Using High-Resolution UAV Imaging to Measure Canopy Height of Diverse Cover Crops and Predict Biomass
Abstract
1. Introduction
2. Materials and Methods
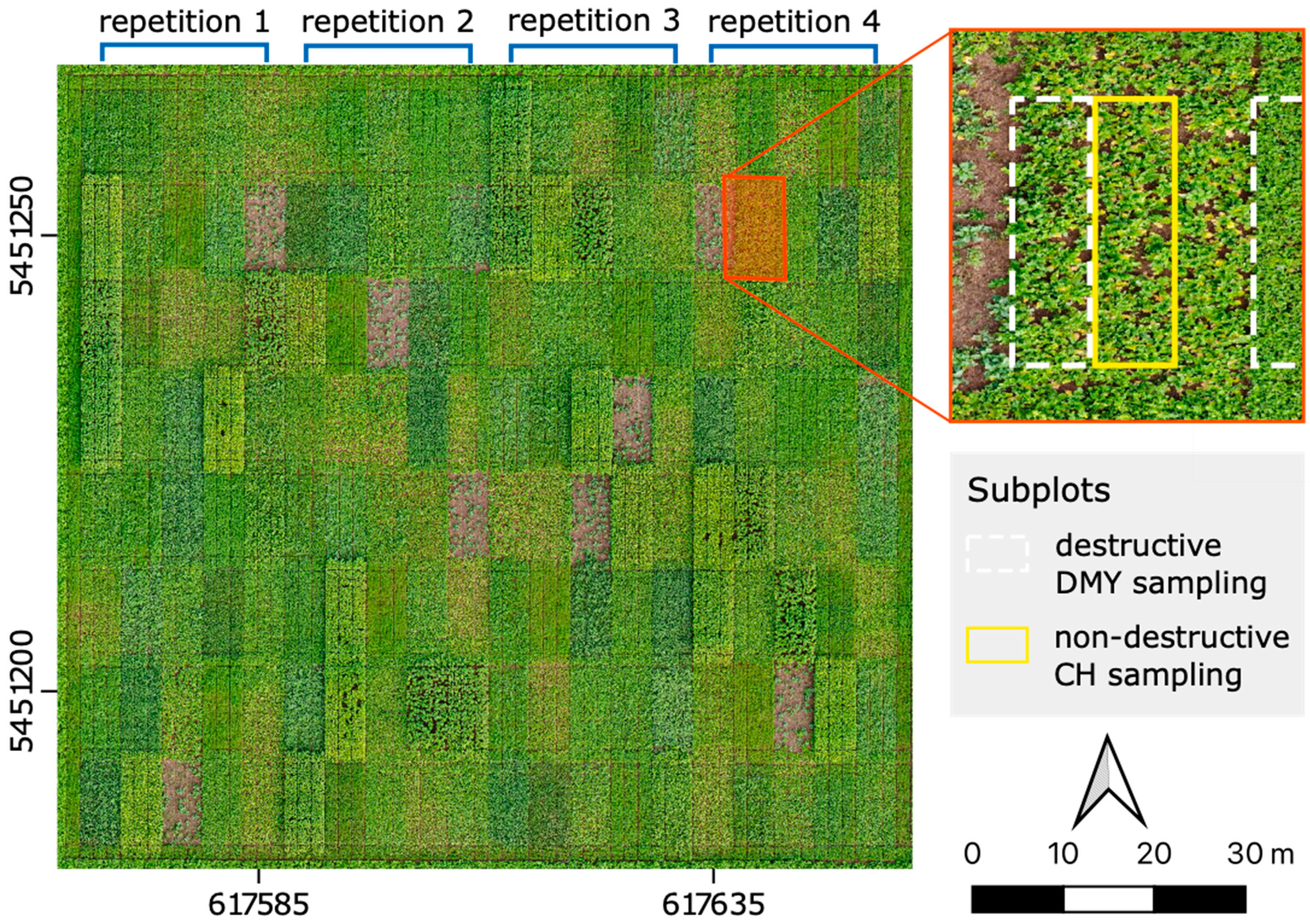
2.1. Study Site and Experimental Design
2.2. Data Acquisition
2.3. Data Processing of Remote-Sensing Images
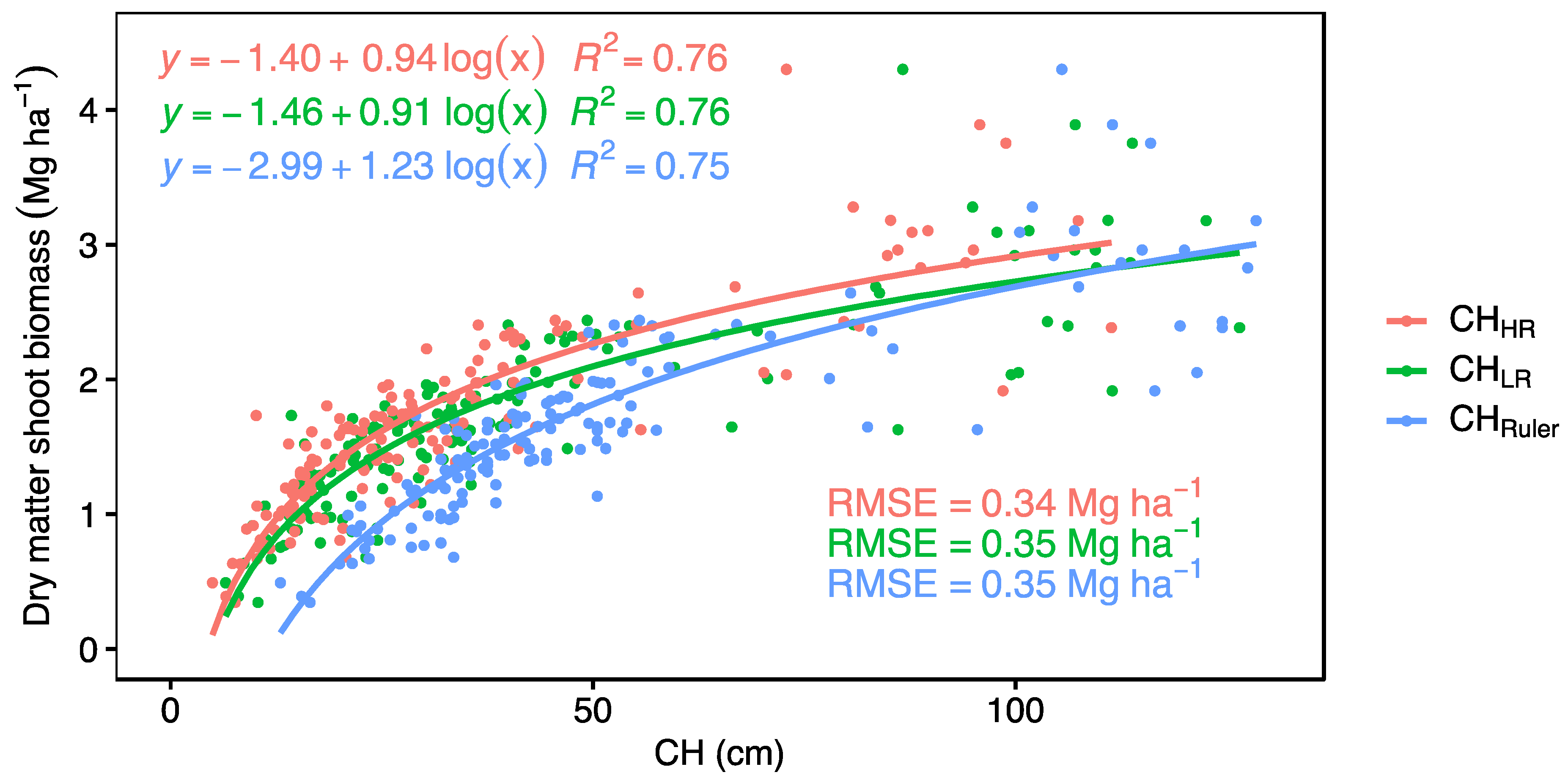
2.4. Statistical Analysis
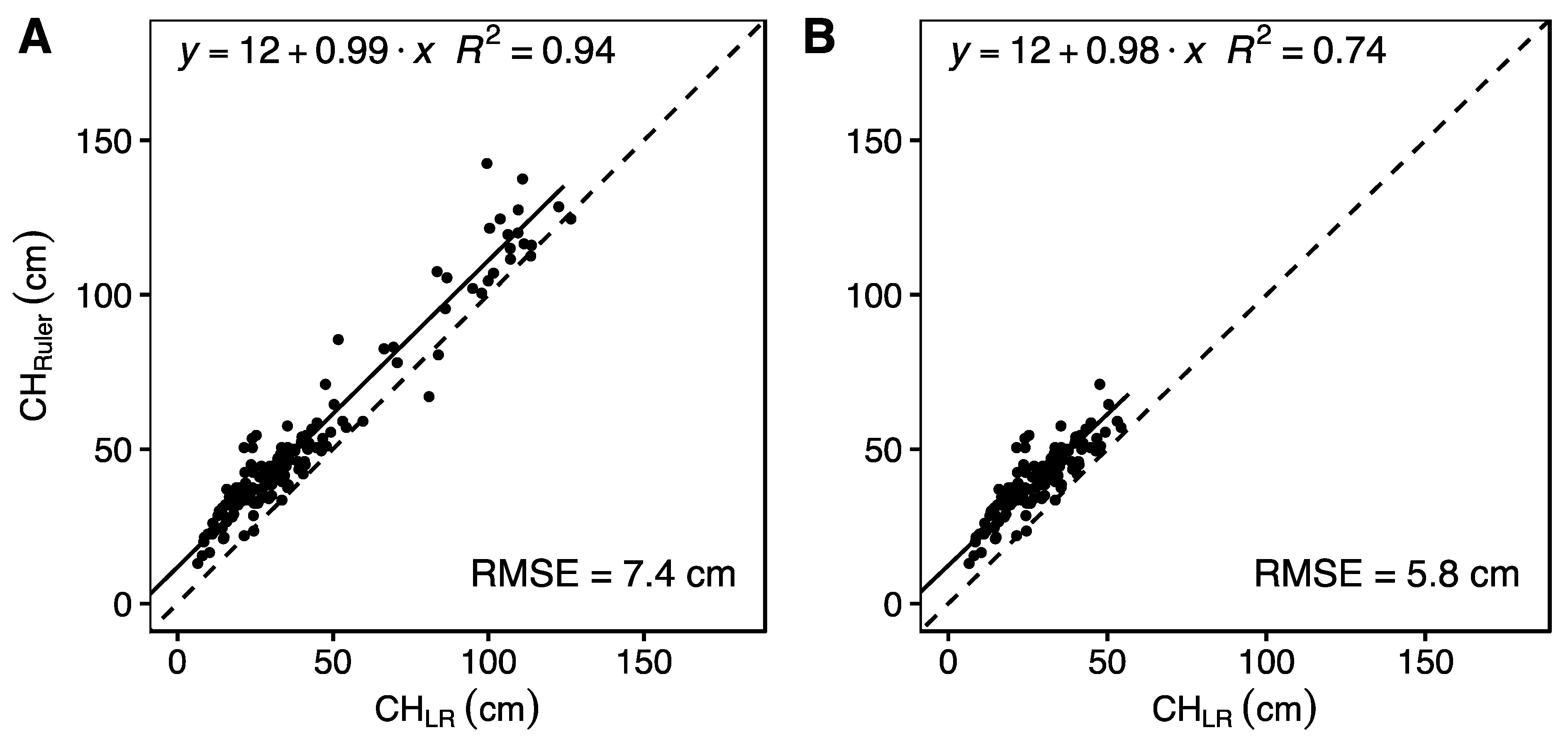
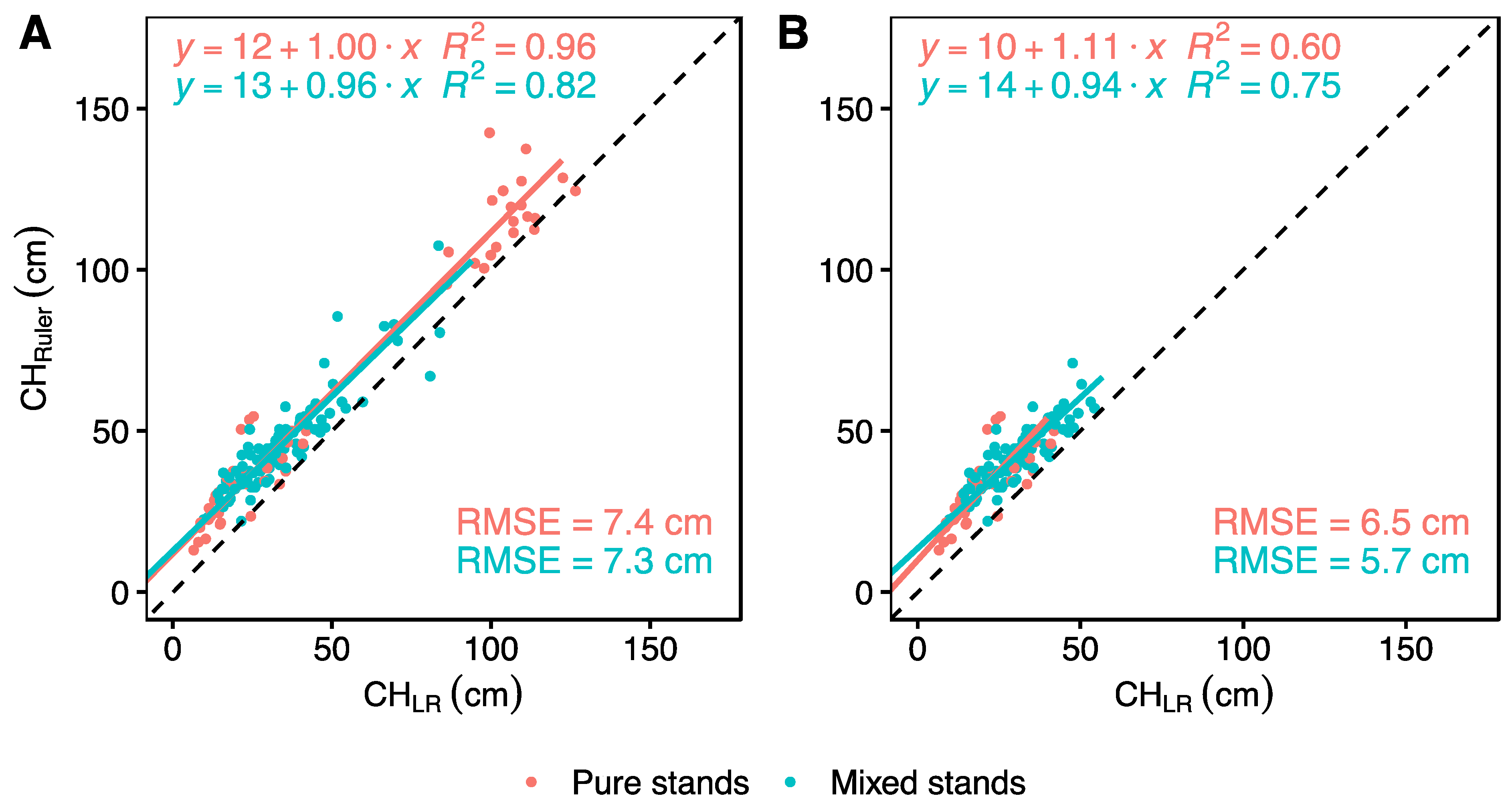
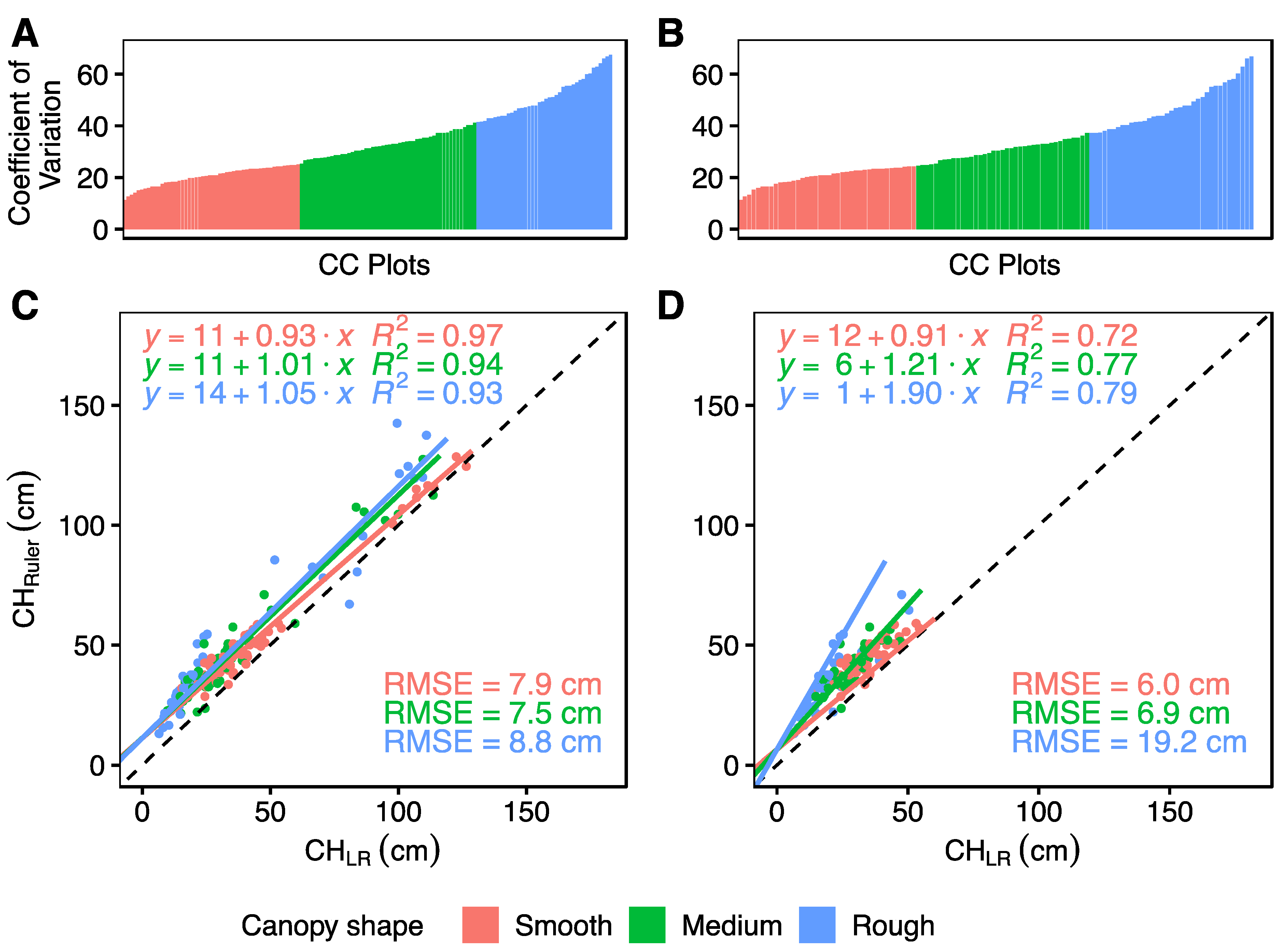
3. Results
4. Discussion
4.1. Plant Height or Canopy Height?
4.2. Is Ground Truth the Truth?
4.3. Predicting Biomass Yield by Canopy Height
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | CHLR | SE | n | CLD |
|---|---|---|---|---|
| Oil Radish | 11.50 | 1.88 | 4 | a |
| Field Pea | 23.13 | 1.88 | 4 | b |
| Egyptian Clover | 23.54 | 1.88 | 4 | b |
| Bristle Oat | 33.25 | 1.88 | 4 | c |
| Phacelia | 39.05 | 1.88 | 4 | c |
| Mustard | 106.96 | 1.88 | 4 | d |

| Cover Crop Treatments | CHLR | SE | n | CLD |
|---|---|---|---|---|
| Pure stands | 33.90 | 2.99 | 56 | a |
| Mixtures | 50.89 | 3.83 | 92 | b |
References
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover Crops and Ecosystem Services: Insights from Studies in Temperate Soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Gentsch, N.; Boy, J.; Batalla, J.D.K.; Heuermann, D.; von Wirén, N.; Schweneker, D.; Feuerstein, U.; Groß, J.; Bauer, B.; Reinhold-Hurek, B.; et al. Catch Crop Diversity Increases Rhizosphere Carbon Input and Soil Microbial Biomass. Biol. Fertil. Soils 2020, 56, 943–957. [Google Scholar] [CrossRef]
- Chahal, I.; Vyn, R.J.; Mayers, D.; van Eerd, L.L. Cumulative Impact of Cover Crops on Soil Carbon Sequestration and Profitability in a Temperate Humid Climate. Sci. Rep. 2020, 10, 13381. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Steinbach, H.S.; de Paepe, J.L. Cover Crop Effects on Soils and Subsequent Crops in the Pampas: A Meta-Analysis. Soil Tillage Res. 2017, 170, 53–65. [Google Scholar] [CrossRef]
