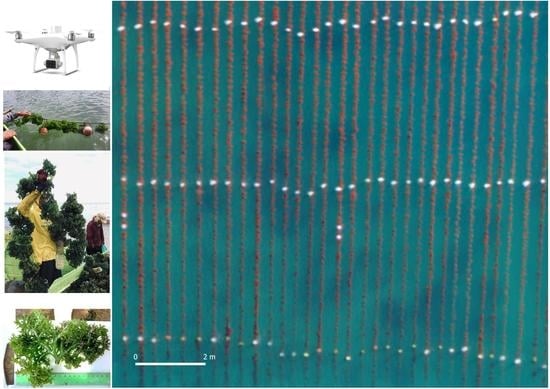
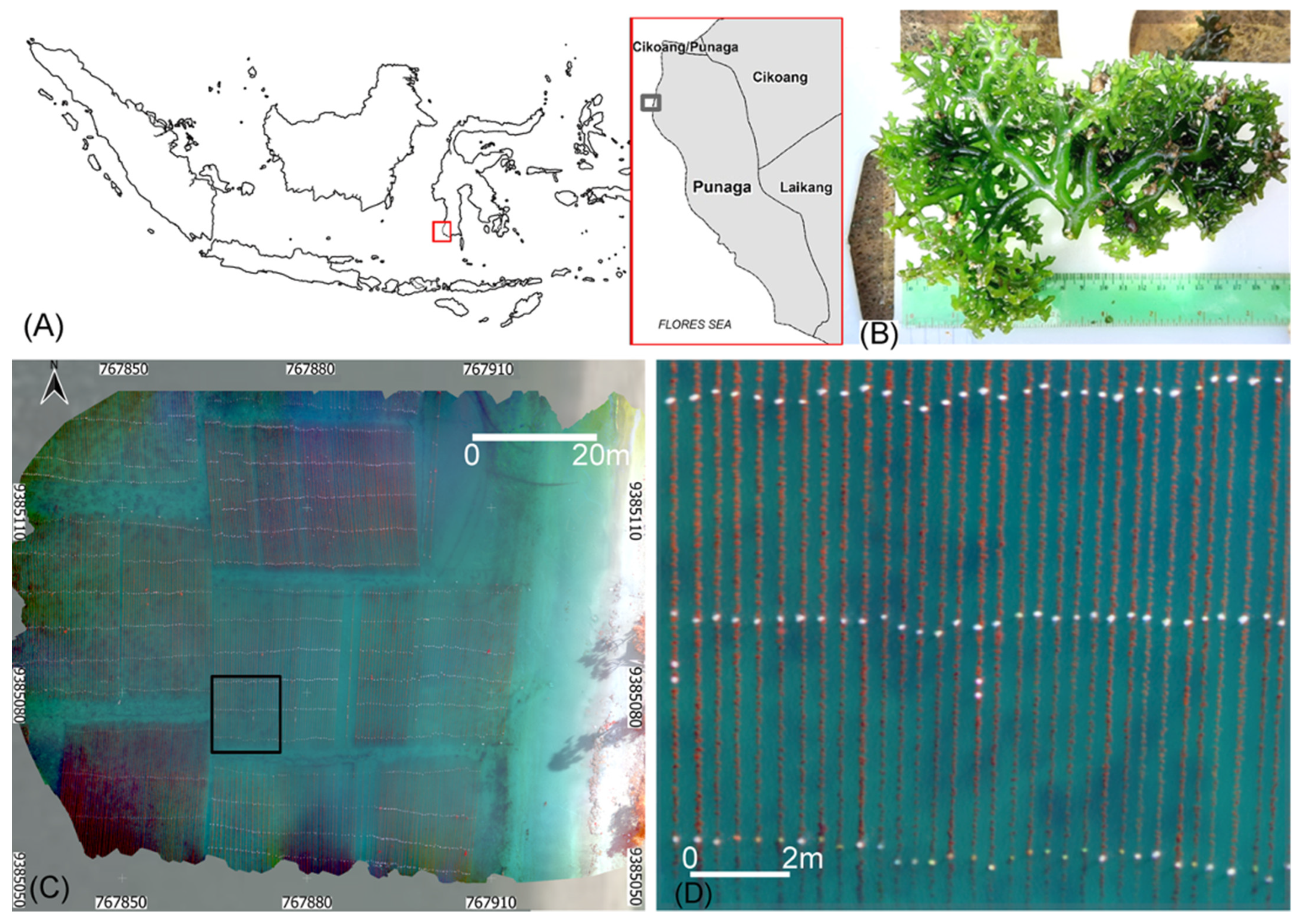
Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation, Biometry and Carrageenan Content
2.2. UAV Data Acquisition
2.3. UAV Image Processing
2.4. Image Classification
2.4.1. Algorithm Introduction
2.4.2. Training/Validation Data
2.4.3. Random Forest Implementation
2.4.4. Classification Accuracy Assessment
2.5. Geospatial Analyses
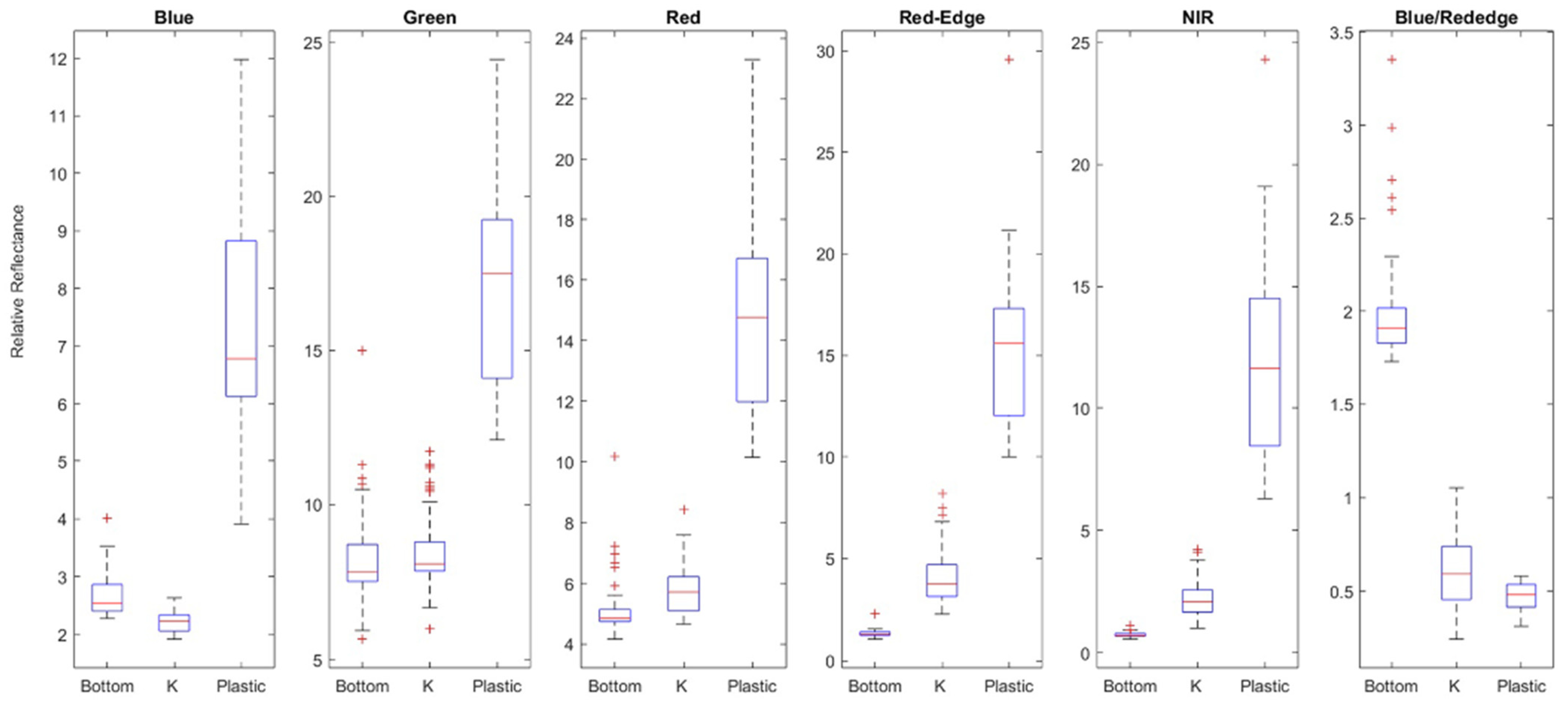
2.6. Statistical Analysis
3. Results
3.1. Biometry and Carrageenan Content
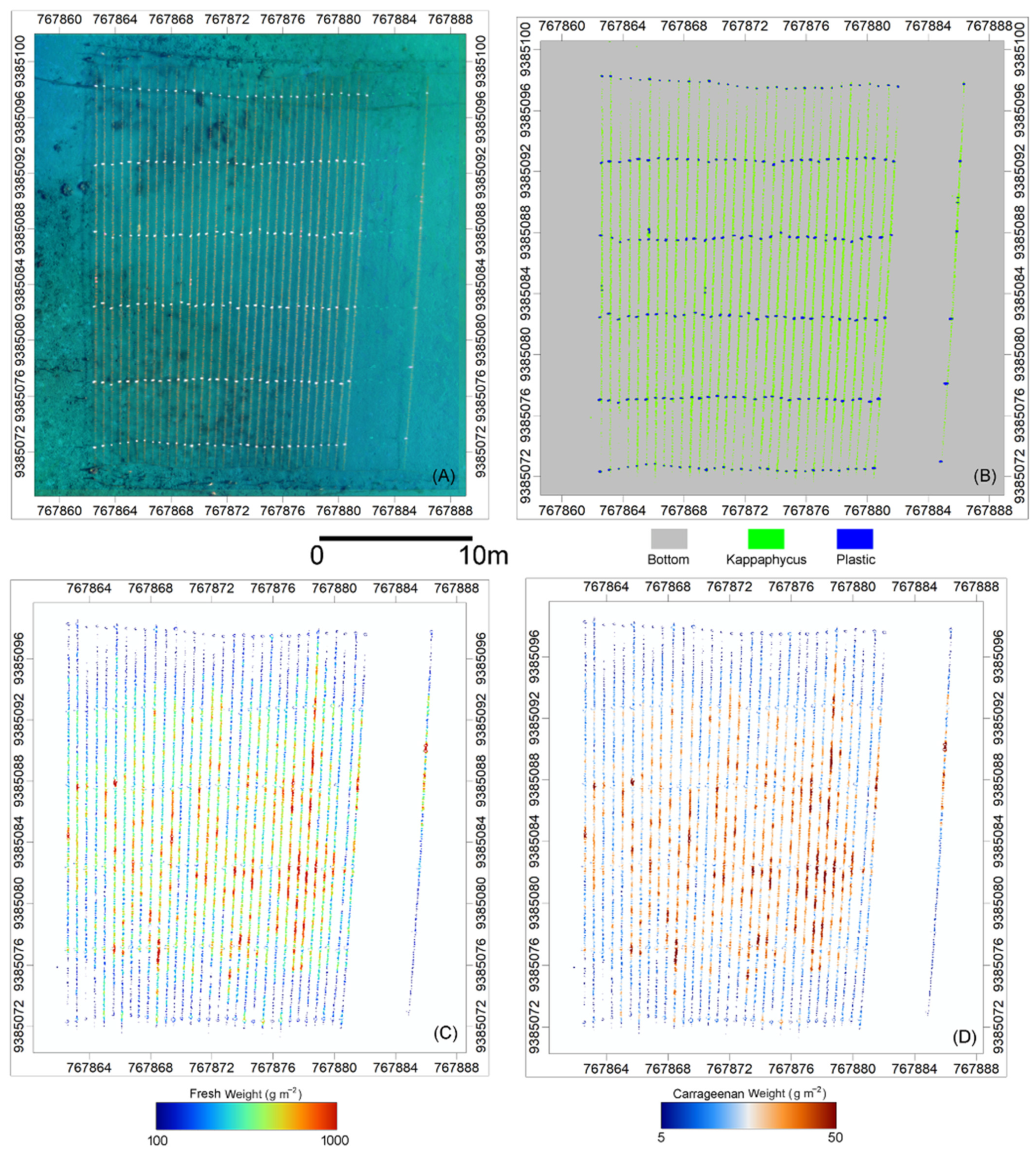
3.2. Random Forest Classification
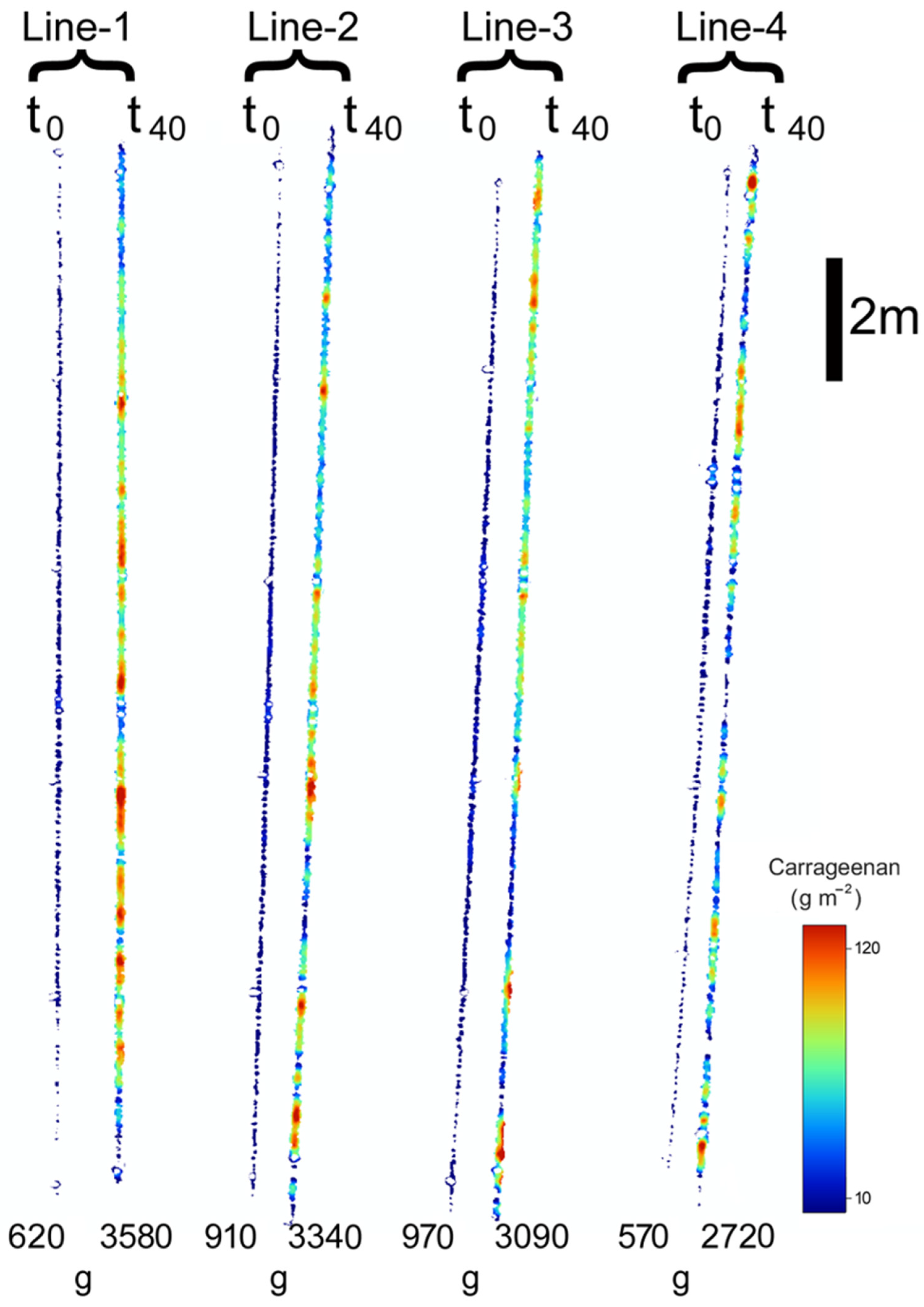
3.3. Spatial Distribution of Fresh Weight and Carrageenan
4. Discussion
4.1. Drone-Based Detection of Kappaphycus at the Scale of Cultivation Lines
4.2. Estimation of Biomass, Carrageenan and Production
4.3. Sources of Error and Constraints
4.4. Implications for Kappaphycus Aquaculture Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Cai, J. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development; FAO Fisheries and Aquaculture Circular; FAO: Rome, Italy, 2021; ISBN 978-92-5-134710-2. [Google Scholar]
- Rimmer, M.A.; Larson, S.; Lapong, I.; Purnomo, A.H.; Pong-Masak, P.R.; Swanepoel, L.; Paul, N.A. Seaweed Aquaculture in Indonesia Contributes to Social and Economic Aspects of Livelihoods and Community Wellbeing. Sustainability 2021, 13, 10946. [Google Scholar] [CrossRef]
- Rimmer, M.A.; Sugama, K.; Rakhmawati, D.; Rofiq, R.; Habgood, R.H. A Review and SWOT Analysis of Aquaculture Development in Indonesia. Rev. Aquac. 2013, 5, 255–279. [Google Scholar] [CrossRef]
- Gentry, R.R.; Froehlich, H.E.; Grimm, D.; Kareiva, P.; Parke, M.; Rust, M.; Gaines, S.D.; Halpern, B.S. Mapping the Global Potential for Marine Aquaculture. Nat. Ecol. Evol. 2017, 1, 1317–1324. [Google Scholar] [CrossRef]
- MMAF-JICA Indonesia Marine and Fisheries Book; Ministry of Marine Affairs and Fisheries & Japan International Cooperation Agency: Tokyo, Japan, 2017.
- Nuryartono, N.; Waldron, S.; Tarman, K.; Siregar, U.; Pasaribu, S.; Langford, Z.; Lusno, M.; Sulfahri, S. An Analysis of the South Sulawesi Seaweed Industry; University of Queensland: Brisbane, Australia, 2021. [Google Scholar]
