Estimating Leymus chinensis Loss Caused by Oedaleus decorus asiaticus Using an Unmanned Aerial Vehicle (UAV)
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
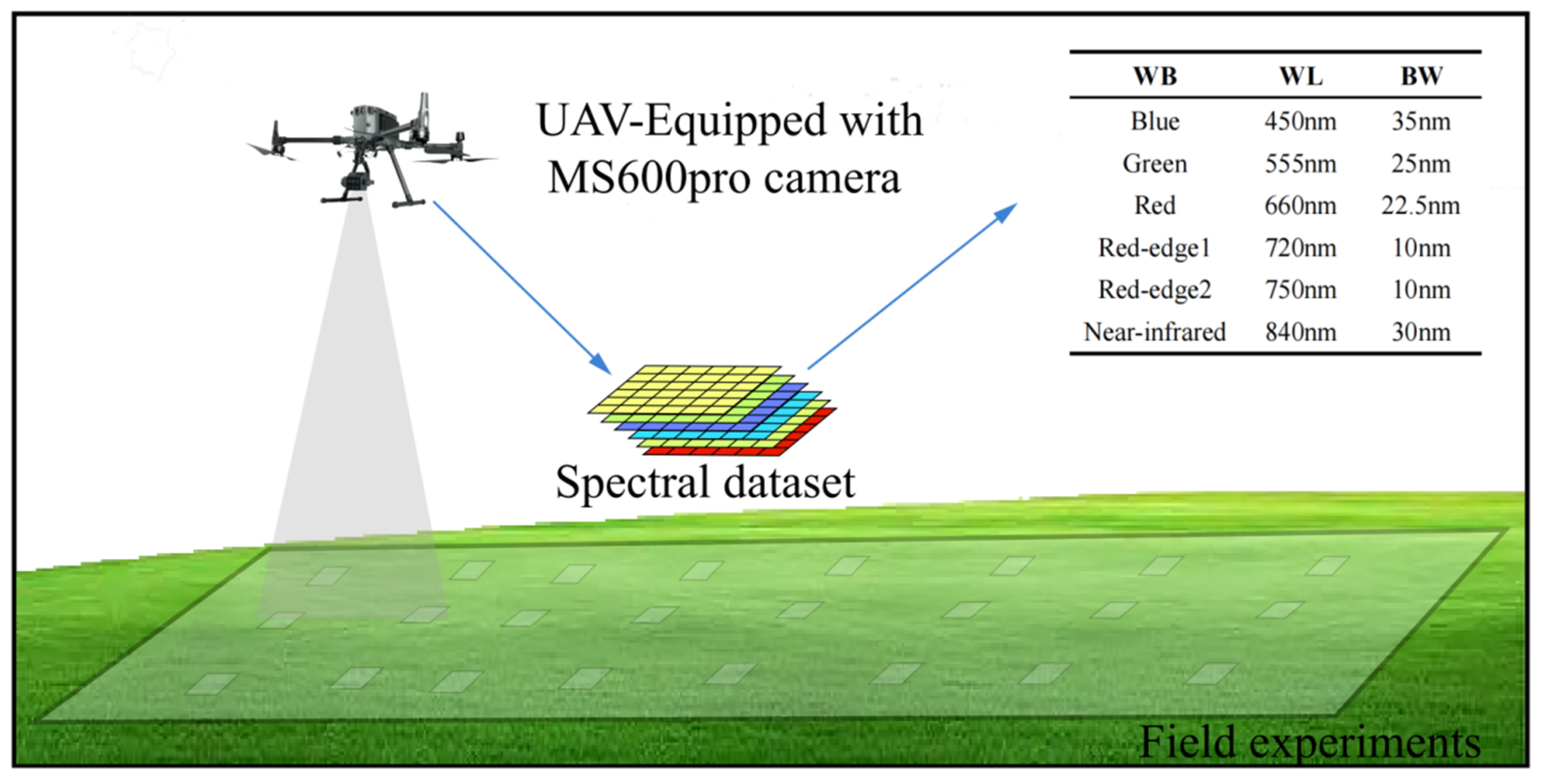
2.2. Unmanned Aerial Vehicle (UAV) Sensor Platform
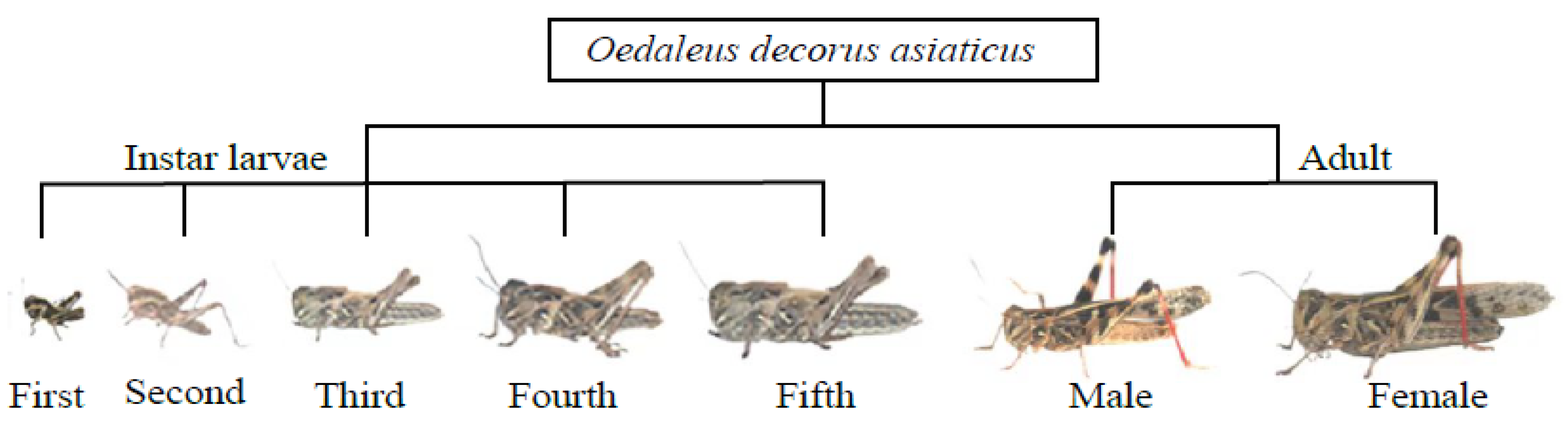
2.3. Field Experiment
- (1)
- On 19 June 2022, multi-spectral images were collected by the UAV system as initial data. Then, all cages were installed and different numbers of O. decorus nymphs (0, 8, 15, 22, 30, 45, 60, and 90 nymphs/m2) were placed in corresponding cages (Figure 1C).
- (2)
- On 28 June 2022, eighty percent of second instar nymphs had transformed to third instar nymphs in most cages. All cages were removed and spectral images were collected using the UAV system. Subsequently, all cages were moved back to continue the experiments.
- (3)
- On 3 July 2022, more than eighty percent of third instar nymphs had transformed to fourth instar nymphs in most cages. All cages were removed. Spectral images of all plot canopies were taken by the UAV system.
- (4)
- On 8 July 2022, fourth instar nymphs in the cages had basically molted. Spectral images were collected after removing all cages.
- (5)
- On 11 July 2022, more than eighty percent of fifth instar nymphs had transformed to adults. However, spectral data could not be collected due to poor weather conditions until the field experiment was completed on the following day.
