Abstract
The diel vertical variations of chlorophyll a (Chl-a) concentration are thought of primarily as an external manifestation of regulating phytoplankton’s biomass, which is essential for dynamically estimating the biogeochemical cycle in inland waters. However, information on these variations is limited due to insufficient measurements. Undersampled observations lead to delayed responses in phytoplankton assessment, impacting accurate evaluations of carbon export and water quality in dynamic inland waters. Here, we report the first lidar-observed diel vertical variations of inland Chl-a concentration. Strong agreement with r2 of 0.83 and a root mean square relative difference (RMSRD) of 9.0% between the lidar-retrieved and in situ measured Chl-a concentration verified the feasibility of the Mie–fluorescence–Raman lidar (MFRL). An experiment conducted at a fixed observatory demonstrated the lidar-observed diel Chl-a concentration variations. The results showed that diel variations of Chl-a and the formation of subsurface phytoplankton layers were driven by light availability and variations in water temperature. Furthermore, the facilitation from solar radiation-regulated water temperature on the phytoplankton growth rate was revealed by the high correlation between water temperature and Chl-a concentration anomalies. Lidar technology is expected to provide new insights into continuous three-dimension observations and be of great importance in dynamic inland water ecosystems.
1. Introduction
Phytoplankton impels biogeochemical processes in inland water environments [1,2], with major impacts on carbon and energy flux, water trophic status, and global climate change to a large extent [3,4,5,6]. Chlorophyll a (Chl-a) concentration is commonly identified as a proxy for phytoplankton biomass [7], and the vertical distributions of phytoplankton in waters tend to be heterogeneous and dynamic in many cases [8,9,10]. Basic physical and physiological processes leading to this phenomenon include swimming behaviors, buoyancy control of phytoplankton, and photoacclimation of pigment content, among which light plays a critical role [11,12]. The diel fluctuation of light is considered an important environmental cue for the underlying heterogeneity pattern [13,14]. Sunlight influences photosynthesis and water temperature, driving the macromolecule biosynthesis that is essential for the growth of phytoplankton. The alternations of day and night affect vertical physiological variations of phytoplankton in lakes and oceans [6,15]. As a consequence, observations of the diel variations of Chl-a profiles are of great significance to fully understand the role of phytoplankton in biogeochemical cycling and to dynamically estimate water quality and trophic status, primary production, and harmful algae blooms in inland water environments [3,7,16,17,18].
Most previous observations of these diel inhomogeneous phenomena in inland waters were carried out through in situ measurement or buoy detection, which advanced research on the diel distribution of Chl-a [6,19,20,21]. However, these approaches are limited by either temporal resolution or complex instrument arrangement, leading to an inadequate understanding of diel vertical variations of Chl-a in lakes or reservoirs. Though passive satellite remote sensing can detect Chl-a concentration in inland waters on a large scale with high efficiency and multispectral information, it cannot operate at night and can only detect information within one optical depth during the day, omitting vertical features of Chl-a [22,23,24,25]. Hence, there is an increasing demand for high spatiotemporal, convenient, and full-day detection of diel vertical distribution of Chl-a concentration in inland waters.
Oceanic lidar is an emerging active optical remote sensing technique [15,26,27,28], which can provide continuous and highly spatiotemporal vertical bio-optical information of phytoplankton on the upper water column to three optical depths day and night [29,30]. Yet, the application of lidar in inland waters has been almost blank in the past decades, especially quantitative detection of Chl-a concentration [12,31]. Different from studies in oceans, the optical complexity of inland waters, which is not only contributed by phytoplankton but also by inorganic suspended particulate matter and colored dissolved organic matter, restricts quantitative observations utilizing lidar [22,24]. Fortunately, a recent study has demonstrated that an integrated lidar, Mie-fluorescence-Raman lidar (MFRL), has the potential to profile Chl-a concentration in inland waters [16]. It will greatly promote the observations of diel vertical variations of phytoplankton in lakes and reservoirs.