- Chahal, I.; van Eerd, L.L. Evaluation of Commercial Soil Health Tests Using a Medium-Term Cover Crop Experiment in a Humid, Temperate Climate. Plant Soil 2018, 427, 351–367. [Google Scholar] [CrossRef]
- Heuermann, D.; Gentsch, N.; Boy, J.; Schweneker, D.; Feuerstein, U.; Groß, J.; Bauer, B.; Guggenberger, G.; von Wirén, N. Interspecific Competition among Catch Crops Modifies Vertical Root Biomass Distribution and Nitrate Scavenging in Soils. Sci. Rep. 2019, 9, 11531. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.C.; Schipanski, M.E.; Burgess, M.H.; Lachance, J.C.; Bradley, B.A.; Barbercheck, M.E.; Kaye, J.P.; Mortensen, D.A. Cover Crop Mixture Effects on Maize, Soybean, and Wheat Yield in Rotation. Agric. Environ. Lett. 2019, 4, 180051. [Google Scholar] [CrossRef]
- Martin, R.C.; Astatkie, T.; Cooper, J.M.; Fredeen, A.H. A Comparison of Methods Used to Determine Biomass on Naturalized Swards. J. Agron. Crop. Sci. 2005, 191, 152–160. [Google Scholar] [CrossRef]
- Punalekar, S.M.; Verhoef, A.; Quaife, T.L.; Humphries, D.; Bermingham, L.; Reynolds, C.K. Application of Sentinel-2A Data for Pasture Biomass Monitoring Using a Physically Based Radiative Transfer Model. Remote Sens. Environ. 2018, 218, 207–220. [Google Scholar] [CrossRef]
- Murphy, W.M.; Silman, J.P.; Barreto, A.D.M. A Comparison of Quadrat, Capacitance Meter, HFRO Sward Stick, and Rising Plate for Estimating Herbage Mass in a Smooth-stalked, Meadowgrass-dominant White Clover Sward. Grass Forage Sci. 1995, 50, 452–455. [Google Scholar] [CrossRef]