2.4. Multi-Spectral Image Processing
2.5. Variation of Vegetation Indices
3. Results
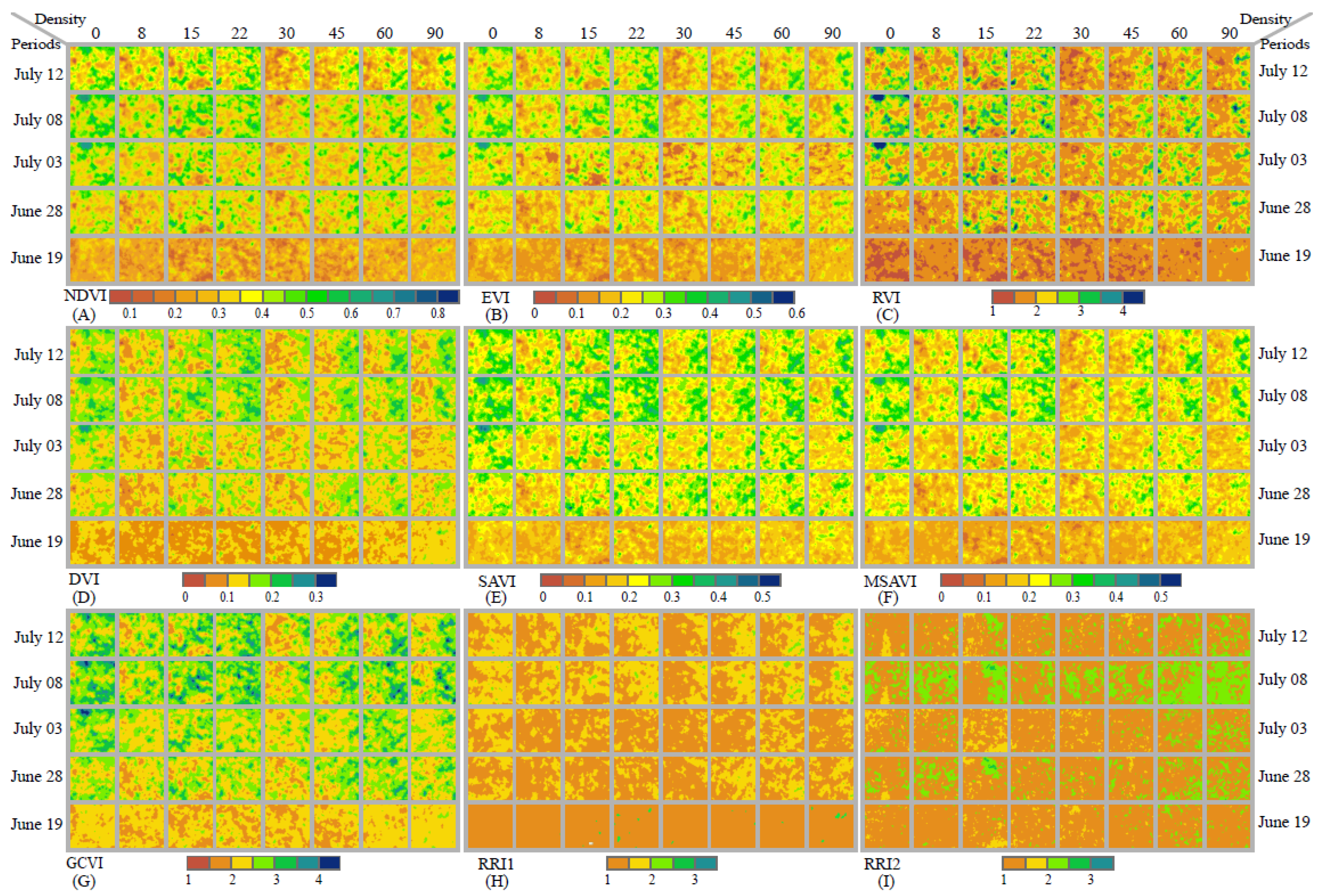
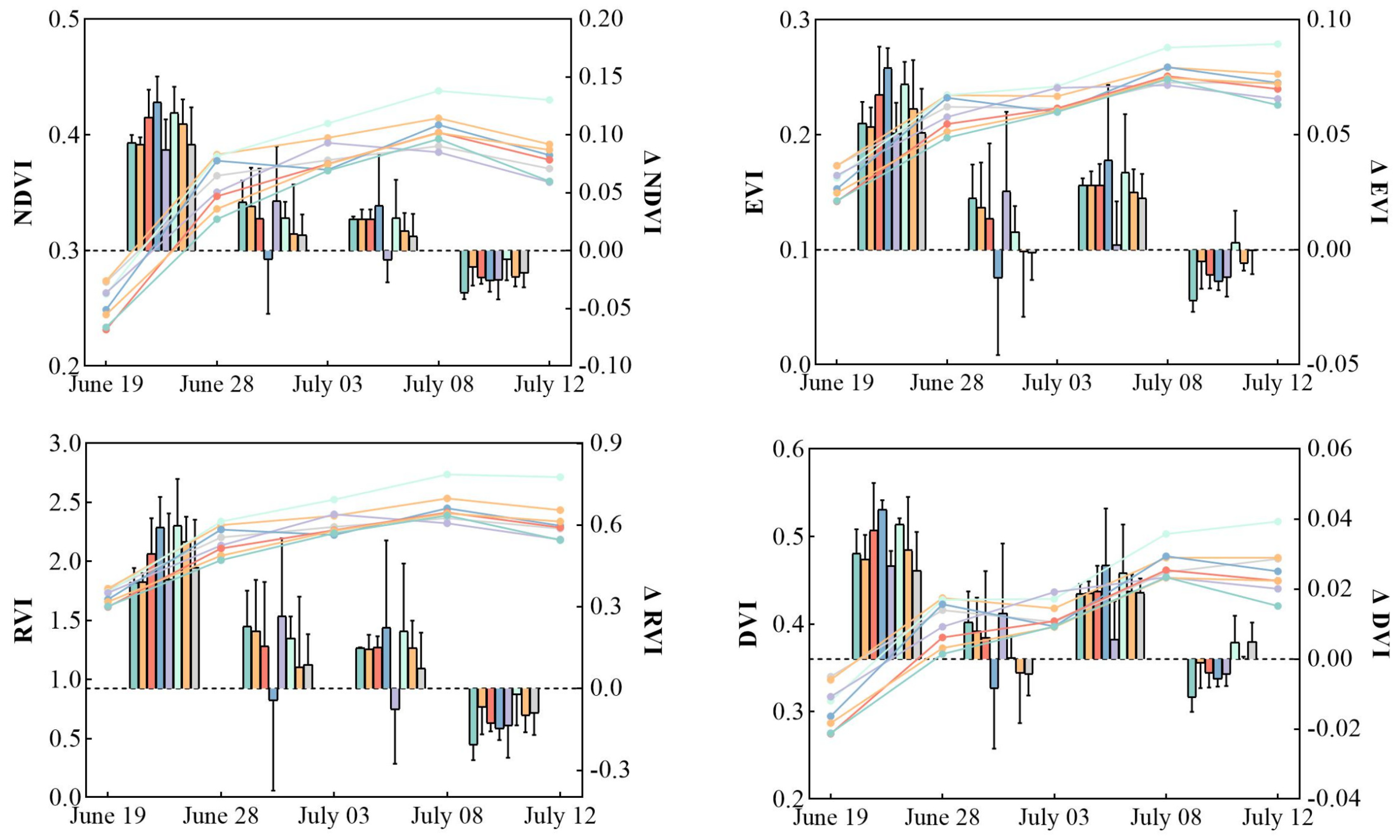
3.1. Response of Plant Vegetation Indices to O. decorus Invasion
3.2. Variation Characteristics of Vegetation Indices Attributable to O. decorus
3.3. Relationship between the Loss Component of Leymus Chinensis and the Density Level of O. decorus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, L.; Wei, L. Progress of acridology in China over the last 60 years. Acta Phytophylacica. Sin. 2022, 49, 4–16. [Google Scholar]
- Mariottini, Y.; Marinelli, C.; Cepeda, R.; DeWysiecki, M.L.; Lange, C.E. Relationship between pest grasshopper densities and climate variables in the southern Pampas of Argentina. Bull. Entomol. Res. 2022, 112, 613–625. [Google Scholar] [CrossRef]
- Song, P.L.; Zheng, X.M.; Li, Y.Y.; Zhang, K.Y.; Huang, J.F.; Li, H.M.; Zhang, H.J.; Liu, L.; Wei, C.W.; Mansararay, L.R.; et al. Estimating reed loss caused by Locusta migratoria manilensis using UAV-based hyperspectral data. Sci. Total Environ. 2020, 719, 137519. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.X.; Ma, N.L.; Zhang, D.Q.; Zhou, Q.; Yue, X.C.; Khoo, S.C.; Yang, H.; Guan, R.R.; Chen, H.L.; Zhang, X.F.; et al. A review of historical and recent locust outbreaks: Links to global warming, food security and mitigation strategies. Environ. Res. 2020, 191, 110046. [Google Scholar] [CrossRef] [PubMed]
- Latchininsky, A.V. Locusts and remote sensing: A review. J. Appl. Remote Sens. 2013, 7, 075099. [Google Scholar] [CrossRef]
- Zhang, L.; Lecoq, M.; Latchininsky, A.; Hunter, D.; Douglas, A.E. Locust and Grasshopper Management. Annu. Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Chen, C.L.; Qian, J.; Chen, X.; Hu, Z.Y.; Sun, J.Y.; Wei, S.J.; Xu, K.B. Geographic Distribution of Desert Locusts in Africa, Asia and Europe Using Multiple Sources of Remote-Sensing Data. Remote Sens. 2020, 12, 3593. [Google Scholar] [CrossRef]
- Ni, S.; Wu, T. Monitoring the intensity of locust damage to vegetation using hyper-spectra data obtained at ground surface. In Proceedings of the Remote Sensing and Modeling of Ecosystems for Sustainability IV. SPIE, San Diego, CA, USA, 26–30 August 2007; Volume 6679, pp. 89–97. [Google Scholar]