In this paper, we report the first lidar-observed diel vertical variations of inland Chl-a concentration and analyze the effects of light-induced variations in water temperature on the variations of Chl-a. Here, we assume that the contribution ratio of the diffuse attenuation coefficient from the upper water column’s biogenic components can be estimated using the surface biogenic component contributions within acceptable error bounds. A diel experiment was conducted at a fixed observatory in a large subtropical reservoir. Chl-a concentration retrieved from MFRL developed by Zhejiang University (ZJU–MFRL) was validated with the in situ data. The availability of light and vertical variations of water temperature were recorded to further analyze the inherent relationships with the diel vertical variations of Chl-a. This study can promote the comprehension of diel activities of phytoplankton and the applications of MFRL in inland waters in future works.
2. Materials and Methods
2.1. Mie-Fluorescence-Raman Lidar System
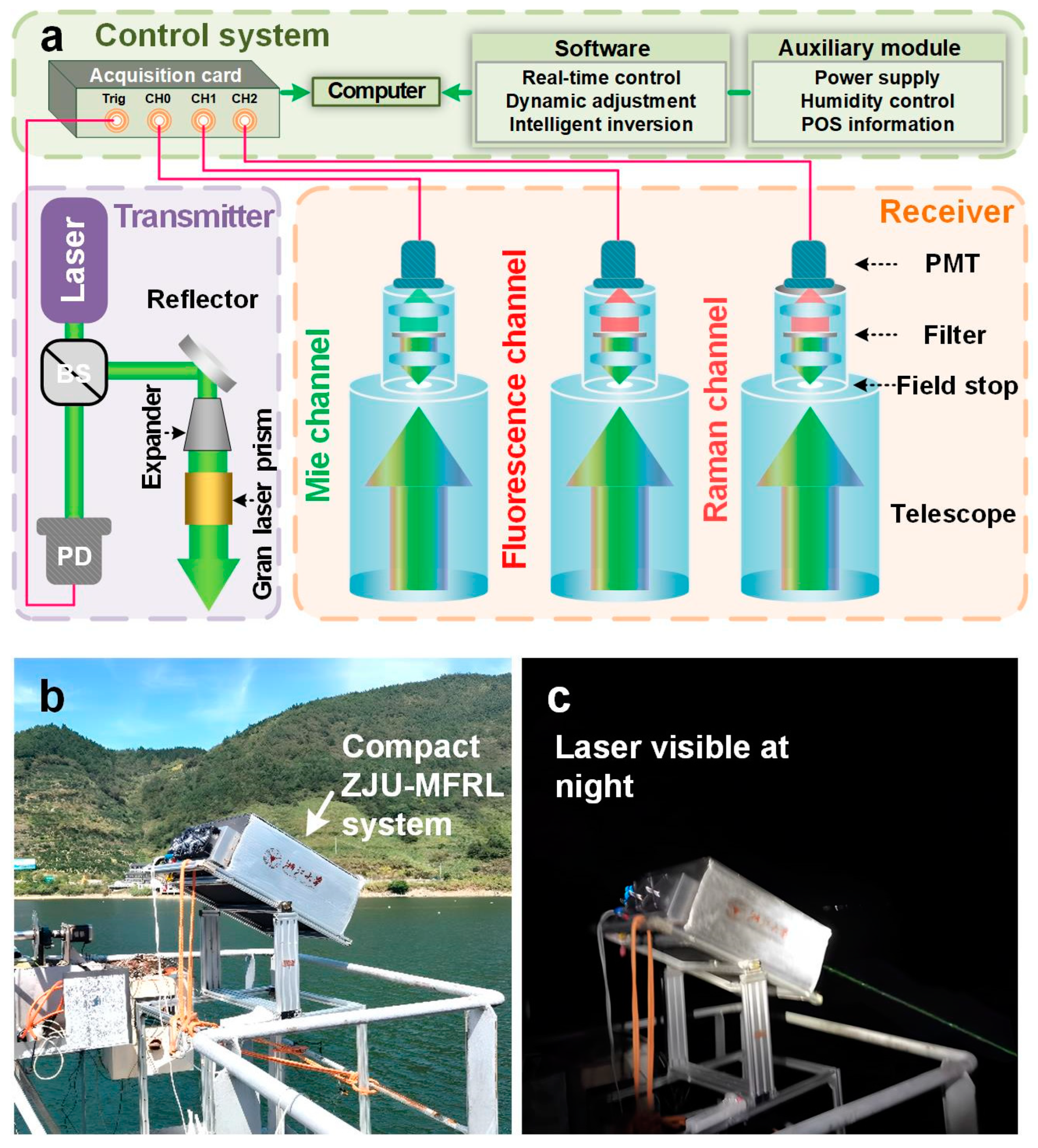
Mie-fluorescence-Raman lidar utilized in this study was developed by Zhejiang University (ZJU–MFRL). The MFRL system originated from an elastic lidar, which had been thoroughly validated by Monte Carlo simulations and analytical models, and was subsequently upgraded to its current form [32,33]. Its simplified schematic diagram is shown in Figure 1a. ZJU–MFRL is composed of a transmitting system, a receiving system, and a control system. The transmitting