- Fehmi, J.S.; Stevens, J.M. A Plate Meter Inadequately Estimated Herbage Mass in a Semi-Arid Grassland. Grass Forage Sci. 2009, 64, 322–327. [Google Scholar] [CrossRef]
- O’Donovan, M.; Dillon, P.; Rath, M.; Stakelum, G. A Comparison of Four Methods of Herbage Mass Estimation. Ir. J. Agric. Food Res. 2002, 41, 17–27. [Google Scholar]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote Sensing for Agricultural Applications: A Meta-Review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Jung, J.; Maeda, M.; Chang, A.; Bhandari, M.; Ashapure, A.; Landivar-Bowles, J. The Potential of Remote Sensing and Artificial Intelligence as Tools to Improve the Resilience of Agriculture Production Systems. Curr. Opin. Biotechnol. 2021, 70, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Taylor, J.; Frewer, L.; Zhao, C.; Yang, G.; Li, Z.; Liu, Z.; Gaulton, R.; Wicks, D.; Mortimer, H.; et al. A Comparative Review of the State and Advancement of Site-Specific Crop Management in the UK and China. Front. Agric. Sci. Eng. 2019, 6, 116. [Google Scholar] [CrossRef]
- Yang, C. Remote Sensing and Precision Agriculture Technologies for Crop Disease Detection and Management with a Practical Application Example. Engineering 2020, 6, 528–532. [Google Scholar] [CrossRef]
- Munnaf, M.A.; Haesaert, G.; van Meirvenne, M.; Mouazen, A.M. Site-Specific Seeding Using Multi-Sensor and Data Fusion Techniques: A Review. Adv. Agron. 2020, 161, 241–323. [Google Scholar] [CrossRef]
- Gerhards, R.; Andújar Sanchez, D.; Hamouz, P.; Peteinatos, G.G.; Christensen, S.; Fernandez-Quintanilla, C. Advances in Site-specific Weed Management in Agriculture—A Review. Weed Res. 2022, 62, 123–133. [Google Scholar] [CrossRef]
- Freeman, K.W.; Girma, K.; Arnall, D.B.; Mullen, R.W.; Martin, K.L.; Teal, R.K.; Raun, W.R. By-Plant Prediction of Corn Forage Biomass and Nitrogen Uptake at Various Growth Stages Using Remote Sensing and Plant Height. Agron. J. 2007, 99, 530–536. [Google Scholar] [CrossRef]