- Moshou, D.; Bravo, C.; West, J.; Wahlen, S.; McCartney, A.; Ramon, H. Automatic detection of ‘yellow rust’ in wheat using reflectance measurements and neural networks. Comput. Electron. Agric. 2004, 44, 173–188. [Google Scholar] [CrossRef]
- Wu, T.; Ni, S.; Li, Y.; Zhou, X.; Chen, J. Monitoring of the damage intensity extent by oriental migratory locust using of hyper-spectra data measured at ground surface. J. Remote Sens.-Beijing 2007, 11, 103–188. [Google Scholar]
- Devesoni, E.D. Satellite normalized difference vegetation index data used in managing Australian plague locusts. J. Appl. Remote Sens. 2013, 7, 075096. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Luo, J.; Huang, L.; Zhou, X. Detection and discrimination of pests and diseases in winter wheat based on spectral indices and kernel discriminant analysis. Comput. Electron. Agric. 2017, 141, 171–180. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Z.; Wang, H.; Wu, H.; Liu, H.; Wang, G.; Zhang, Z. The effects of hyper spectral change on grassland biomass after damage by Calliptamus abbreviates populations of different densities. Acta Prataculturae Sin. 2015, 24, 195–203. [Google Scholar]
- Zheng, X.; Song, P.; Li, Y.; Zhang, K.; Zhang, H.; Liu, L.; Huang, J. Monitoring Locusta migratoria manilensis damage using ground level hyperspectral data. In Proceedings of the 2019 8th International Conference on Agro-Geoinformatics, Istanbul, Turke, 16–19 July 2019; pp. 1–5. [Google Scholar]
- Lausch, A.; Heurich, M.; Gordalla, D.; Dobner, H.J.; Gwillym-Margianto, S.; Salbach, C. Forecasting potential bark beetle outbreaks based on spruce forest vitality using hyperspectral remote-sensing techniques at different scales. For. Ecol. Manag. 2013, 308, 76–89. [Google Scholar] [CrossRef]
- Du, B.B.; Wei, J.; Lin, K.; Lu, L.H.; Ding, X.L.; Ye, H.C.; Huang, W.J.; Wang, N. Spatial and Temporal Variability of Grassland Grasshopper Habitat Suitability and Its Main Influencing Factors. Remote Sens. 2022, 14, 3910. [Google Scholar] [CrossRef]
- Guo, J.; Lu, L.H.; Dong, Y.Y.; Huang, W.J.; Zhang, B.; Du, B.B.; Ding, C.; Ye, H.C.; Wang, K.; Huang, Y.R.; et al. Spatiotemporal Distribution and Main Influencing Factors of Grasshopper Potential Habitats in Two Steppe Types of Inner Mongolia, China. Remote Sens. 2023, 15, 886. [Google Scholar] [CrossRef]