- Simatupang, N.F.; Pong-Masak, P.R.; Ratnawati, P.; Agusman; Paul, N.A.; Rimmer, M.A. Growth and Product Quality of the Seaweed Kappaphycus Alvarezii from Different Farming Locations in Indonesia. Aquac. Rep. 2021, 20, 100685. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.-E.; Phang, S.-M.; Hurtado, A.Q. Biodiversity, Biogeography and Molecular Genetics of the Commercially Important Genera Kappaphycus and Eucheuma. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Developments in Applied, Phycology; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–43. ISBN 978-3-319-63498-2. [Google Scholar]
- Hurtado, A.Q.; Critchley, A.T.; Neish, I.C. (Eds.) Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-63497-5. [Google Scholar]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N. (Eds.) Social and Economic Dimensions of Carrageenan Seaweed Farming; Fisheries and Aquaculture Technical Paper No. 580; FAO: Rome, Italy, 2013; p. 204. ISBN 978-92-5-107746-7. [Google Scholar]
- Hayashi, L.; Reis, R.P.; dos Santos, A.A.; Castelar, B.; Robledo, D.; de Vega, G.B.; Msuya, F.E.; Eswaran, K.; Yasir, S.M.D.; Ali, M.K.M.; et al. The Cultivation of Kappaphycus and Eucheuma in Tropical and Sub-Tropical Waters. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Developments in Applied Phycology; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–90. ISBN 978-3-319-63498-2. [Google Scholar]
- Syamsuddin, R. Seaweed Kappaphycus Alvarezii Cultivation for Seagrass Ecosystem Conservation; IntechOpen: London, UK, 2023; ISBN 978-1-83968-460-9. [Google Scholar]
- Ward, G.M.; Kambey, C.S.B.; Faisan, J.P.; Tan, P.; Daumich, C.C.; Matoju, I.; Stentiford, G.D.; Bass, D.; Lim, P.; Brodie, J.; et al. Ice-Ice Disease: An Environmentally and Microbiologically Driven Syndrome in Tropical Seaweed Aquaculture. Rev. Aquac. 2021, 14, 414–439. [Google Scholar] [CrossRef]
- Ateweberhan, M.; Rougier, A.; Rakotomahazo, C. Influence of Environmental Factors and Farming Technique on Growth and Health of Farmed Kappaphycus Alvarezii (Cottonii) in South-West Madagascar. J. Appl. Phycol. 2015, 27, 923–934. [Google Scholar] [CrossRef]
- Kumar, Y.N.; Poong, S.-W.; Gachon, C.; Brodie, J.; Sade, A.; Lim, P.-E. Impact of Elevated Temperature on the Physiological and Biochemical Responses of Kappaphycus Alvarezii (Rhodophyta). PLoS ONE 2020, 15, e0239097. [Google Scholar] [CrossRef]