- Yin, X.; McClure, M.A.; Jaja, N.; Tyler, D.D.; Hayes, R.M. In-Season Prediction of Corn Yield Using Plant Height under Major Production Systems. Agron. J. 2011, 103, 923–929. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Yang, H.; Xu, B.; Li, Z.; Yang, X. Clustering Field-Based Maize Phenotyping of Plant-Height Growth and Canopy Spectral Dynamics Using a UAV Remote-Sensing Approach. Front. Plant Sci. 2018, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Bendig, J.; Bolten, A.; Bennertz, S.; Broscheit, J.; Eichfuss, S.; Bareth, G. Estimating Biomass of Barley Using Crop Surface Models (CSMs) Derived from UAV-Based RGB Imaging. Remote Sens. 2014, 6, 10395–10412. [Google Scholar] [CrossRef]
- Stanton, C.; Starek, M.J.; Elliott, N.; Brewer, M.; Maeda, M.M.; Chu, T. Unmanned Aircraft System-Derived Crop Height and Normalized Difference Vegetation Index Metrics for Sorghum Yield and Aphid Stress Assessment. J. Appl. Remote Sens. 2017, 11, 026035. [Google Scholar] [CrossRef]
- ten Harkel, J.; Bartholomeus, H.; Kooistra, L. Biomass and Crop Height Estimation of Different Crops Using UAV-Based Lidar. Remote Sens. 2019, 12, 17. [Google Scholar] [CrossRef]
- Capalbo, S.M.; Antle, J.M.; Seavert, C. Next Generation Data Systems and Knowledge Products to Support Agricultural Producers and Science-Based Policy Decision Making. Agric. Syst. 2017, 155, 191–199. [Google Scholar] [CrossRef]
- Atzberger, C. Advances in Remote Sensing of Agriculture: Context Description, Existing Operational Monitoring Systems and Major Information Needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- Roth, L.; Streit, B. Predicting Cover Crop Biomass by Lightweight UAS-Based RGB and NIR Photography: An Applied Photogrammetric Approach. Precis Agric. 2018, 19, 93–114. [Google Scholar] [CrossRef]