system primarily includes a 532 nm diode-pumped Nd:YAG solid-state laser, a reflector, a beam expander, and a Gran laser prism [34,35]. The transmitting direction and the divergence angle of the laser are adjusted by the reflector and the beam expander, respectively. The Gran laser prism is used to improve the polarization degree [33]. Three channels are employed in the receiving system for receiving Mie scattering, fluorescence scattering, and Raman scattering. The central wavelengths of optical filters for the three channels are 532 nm, 685 nm, and 650 nm, respectively. Photodetectors are utilized to convert light signals to electrical signals [36,37]. The control system comprises an acquisition card, computer, relevant software, and auxiliary modules. The acquisition card records digital electrical signals from analog electrical signals output by the photodetectors with a sampling rate of 400 MSa/s and a bandwidth of 200 MHz. Real-time remote control and adjustment can be achieved through the software module. The auxiliary system drives the power supply and uninterrupted power supply (UPS) to ensure a stable power supply. Additionally, a thermo electric cooler (TEC) and a hygrothermograph are utilized to monitor and control the system’s temperature and humidity. A position module and external antenna provide geographic coordinates, speed, and instrument attitude angles of the system. Detailed parameters of ZJU–MFRL are displayed in Table 1. The lidar system was installed on a fixed observatory with an angle of inclination away from the influence of strong specular reflection of the water surface, as shown in Figure 1b,c.