- Zha, Y.; Gao, J.; Ni, S.; Shen, N. Temporal filtering of successive MODIS data in monitoring a locust outbreak. Int. J. Remote Sens. 2005, 26, 5665–5674. [Google Scholar] [CrossRef]
- Rong, J.; Xia, Z.; Baoyu, X.; Zhe, L.; Tuanji, L.; Chuang, L.; Dianmo, L. Use of MODIS data of detect the Oriental migratory locust plague: A case study in Nandagang, Hebei Province. Acta Entomol. Sin. 2003, 46, 713–719. [Google Scholar]
- Lehmann, J.R.K.; Nieberding, F.; Prinz, T.; Knoth, C. Analysis of Unmanned Aerial System-Based CIR Images in Forestry-A New Perspective to Monitor Pest Infestation Levels. Forests 2015, 6, 594–612. [Google Scholar] [CrossRef]
- Rango, A.; Laliberte, A.; Steele, C.; Herrick, J.E.; Bestelmeyer, B.; Schmugge, T.; Roanhorse, A.; Jenkins, V. Using Unmanned Aerial Vehicles for Rangelands: Current Applications and Future Potentials. Environ. Pract. 2006, 8, 159–168. [Google Scholar] [CrossRef]
- Xi, G.L.; Huang, X.J.; Bao, Y.H.; Bao, G.; Tong, S.Q.; Dashzebegd, G.; Nanzadd, T.; Dorjsurene, A.; Davaadorj, E.; Ariuaad, M. Hyperspectral Discrimination of Different Canopy Colors in Erannis Jacobsoni Djak-Infested Larch. Spectrosc. Spectr. Anal. 2020, 40, 2925–2931. [Google Scholar]
- Nasi, R.; Honkavaara, E.; Lyytikainen-Saarenmaa, P.; Blomqvist, M.; Litkey, P.; Hakala, T.; Viljanen, N.; Kantola, T.; Tanhuanpaa, T.; Holopainen, M. Using UAV-Based Photogrammetry and Hyperspectral Imaging for Mapping Bark Beetle Damage at Tree-Level. Remote Sens. 2015, 7, 15467–15493. [Google Scholar] [CrossRef]
- Tong, S.; Dong, Z.; Zhang, J.Q.; Bao, Y.B.; Guna, A.; Bao, Y.H. Spatiotemporal Variations of Land Use/Cover Changes in Inner Mongolia (China) during 1980–2015. Sustainability 2018, 10, 4730. [Google Scholar] [CrossRef]
- Wu, T.; Hao, S.; Kang, L. Effects of Soil Temperature and Moisture on the Development and Survival of Grasshopper Eggs in Inner Mongolian Grasslands. Front. Ecol. Evol. 2021, 9, 727911. [Google Scholar] [CrossRef]
- Du, G.L.; Zhao, H.L.; Tu, X.B.; Zhang, Z.H. Division of the inhabitable areas for Oedaleus decorus asiaticus in Inner Mongolia. Plant Prot. 2018, 44, 24–31. [Google Scholar]