- Malambo, L.; Popescu, S.C.; Murray, S.C.; Putman, E.; Pugh, N.A.; Horne, D.W.; Richardson, G.; Sheridan, R.; Rooney, W.L.; Avant, R.; et al. Multitemporal Field-Based Plant Height Estimation Using 3D Point Clouds Generated from Small Unmanned Aerial Systems High-Resolution Imagery. Int. J. Appl. Earth Obs. Geoinf. 2018, 64, 31–42. [Google Scholar] [CrossRef]
- Chu, T.; Starek, M.J.; Brewer, M.J.; Murray, S.C.; Pruter, L.S. Characterizing Canopy Height with UAS Structure-from-Motion Photogrammetry—Results Analysis of a Maize Field Trial with Respect to Multiple Factors. Remote Sens. Lett. 2018, 9, 753–762. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot 2016, 64, 715. [Google Scholar] [CrossRef]
- Jimenez-Berni, J.A.; Deery, D.M.; Rozas-Larraondo, P.; Condon, A.G.; Rebetzke, G.J.; James, R.A.; Bovill, W.D.; Furbank, R.T.; Sirault, X.R.R. High Throughput Determination of Plant Height, Ground Cover, and Above-Ground Biomass in Wheat with LiDAR. Front. Plant Sci. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Grenzdörffer, G.J. Crop Height Determination with UAS Point Clouds. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, XL-1, 135–140. [Google Scholar] [CrossRef]
- Wendling, M.; Büchi, L.; Amossé, C.; Sinaj, S.; Walter, A.; Charles, R. Influence of Root and Leaf Traits on the Uptake of Nutrients in Cover Crops. Plant Soil 2016, 409, 419–434. [Google Scholar] [CrossRef]
- Sieling, K. Improved N Transfer by Growing Catch Crops—A Challenge. J. Kult. 2019, 71, 145–160. [Google Scholar] [CrossRef]
- Muñoz, J.D.; Finley, A.O.; Gehl, R.; Kravchenko Sasha, S. Nonlinear Hierarchical Models for Predicting Cover Crop Biomass Using Normalized Difference Vegetation Index. Remote Sens. Environ. 2010, 114, 2833–2840. [Google Scholar] [CrossRef]