Figure 1.
(a) Schematic diagram of ZJU-MFRL system. BS: beam splitter; PD: photodetector; PMT: photomultiplier tube. (b) MFRL system worked during the day. (c) MFRL system worked at night.

Table 1.
ZJU-MFRL system parameters.
2.2. Study Area and Auxiliary Measurements
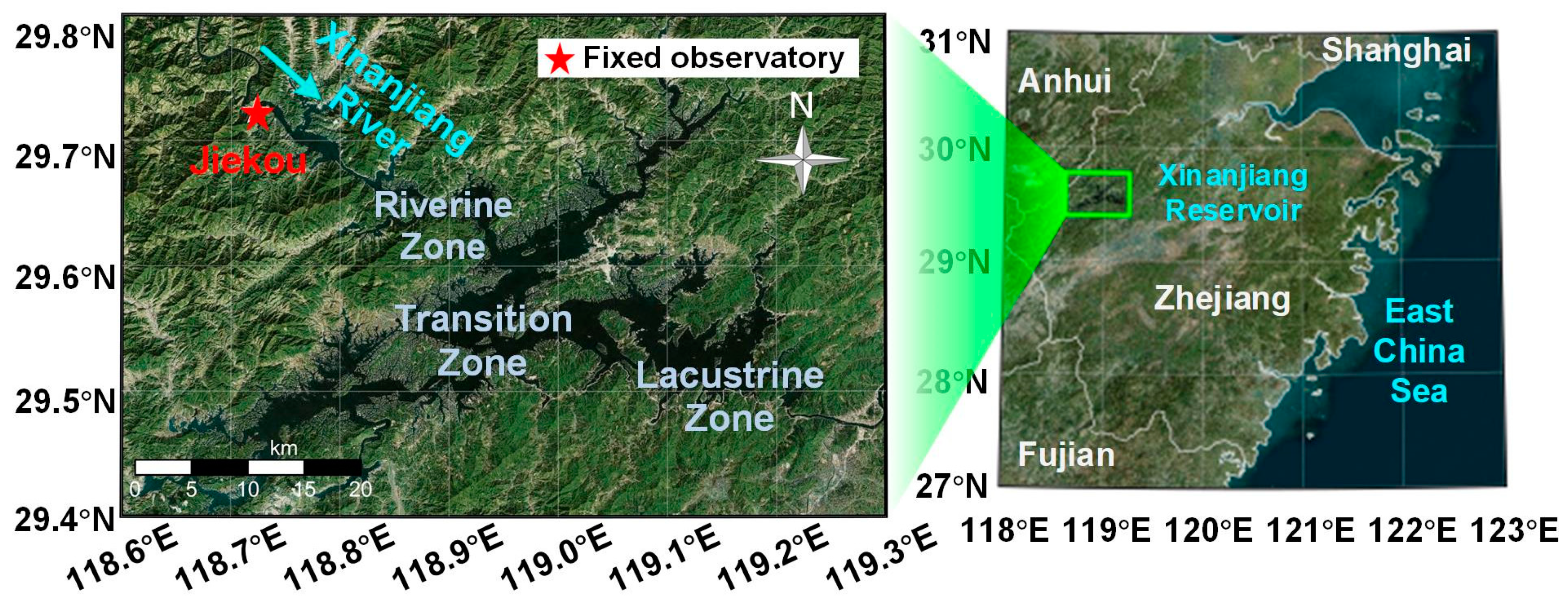
The diel experiment was conducted at Jiekou station (29°44′N, 118°433′E) in Xinanjiang Reservoir (29°22′–29°50′N, 118°36′–119°14′E) from 17:24, 23 September to 16:51, 24 September 2021. Xinanjiang Reservoir is the largest subtropical artificial reservoir in China and a crucial water source for the Yangtze River Delta region, owning the characteristics of diverse nutrient levels, turbidity, and patterns of vertical distributions of Chl-a, which can provide advantages for detecting diel phytoplankton variations [20]. Jiekou station, located at the entrance of the riverine zone where the Xinanjiang River flows into (Figure 2), serves as the main inlet of Xinanjiang Reservoir with an average water depth of 16 m. Xinanjiang River is the principal inflow of Xinanjiang Reservoir, and the average annual inflow from Xinanjiang River accounts for over 70% of the total inflow [7,38], bringing rich nutrients, which makes phytoplankton activities vigorous at Jiekou station [39].

Figure 2.
Map of Jiekou station in Xinanjiang Reservoir. The fixed observatory was marked with a red pentagram.