- Lu, L.H.; Kong, W.Q.; Eerdengqimuge; Ye, H.C.; Sun, Z.X.; Wang, N.; Du, B.B.; Zhou, Y.T.; Wei, J. Detecting Key Factors of Grasshopper Occurrence in Typical Steppe and Meadow Steppe by Integrating Machine Learning Model and Remote Sensing Data. Insects 2022, 13, 894. [Google Scholar] [CrossRef]
- Zhou, X.R.; Chen, Y.; Guo, Y.H.; Pang, B.P. Population dynamics of Oedaleus asiaticus on desert grasslands in Inner Mongolia. Chin. J. Appl. Entomol. 2012, 49, 1598–1603. [Google Scholar]
- Liu, H.Q.; Huete, A. A feedback based modification of the NDVI to minimize canopy background and atmospheric noise. IEEE Trans. Geosci. Remote Sens. 1995, 33, 457–465. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Vina, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 825. [Google Scholar] [CrossRef]
- Gomez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J.L. Machine learning approach to locate desert locust breeding areas based on ESA CCI soil moisture. J. Appl. Remote Sens. 2018, 12, 036011. [Google Scholar] [CrossRef]
- Lu, H.; Yu, M.; Zhang, L.S.; Zhang, Z.H.; Long, R.J. Effects of foraging by different instars and densities of Oodles asiaticus Oedaleus asiaticus on forage yield. Plant Prot. 2005, 31, 55–58. [Google Scholar]


| Vegetation Indices | Formula | Order |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (1) | |
| Enhanced Vegetation Index (EVI) | (2) | |
| Ratio Vegetation Index (RVI) | (3) | |
| Difference Vegetation Index (DVI) | (4) | |
| Soil-Adjusted Vegetation Index (SAVI) | (5) | |
| Modified Soil-Adjusted Vegetation Index (MSAVI) | (6) | |
| Green Chlorophyll Vegetation Index (GCVI) | (7) | |
| Red Edge Ratio Index (RRI 1) | (8) | |
| Red Edge Ratio Index (RRI 2) | (9) |
| Growth Stages | Second Instar Larvae | Third Instar Larvae | Fourth Instar Larvae | Fifth Instar Larvae | ||||
|---|---|---|---|---|---|---|---|---|
| VIs | Equation | Equation | Equation | Equation | ||||
| NDVI | y = −7 × 10−6x2 + 0.0005x + 0.0077 | R2 = 0.2422 | y = −6 × 10−6x2 + 0.0005x − 0.0268 | R2 = 0.1085 | y = −0.0002x − 0.0011 | R2 = 0.3205 | y = −2 × 10−7 × 2 + 8E−05x + 0.0137 | R2 = 0.1143 |
| EVI | y = −7 × 10−6x2 + 0.0005x + 0.0012 | R2 = 0.3809 | y = 8 × 10−7x2 − 0.0003x − 0.0068 | R2 = 0.1302 | y = −6 × 10−5x − 0.0004 | R2 = 0.1048 | y = 0.0001x + 0.0109 | R2 = 0.1978 |