- Xu, M.; Lacey, C.G.; Armstrong, S.D. The Feasibility of Satellite Remote Sensing and Spatial Interpolation to Estimate Cover Crop Biomass and Nitrogen Uptake in a Small Watershed. J. Soil Water Conserv. 2018, 73, 682–692. [Google Scholar] [CrossRef]
- Breunig, F.M.; Galvão, L.S.; Dalagnol, R.; Dauve, C.E.; Parraga, A.; Santi, A.L.; della Flora, D.P.; Chen, S. Delineation of Management Zones in Agricultural Fields Using Cover–Crop Biomass Estimates from PlanetScope Data. Int. J. Appl. Earth Obs. Geoinf. 2020, 85, 102004. [Google Scholar] [CrossRef]
- Chapagain, T.; Lee, E.A.; Raizada, M.N. The Potential of Multi-Species Mixtures to Diversify Cover Crop Benefits. Sustainability 2020, 12, 2058. [Google Scholar] [CrossRef]
- Finney, D.M.; Murrell, E.G.; White, C.M.; Baraibar, B.; Barbercheck, M.E.; Bradley, B.A.; Cornelisse, S.; Hunter, M.C.; Kaye, J.P.; Mortensen, D.A.; et al. Ecosystem Services and Disservices Are Bundled in Simple and Diverse Cover Cropping Systems. Agric. Environ. Lett. 2017, 2, 170033. [Google Scholar] [CrossRef]
- Michell, P. Value of a Rising-Plate Meter for Estimating Herbage Mass of Grazed Perennial Ryegrass-White Clover Swards. Grass Forage Sci. 1982, 37, 81–87. [Google Scholar] [CrossRef]
- Westoby, M.J.; Brasington, J.; Glasser, N.F.; Hambrey, M.J.; Reynolds, J.M. ‘Structure-from-Motion’ Photogrammetry: A Low-Cost, Effective Tool for Geoscience Applications. Geomorphology 2012, 179, 300–314. [Google Scholar] [CrossRef]
- Agisoft LLC. Agisoft Metashape User Manual Professional Edition; Version 1.6; Agisoft LLC: St. Petersburg, Russia, 2020. [Google Scholar]
- Metashape Scripts. Available online: https://github.com/agisoft-llc/metashape-scripts (accessed on 30 January 2023).