- Neish, I.C. Monograph No. HB2F 1008 V3 GAP; SEAPlant.net: Kuala Lumpur, Malaysia, 2008. [Google Scholar]
- Setyawidati, N.; Liabot, P.O.; Perrot, T.; Radiarta, N.; Deslandes, E.; Bourgougnon, N.; Rossi, N.; Stiger-Pouvreau, V. In Situ Variability of Carrageenan Content and Biomass in the Cultivated Red Macroalga Kappaphycus Alvarezii with an Estimation of Its Carrageenan Stock at the Scale of the Malasoro Bay (Indonesia) Using Satellite Image Processing. J. Appl. Phycol. 2017, 29, 2307–2321. [Google Scholar] [CrossRef]
- Langford, A.; Waldron, S.; Sulfahri; Saleh, H. Monitoring the COVID-19-Affected Indonesian Seaweed Industry Using Remote Sensing Data. Mar. Policy 2021, 127, 104431. [Google Scholar] [CrossRef]
- Eugenio, F.; Marcello, J.; Martin, J.; Rodríguez-Esparragón, D. Benthic Habitat Mapping Using Multispectral High-Resolution Imagery: Evaluation of Shallow Water Atmospheric Correction Techniques. Sensors 2017, 17, 2639. [Google Scholar] [CrossRef] [Green Version]
- Kutser, T.; Hedley, J.; Giardino, C.; Roelfsema, C.; Brando, V.E. Remote Sensing of Shallow Waters—A 50 Year Retrospective and Future Directions. Remote Sens. Environ. 2020, 240, 111619. [Google Scholar] [CrossRef]
- Phinn, S.; Roelfsema, C.; Dekker, A.; Brando, V.; Anstee, J. Mapping Seagrass Species, Cover and Biomass in Shallow Waters: An Assessment of Satellite Multi-Spectral and Airborne Hyper-Spectral Imaging Systems in Moreton Bay (Australia). Remote Sens. Environ. 2008, 112, 3413–3425. [Google Scholar] [CrossRef]
- Rossiter, T.; Furey, T.; McCarthy, T.; Stengel, D.B. UAV-Mounted Hyperspectral Mapping of Intertidal Macroalgae. Estuar. Coast. Shelf Sci. 2020, 242, 106789. [Google Scholar] [CrossRef]
- Tait, L.; Bind, J.; Charan-Dixon, H.; Hawes, I.; Pirker, J.; Schiel, D. Unmanned Aerial Vehicles (UAVs) for Monitoring Macroalgal Biodiversity: Comparison of RGB and Multispectral Imaging Sensors for Biodiversity Assessments. Remote Sens. 2019, 11, 2332. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, S.; Byfield, V.; Felgate, S.L.; Price, D.M.; Andrade, V.; Cobb, E.; Strong, J.; Lichtschlag, A.; Brittain, H.; Barry, C.; et al. Using Unoccupied Aerial Vehicles (UAVs) to Map Seagrass Cover from Sentinel-2 Imagery. Remote Sens. 2022, 14, 477. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Cavanaugh, K.C.; Bell, T.W.; Hockridge, E.G. An Automated Method for Mapping Giant Kelp Canopy Dynamics from UAV. Front. Environ. Sci. 2021, 8, 587354. [Google Scholar] [CrossRef]