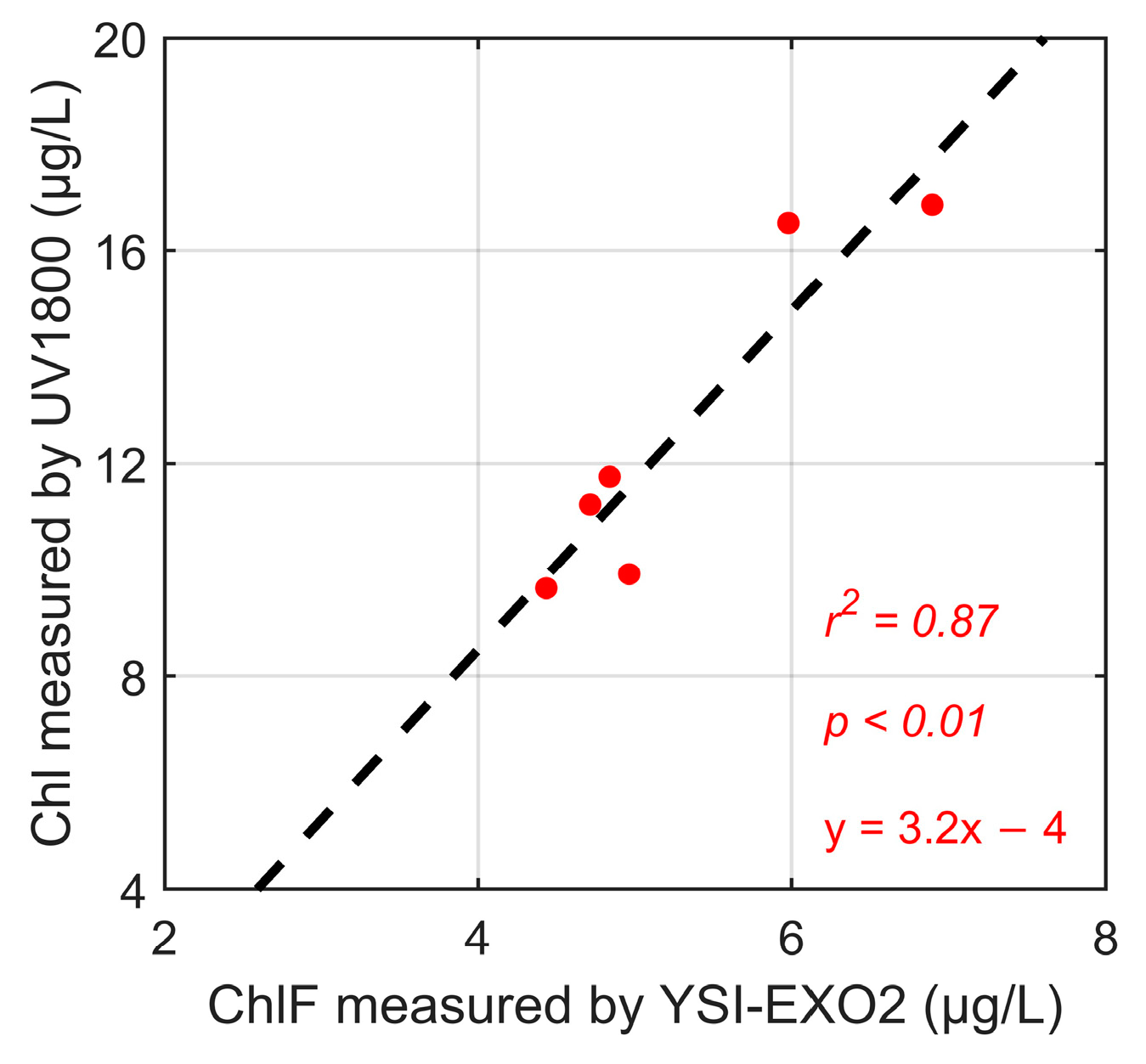
Synchronous in situ measurements were conducted to validate the retrieved results from MFRL. Vertical distribution of chlorophyll fluorescence (ChlF) profiles was collected by a multiparameter water quality sonde (YSI-EXO2 Multiparameter Sonde, YSI Inc., Yellow Springs, OH, USA) equipped with a chlorophyll fluorescence (ChlF) sensor every three hours starting from 20:02, 23 September to 16:51, 24 September. It meant that a total of eight sets of ChlF profiles measured by YSI-EXO2 were recorded and used to validate the lidar results subsequently. The profiles were binned to a depth resolution of 0.5 m for further analysis. However, due to the limitations of the ChlF sensor [40,41,42], the calibration of ChlF was necessary to be done by discrete Chl-a measured via spectrophotometry. Two sets of spectrophotometry results were collected to calibrate the YSI-EXO2 data. The procedures for implementing spectrophotometry are as follows. Discrete measurements of Chl-a were determined through water samplings taken from 1 m, 5 m, and 7 m below the water surface around 20:02, 23 September, and 08:17, 24 September, respectively. The water samples were initially filtered through Whatman GF/F filters. Subsequently, the samples were stored in a refrigerator at temperatures below −20 °C until arriving at the laboratory in 24 h (GB17378.7-2007 [43], Chinese Water Quality Standards). Upon returning to room temperature, Chl-a was extracted using 90% acetone according to NASA protocols and measured using a laboratory spectrophotometer (UV-1800, SHIMADZU Co., Ltd., Kyoto, Japan) at wavelengths of 630 nm, 647 nm, 664 nm, and 750 nm through absorption photometry (Table A1) [44]. ChlF profiles were calibrated by Chl-a measured through spectrophotometry with r2 of 0.87 (Figure A1), indicating the accuracy of in situ detection in this work [16,45]. The vertical distribution of water temperature profiles was measured by a high-frequency buoy attached to a YSI-EXO2 multiparameter sonde equipped with a temperature sensor. The buoy was measured every three hours at the top of the hour. The comprehensive sampling results with wider coverage time were conducive to subsequent pattern analysis.