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 3.6-3. Available online: https://CRAN.R-project.org/package=raster (accessed on 30 January 2023).
- Smith, R.J. Use and Misuse of the Reduced Major Axis for Line-Fitting. Am. J. Phys. Anthropol. 2009, 140, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, M.; Höfle, B. Effects of Reduced Terrestrial LiDAR Point Density on High-Resolution Grain Crop Surface Models in Precision Agriculture. Sensors 2014, 14, 24212–24230. [Google Scholar] [CrossRef]
- Friedli, M.; Kirchgessner, N.; Grieder, C.; Liebisch, F.; Mannale, M.; Walter, A. Terrestrial 3D Laser Scanning to Track the Increase in Canopy Height of Both Monocot and Dicot Crop Species under Field Conditions. Plant Methods 2016, 12, 9. [Google Scholar] [CrossRef]
- Batistoti, J.; Marcato, J.; Ítavo, L.; Matsubara, E.; Gomes, E.; Oliveira, B.; Souza, M.; Siqueira, H.; Filho, G.S.; Akiyama, T.; et al. Estimating Pasture Biomass and Canopy Height in Brazilian Savanna Using UAV Photogrammetry. Remote Sens. 2019, 11, 2447. [Google Scholar] [CrossRef]
- Grüner, E.; Astor, T.; Wachendorf, M. Biomass Prediction of Heterogeneous Temperate Grasslands Using an SfM Approach Based on UAV Imaging. Agronomy 2019, 9, 54. [Google Scholar] [CrossRef]
- Scotford, I.M.; Miller, P.C.H. Combination of Spectral Reflectance and Ultrasonic Sensing to Monitor the Growth of Winter Wheat. Biosyst. Eng. 2004, 87, 27–38. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Rotz, C.A.; Fultz, S.W.; Rayburn, E.B. Estimating Forage Mass with a Commercial Capacitance Meter, Rising Plate Meter, and Pasture Ruler. Agron. J. 2001, 93, 1281–1286. [Google Scholar] [CrossRef]
- Rueda-Ayala, V.P.; Peña, J.M.; Höglind, M.; Bengochea-Guevara, J.M.; Andújar, D. Comparing UAV-Based Technologies and RGB-D Reconstruction Methods for Plant Height and Biomass Monitoring on Grass Ley. Sensors 2019, 19, 535. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Y.; Chang, L.; Qin, Y.; Chen, J.; Qin, Y.; Du, J.; Yi, S.; Wang, Y. Estimation of Grassland Canopy Height and Aboveground Biomass at the Quadrat Scale Using Unmanned Aerial Vehicle. Remote Sens. 2018, 10, 851. [Google Scholar] [CrossRef]
- Becirevic, D.; Klingbeil, L.; Honecker, A.; Schumann, H.; Rascher, U.; Léon, J.; Kuhlmann, H. On the derivation of crop heights from multitemporal UAV based imagery. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, IV-2/W5, 95–102. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, D.; Geng, C.; Zhang, Z.; Xiang, Y.; Hu, T. Combining Plant Height, Canopy Coverage and Vegetation Index from UAV-Based RGB Images to Estimate Leaf Nitrogen Concentration of Summer Maize. Biosyst. Eng. 2021, 202, 42–54. [Google Scholar] [CrossRef]
- Borra-Serrano, I.; de Swaef, T.; Muylle, H.; Nuyttens, D.; Vangeyte, J.; Mertens, K.; Saeys, W.; Somers, B.; Roldán-Ruiz, I.; Lootens, P. Canopy Height Measurements and Non-destructive Biomass Estimation of Lolium Perenne Swards Using UAV Imagery. Grass Forage Sci. 2019, 74, gfs.12439. [Google Scholar] [CrossRef]
- Lussem, U.; Bolten, A.; Menne, J.; Gnyp, M.L.; Schellberg, J.; Bareth, G. Estimating Biomass in Temperate Grassland with High Resolution Canopy Surface Models from UAV-Based RGB Images and Vegetation Indices. J. Appl. Remote Sens. 2019, 13, 034525. [Google Scholar] [CrossRef]
- Bareth, G.; Bendig, J.; Tilly, N.; Hoffmeister, D.; Aasen, H.; Bolten, A. A Comparison of UAV-and TLS-Derived Plant Height for Crop Monitoring: Using Polygon Grids for the Analysis of Crop Surface Models (CSMs). Photogramm. Fernerkund. Geoinf. (PFG) 2016, 2, 85–94. [Google Scholar] [CrossRef]