- Hamad, I.Y.; Staehr, P.A.U.; Rasmussen, M.B.; Sheikh, M. Drone-Based Characterization of Seagrass Habitats in the Tropical Waters of Zanzibar. Remote Sens. 2022, 14, 680. [Google Scholar] [CrossRef]
- Borges, D.; Padua, L.; Azevedo, I.C.; Silva, J.; Sousa, J.J.; Sousa–Pinto, I.; Gonçalves, J.A. Classification of an Intertidal Reef by Machine Learning Techniques Using UAV Based RGB and Multispectral Imagery. In Proceedings of the 2021 IEEE International Geoscience and Remote Sensing Symposium IGARSS, Brussels, Belgium, 11–14 July 2021; pp. 64–67. [Google Scholar]
- Murfitt, S.L.; Allan, B.M.; Bellgrove, A.; Rattray, A.; Young, M.A.; Ierodiaconou, D. Applications of Unmanned Aerial Vehicles in Intertidal Reef Monitoring. Sci. Rep. 2017, 7, 10259. [Google Scholar] [CrossRef] [Green Version]
- Hu, C. A Novel Ocean Color Index to Detect Floating Algae in the Global Oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Davies, B.F.R.; Gernez, P.; Geraud, A.; Oiry, S.; Rosa, P.; Zoffoli, M.L.; Barillé, L. Multi- and Hyperspectral Classification of Soft-Bottom Intertidal Vegetation Using a Spectral Library for Coastal Biodiversity Remote Sensing. Remote Sens. Environ. 2023, 290, 113554. [Google Scholar] [CrossRef]
- Román, A.; Tovar-Sánchez, A.; Olivé, I.; Navarro, G. Using a UAV-Mounted Multispectral Camera for the Monitoring of Marine Macrophytes. Front. Mar. Sci. 2021, 8, 722698. [Google Scholar] [CrossRef]
- Bell, T.W.; Nidzieko, N.J.; Siegel, D.A.; Miller, R.J.; Cavanaugh, K.C.; Nelson, N.B.; Reed, D.C.; Fedorov, D.; Moran, C.; Snyder, J.N.; et al. The Utility of Satellites and Autonomous Remote Sensing Platforms for Monitoring Offshore Aquaculture Farms: A Case Study for Canopy Forming Kelps. Front. Mar. Sci. 2020, 7, 520223. [Google Scholar] [CrossRef]
- Che, S.; Du, G.; Wang, N.; He, K.; Mo, Z.; Sun, B.; Chen, Y.; Cao, Y.; Wang, J.; Mao, Y. Biomass Estimation of Cultivated Red Algae Pyropia Using Unmanned Aerial Platform Based Multispectral Imaging. Plant Methods 2021, 17, 12. [Google Scholar] [CrossRef]