2.3. MFRL Bio-Optical Properties Retrieval Model
An MFRL bio-optical properties retrieval model was proposed for the lidar attenuation coefficient klidar and Chl-a concentration based on the Fernald method and bio-optical model [16,46,47]. Herein, klidar was retrieved first from Mie signals. The values of the diffusion attenuation coefficient Kd were approximated by the values of klidar. The wide field of view of 200 mrad in MFRL ensured that klidar was very close to the values of Kd [48,49]. Then, Kd can be calculated from the Mie signal as
where z is the depth, D(z) is the range-corrected Mie signal, Kd,p is the diffuse attenuation coefficient of the biogenic and inorganic components, Kd,w is the diffuse attenuation coefficient of the pure water (0.0452 m−1) [50], RS is the ratio between the lidar ratios of the suspended matter R (50 sr) and water molecules Rw (233 sr) [16,50], can be expressed as , zc is the boundary depth, and Kd,p(zc) can be estimated by the slope method [51].
In Case 1 water, Kd was utilized directly to characterize Chl-a through the bio-optical model, as shown in (2)
where Chla represents Chl-a concentration, Kd,bio(λ) is the particle diffuse attenuation coefficient calculated as a function of Chl-a, χ(λ) and e(λ) are scaling factors, which are 0.0474 and 0.6667 at 532 nm, respectively [47]. The total Kd is generally regarded as the sum of Kd,w, Kd,bio, and Kd,x, which represent the optical properties of pure water, the combined impacts of all biogenic components, and the influences of all inorganic components, respectively. Here, we assume that the contribution ratio of the diffuse attenuation coefficient from the upper water column’s biogenic components can be estimated using the surface biogenic component contributions within acceptable error bounds. In other words, the ratio Kd,bio/(Kd − Kd,w) can be regarded as a constant that can be acquired from surface values to some extent. Consequently, Chl-a concentrations can be derived after calibration at the water surface using the Raman channel signal and fluorescence channel signal, which can be depicted as
where z is depth, F{*} is the bio-optical model to transfer the attenuation to the Chl-a, Kd,bio(0) is the Chl-a component of the diffuse attenuation coefficient from the surface Chl-a, which is calculated from the bio-optical model.
2.4. Statistical Analysis
Statistical analysis was conducted to verify the accuracy of the lidar-retrieved Chl-a concentration. The root-mean-square relative difference (RMSRD) was utilized here for reflecting the ZJU-MFRL performance realistically, that is
where N is the total number of sampling points, is the lidar-measured value, and is the in situ-measured value. In addition, Pearson’s correlation coefficient and paired sample t-test were utilized to analyze correlation. Notations and their definitions and dimensions used in this paper are shown in Table A2.