- Heuermann, D.; Gentsch, N.; Guggenberger, G.; Reinhold-Hurek, B.; Schweneker, D.; Feuerstein, U.; Heuermann, M.C.; Groß, J.; Kümmerer, R.; Bauer, B.; et al. Catch Crop Mixtures Have Higher Potential for Nutrient Carry-over than Pure Stands under Changing Environments. Eur. J. Agron. 2022, 136, 126504. [Google Scholar] [CrossRef]
- Gentsch, N.; Heuermann, D.; Boy, J.; Schierding, S.; von Wirén, N.; Schweneker, D.; Feuerstein, U.; Kümmerer, R.; Bauer, B.; Guggenberger, G. Soil Nitrogen and Water Management by Winter-Killed Catch Crops. SOIL 2022, 8, 269–281. [Google Scholar] [CrossRef]
- Holzhauser, K.; Räbiger, T.; Rose, T.; Kage, H.; Kühling, I. Estimation of Biomass and N Uptake in Different Winter Cover Crops from UAV-Based Multispectral Canopy Reflectance Data. Remote Sens. 2022, 14, 4525. [Google Scholar] [CrossRef]
- Walter, J.; Edwards, J.; McDonald, G.; Kuchel, H. Photogrammetry for the Estimation of Wheat Biomass and Harvest Index. Field Crops Res. 2018, 216, 165–174. [Google Scholar] [CrossRef]
- Gil-Docampo, M.L.; Arza-García, M.; Ortiz-Sanz, J.; Martínez-Rodríguez, S.; Marcos-Robles, J.L.; Sánchez-Sastre, L.F. Above-Ground Biomass Estimation of Arable Crops Using UAV-Based SfM Photogrammetry. Geocarto Int. 2020, 35, 687–699. [Google Scholar] [CrossRef]



| Cover-Crop Species | Seed Density (Seeds m−2) |
|---|---|
| White Mustard (Sinapis alba L.) | 250 |
| Phacelia (Phacelia tanacetifolia Benth.) | 450 |
| Egyptian Clover (Trifolium alexandrinum L.) | 1000 |
| Oil Radish (Raphanus sativus var. oleiformis Pers.) | 180 |
| Field Pea (Pisum sativum L.) | 80 |
| Bristle Oat (Avena strigosa Schreb.) | 500 |
| Mission | Low Resolution (LR) | High Resolution (HR) |
|---|---|---|
| UAV | Parrot Bluegrass quadrocopter | DJI Mavic Air quadrocopter |
| Camera | Parrot Sequoia | DJI FC2103 |
| Sensor | monochrome | 1/2.3″ CMOS RGB |
| Focal length | 4 mm | 4.3 mm |
| Resolution | 1.2 MP | 12.3 MP |
| Aperture | f/2.2 | f/2.8 |
| Field of view | 61.9° | 73.7° |
| Mission planning | ParrotFields | Pix4Dcapture |
| Flight altitude above ground | 45 m | 15 m |
| Front overlap | 80% | 75% |
| Side overlap | 60% | 75% |
| Ground sample distance | 36.9 mm/px | 4.1 mm/px |
| Database | Cover-Crop Treatments | Canopy Shapes | ||||
|---|---|---|---|---|---|---|
| Method | All | Pure Stands | Mixture | Smooth | Medium | Rough |
| HR | 0.76 | 0.77 | 0.72 | 0.68 | 0.80 | 0.80 |
| LR | 0.75 | 0.77 | 0.71 | 0.67 | 0.79 | 0.82 |
| Ruler | 0.75 | 0.77 | 0.70 | 0.71 | 0.83 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kümmerer, R.; Noack, P.O.; Bauer, B. Using High-Resolution UAV Imaging to Measure Canopy Height of Diverse Cover Crops and Predict Biomass. Remote Sens. 2023, 15, 1520. https://doi.org/10.3390/rs15061520
Kümmerer R, Noack PO, Bauer B. Using High-Resolution UAV Imaging to Measure Canopy Height of Diverse Cover Crops and Predict Biomass. Remote Sensing. 2023; 15(6):1520. https://doi.org/10.3390/rs15061520
Chicago/Turabian StyleKümmerer, Robin, Patrick Ole Noack, and Bernhard Bauer. 2023. "Using High-Resolution UAV Imaging to Measure Canopy Height of Diverse Cover Crops and Predict Biomass" Remote Sensing 15, no. 6: 1520. https://doi.org/10.3390/rs15061520
APA StyleKümmerer, R., Noack, P. O., & Bauer, B. (2023). Using High-Resolution UAV Imaging to Measure Canopy Height of Diverse Cover Crops and Predict Biomass. Remote Sensing, 15(6), 1520. https://doi.org/10.3390/rs15061520