- Ubina, N.A.; Cheng, S.-C. A Review of Unmanned System Technologies with Its Application to Aquaculture Farm Monitoring and Management. Drones 2022, 6, 12. [Google Scholar] [CrossRef]
- Xing, Q.; An, D.; Zheng, X.; Wei, Z.; Wang, X.; Li, L.; Tian, L.; Chen, J. Monitoring Seaweed Aquaculture in the Yellow Sea with Multiple Sensors for Managing the Disaster of Macroalgal Blooms. Remote Sens. Environ. 2019, 231, 111279. [Google Scholar] [CrossRef]
- Zhang, C.; Kovacs, J.M. The Application of Small Unmanned Aerial Systems for Precision Agriculture: A Review. Precis. Agric. 2012, 13, 693–712. [Google Scholar] [CrossRef]
- Radoglou-Grammatikis, P.; Sarigiannidis, P.; Lagkas, T.; Moscholios, I. A Compilation of UAV Applications for Precision Agriculture. Comput. Netw. 2020, 172, 107148. [Google Scholar] [CrossRef]
- Dawes, C.J.; Trono, G.C.; Lluisma, A.O. Clonal Propagation of Eucheuma Denticulatum and Kappaphycus Alvarezii for Philippine Seaweed Farms. Hydrobiologia 1993, 260–261, 379–383. [Google Scholar] [CrossRef]
- Winarno, F.G. Teknologi Pengolahan Rumput Laut; Jakarta Pustaka Sinar Harapan: East Jakarta, Indonesia, 1990; p. 112. [Google Scholar]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for Automated Geoscientific Analyses (SAGA) v. 2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Belgiu, M.; Drăguţ, L. Random Forest in Remote Sensing: A Review of Applications and Future Directions. ISPRS J. Photogramm. Remote Sens. 2016, 114, 24–31. [Google Scholar] [CrossRef]
- Brunier, G.; Oiry, S.; Gruet, Y.; Dubois, S.F.; Barillé, L. Topographic Analysis of Intertidal Polychaete Reefs (Sabellaria Alveolata) at a Very High Spatial Resolution. Remote Sens. 2022, 14, 307. [Google Scholar] [CrossRef]
- Oiry, S.; Barillé, L. Using Sentinel-2 Satellite Imagery to Develop Microphytobenthos-Based Water Quality Indices in Estuaries. Ecol. Indic. 2021, 121, 107184. [Google Scholar] [CrossRef]
- Stephens, D.; Diesing, M. A Comparison of Supervised Classification Methods for the Prediction of Substrate Type Using Multibeam Acoustic and Legacy Grain-Size Data. PLoS ONE 2014, 9, e93950. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, R.; Kim, K.; Zhang, J.; Cui, T. A Random Forest-Based Algorithm to Distinguish Ulva Prolifera and Sargassum from Multispectral Satellite Images. IEEE Trans. Geosci. Remote Sens. 2022, 60, 1–15. [Google Scholar] [CrossRef]