| RVI | y = −5 × 10−5x2 + 0.0033x + 0.0455 | R2 = 0.788 | y = −6 × 10−5x2 + 0.0074x − 0.2479 | R2 = 0.6113 | y = −0.0022x + 0.0659 | R2 = 0.6222 | y = −0.0002x + 0.0826 | R2 = 0.2205 |
| DVI | y = −4 × 10−6x2 + 0.0003x + 0.0005 | R2 = 0.2948 | y = 6 × 10−7x2 − 0.0002x − 0.0028 | R2 = 0.2522 | y = 5 × 10−6x + 0.0002 | R2 = 0.0001 | y = 0.0001x + 0.006 | R2 = 0.3165 |
| SAVI | y = −6 × 10−6x2 + 0.0005x + 0.0011 | R2 = 0.1832 | y = −0.0002x − 0.007 | R2 = 0.2342 | y = 1 × 10−6x2 − 0.0001x + 0.0015 | R2 = 0.1048 | y = 0.0001x + 0.0101 | R2 = 0.2855 |
| MSAVI | y = −−6 × 10−6x2 + 0.0005x + 0.0006 | R2 = 0.2834 | y = −0.0002x − 0.0065 | R2 = 0.1369 | y = −2 × 10−5x + 5E − 05 | R2 = 0.2011 | y = 0.0001x + 0.0099 | R2 = 0.3136 |
| GCVI | y = −6 × 10−5x2 + 0.0064x − 0.0381 | R2 = 0.4125 | y = 7 × 10−6x2 − 0.0019x − 0.0165 | R2 = 0.1313 | y = −3 × 10−5x2 + 0.0034x − 0.0358 | R2 = 0.2251 | y = 9 × 10−6x2 − 0.0012x + 0.0946 | R2 = 0.0174 |
| RRI 1 | y = −3 × 10−5x2 + 0.0031x − 0.0311 | R2 = 0.352 | y = −0.0006x − 0.0146 | R2 = 0.2636 | y = −3 × 10−5x2 + 0.003x − 0.0276 | R2 = 0.3377 | y = 1 × 10−5x2 − 0.0012x + 0.0511 | R2 = 0.2464 |
| RRI 2 | y = −7 × 10−5x2 + 0.0073x − 0.0182 | R2 = 0.1781 | y = −5 × 10−6x2 − 0.0003x − 0.0705 | R2 = 0.2087 | y = −7 × 10−6x2 + 0.0002x − 0.0139 | R2 = 0.1054 | y = −4 × 10−6x2 + 0.0008x + 0.0888 | R2 = 0.1115 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Ding, X.; Ji, C.; Lin, K.; Guo, J.; Lu, L.; Dong, Y.; Huang, W.; Wang, N. Estimating Leymus chinensis Loss Caused by Oedaleus decorus asiaticus Using an Unmanned Aerial Vehicle (UAV). Remote Sens. 2023, 15, 4352. https://doi.org/10.3390/rs15174352
Du B, Ding X, Ji C, Lin K, Guo J, Lu L, Dong Y, Huang W, Wang N. Estimating Leymus chinensis Loss Caused by Oedaleus decorus asiaticus Using an Unmanned Aerial Vehicle (UAV). Remote Sensing. 2023; 15(17):4352. https://doi.org/10.3390/rs15174352
Chicago/Turabian StyleDu, Bobo, Xiaolong Ding, Chao Ji, Kejian Lin, Jing Guo, Longhui Lu, Yingying Dong, Wenjiang Huang, and Ning Wang. 2023. "Estimating Leymus chinensis Loss Caused by Oedaleus decorus asiaticus Using an Unmanned Aerial Vehicle (UAV)" Remote Sensing 15, no. 17: 4352. https://doi.org/10.3390/rs15174352






