15133244
APA StyleVishnu, P. S., & Costa, M. (2023). Evaluating the Performance of Sentinel-3A OLCI Products in the Subarctic Northeast Pacific. Remote Sensing, 15(13), 3244. https://doi.org/10.3390/rs15133244