- Zhang, C. Applying Data Fusion Techniques for Benthic Habitat Mapping and Monitoring in a Coral Reef Ecosystem. ISPRS J. Photogramm. Remote Sens. 2015, 104, 213–223. [Google Scholar] [CrossRef]
- VIGRA Homepage. Available online: http://ukoethe.github.io/vigra/ (accessed on 2 March 2023).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Paleontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ambo-Rappe, R.; Moore, A.M. Chapter 25—Sulawesi Seas, Indonesia. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 559–581. ISBN 978-0-08-100853-9. [Google Scholar]
- Febriyanti, F.; Aslan, L.O.M.; Iba, W.; Patadjai, A.B.; Nurdin, A.R. Effect of Various Planting Distances on Growth and Carrageenan Yield of Kappaphycus Alvarezii (Doty) Using Seedlings Produced from Mass Selection Combined with Tissue-Cultured Method. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012027. [Google Scholar] [CrossRef]
- Kasim, M.; Mustafa, A. Comparison Growth of Kappaphycus Alvarezii (Rhodophyta, Solieriaceae) Cultivation in Floating Cage and Longline in Indonesia. Aquac. Rep. 2017, 6, 49–55. [Google Scholar] [CrossRef]
- Periyasamy, C.; Subba Rao, P.V.; Anantharaman, P. Harvest Optimization to Assess Sustainable Growth and Carrageenan Yield of Cultivated Kappaphycus Alvarezii (Doty) Doty in Indian Waters. J. Appl. Phycol. 2019, 31, 587–597. [Google Scholar] [CrossRef]
- Ali, M.K.M.; Fudholi, A.; Sulaiman, J.; Muthuvalu, M.S.; Ruslan, M.H.; Yasir, S.M.D.; Hurtado, A.Q. Post-Harvest Handling of Eucheumatoid Seaweeds. In Tropical Seaweed Farming Trends, Problems and Opportunities: Focus on Kappaphycus and Eucheuma of Commerce; Developments in Applied Phycology; Hurtado, A.Q., Critchley, A.T., Neish, I.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 131–145. ISBN 978-3-319-63498-2. [Google Scholar]
- Mulyati, H.; Geldermann, J. Managing Risks in the Indonesian Seaweed Supply Chain. Clean Technol. Environ. Policy 2017, 19, 175–189. [Google Scholar] [CrossRef] [Green Version]
- Neish, I.C. Social and economic dimensions of carrageenan seaweed farming in Indonesia. In Social and Economic Dimensions of Carrageenan Seaweed Farming; Valderrama, D., Cai, J., Hishamunda, N., Ridler, N., Eds.; Fisheries and Aquaculture, Technical Paper No. 580; FAO: Rome, Italy, 2013; p. 204. ISBN 978-92-5-107746-7. [Google Scholar]
- Wang, M.; Hu, C. Satellite Remote Sensing of Pelagic Sargassum Macroalgae: The Power of High Resolution and Deep Learning. Remote Sens. Environ. 2021, 264, 112631. [Google Scholar] [CrossRef]
- Descloitres, J.; Minghelli, A.; Steinmetz, F.; Chevalier, C.; Chami, M.; Berline, L. Revisited Estimation of Moderate Resolution Sargassum Fractional Coverage Using Decametric Satellite Data (S2-MSI). Remote Sens. 2021, 13, 5106. [Google Scholar] [CrossRef]
- Schamberger, L.; Minghelli, A.; Chami, M. Quantification of Underwater Sargassum Aggregations Based on a Semi-Analytical Approach Applied to Sentinel-3/OLCI (Copernicus) Data in the Tropical Atlantic Ocean. Remote Sens. 2022, 14, 5230. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis, 2nd ed.; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar] [CrossRef]




| Cycle | Date | FW | SGR | Carrageenan |
|---|---|---|---|---|
| (g) | (%) | (%) | ||
| 1 | 10/03 | 24.0 ± 2.1 | 48.8 ± 4.3 | |
| 1 | 29/03 | 103.0 ± 24.7 | 48.4 ± 2.4 | |
| 1 | 10/04 | 229.3 ± 50.9 | 57.3 ± 4.8 | |
| 1 | 16/04 | 299.3 ± 51.4 | 6.9 ± 0.7 | 54.9 ± 2.8 |
| 2 | 19/04 | 24.0 ± 2.0 | 61.2 ± 1.5 | |
| 2 | 28/04 | 50.7 ± 11.6 | 56.6 ± 4.2 | |
| 2 | 10/05 | 104.7 ± 23.5 | 54.1 ± 4.9 | |
| 2 | 20/05 | 180.7 ± 41.1 | - | |
| 2 | 30/05 | 268.0 ± 60.7 | 5.8 ± 0.7 | 55.7 ± 4.5 |
| 3 | 08/06 | 30.7 ± 6.2 | 53.6 ± 1.2 | |
| 3 | 17/06 | 68.3 ± 11.9 | 55.4 ± 4.5 | |
| 3 | 27/06 | 120.0 ± 37.6 | 55.6 ± 2.6 | |
| 3 | 08/07 | 201.3 ± 68.5 | 60.8 ± 2.7 | |
| 3 | 19/07 | 301.4 ± 77.0 | 5.4 ± 0.8 | 61.6 ± 2.3 |
| Cycle-1 | |||||
|---|---|---|---|---|---|
| CLASS | Kappaphycus | Bottom | Plastic | Sum User | User accuracy% (mean) |
| Kappaphycus | 170 | 0 | 17 | 187 | 88.5 |
| Bottom | 11 | 281 | 6 | 298 | 94.5 |
| Plastic | 0 | 0 | 76 | 76 | 100.0 |
| Sum Producer | 181 | 281 | 99 | ||
| Producer accuracy% (mean) | 90.5 | 100.0 | 76.7 | ||
| Cycle-2 | |||||
| CLASS | Kappaphycus | Bottom | Plastic | Sum User | User accuracy% (mean) |
| Kappaphycus | 482 | 5 | 32 | 519 | 92.0 |
| Bottom | 41 | 442 | 12 | 495 | 90.6 |
| Plastic | 0 | 0 | 139 | 139 | 100.0 |
| Sum Producer | 523 | 447 | 183 | ||
| Producer accuracy% (mean) | 91.0 | 97.6 | 74.8 | ||
| Cycle-3 | |||||
| CLASS | Kappaphycus | Bottom | Plastic | Sum User | User accuracy% (mean) |
| Kappaphycus | 238 | 7 | 25 | 270 | 87.9 |
| Bottom | 19 | 112 | 25 | 156 | 78.8 |
| Plastic | 0 | 0 | 70 | 70 | 100.0 |
| Sum Producer | 257 | 119 | 120 | ||
| Producer accuracy% (mean) | 93.8 | 94.8 | 59.1 | ||
| OVERALL | |||||
| Cycle-1 | Cycle-2 | Cycle-3 | |||
| Accuracy% (mean) | 94.0 | 91.1 | 85.9 | ||
| Kappa (mean) | 0.9 | 0.85 | 0.77 | ||
| Cycle | Date | FW Drone | Carrageenan Drone | BNP Drone | SGR Drone |
|---|---|---|---|---|---|
| (g·m−1) | (g·m−1) | (g·m−1) | (%) | ||
| 1 | 15/03 | 550 ± 128 | 27 ± 6 | ||
| 1 | 29/03 | - | - | ||
| 1 | 10/04 | - | - | ||
| 1 | 16/04 | 1912 ± 356 | 105 ± 19 | 1516 ± 225 | 4.7 ± 0.4 |
| 2 | 19/04 | 344 ± 157 | 21 ± 10 | ||
| 2 | 28/04 | 1019 ± 343 | 58 ± 19 | ||
| 2 | 10/05 | - | - | ||
| 2 | 20/05 | 1945 ± 266 | 97 ± 15 | ||
| 2 | 30/05 | 2151 ± 227 | 120 ± 13 | 1807 ± 296 | 4.9 ± 1.0 |
| 3 | 08/06 | 456 ± 141 | 24 ± 8 | ||
| 3 | 17/06 | 810 ± 159 | 45 ± 9 | ||
| 3 | 27/06 | - | - | ||
| 3 | 08/07 | - | - | ||
| 3 | 19/07 | 1435 ± 388 | 88 ± 24 | 903 ± 314 | 3.6 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurdin, N.; Alevizos, E.; Syamsuddin, R.; Asis, H.; Zainuddin, E.N.; Aris, A.; Oiry, S.; Brunier, G.; Komatsu, T.; Barillé, L. Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan. Remote Sens. 2023, 15, 3674. https://doi.org/10.3390/rs15143674
Nurdin N, Alevizos E, Syamsuddin R, Asis H, Zainuddin EN, Aris A, Oiry S, Brunier G, Komatsu T, Barillé L. Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan. Remote Sensing. 2023; 15(14):3674. https://doi.org/10.3390/rs15143674
Chicago/Turabian StyleNurdin, Nurjannah, Evangelos Alevizos, Rajuddin Syamsuddin, Hasni Asis, Elmi Nurhaidah Zainuddin, Agus Aris, Simon Oiry, Guillaume Brunier, Teruhisa Komatsu, and Laurent Barillé. 2023. "Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan" Remote Sensing 15, no. 14: 3674. https://doi.org/10.3390/rs15143674
APA StyleNurdin, N., Alevizos, E., Syamsuddin, R., Asis, H., Zainuddin, E. N., Aris, A., Oiry, S., Brunier, G., Komatsu, T., & Barillé, L. (2023). Precision Aquaculture Drone Mapping of the Spatial Distribution of Kappaphycus alvarezii Biomass and Carrageenan. Remote Sensing, 15(14), 3674. https://doi.org/10.3390/rs15143674