3. Results
3.1. Consistency Check
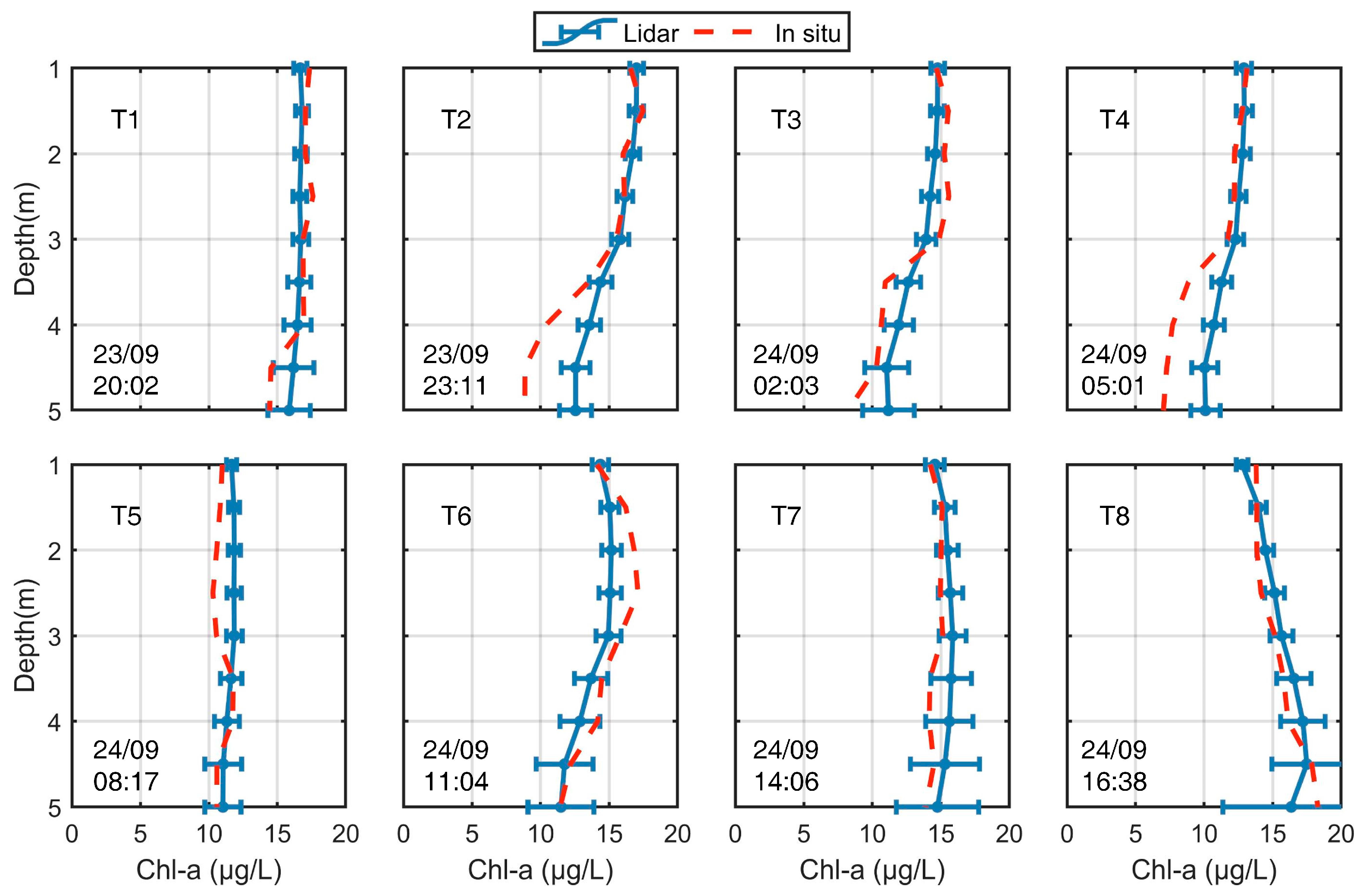
The performance of ZJU-MFRL was estimated from the detection accuracy of Chl-a concentration. In situ measurements of Chl-a concentration were carried out at eight different moments (T1-T8) to validate the reliability of lidar-retrieved Chl-a concentration, as shown in Figure 3. In the general case, the results showed that the lidar-retrieved Chl-a concentration matched relatively well with the in situ data in terms of variation trends and values at almost all moments. It could be found that there were relatively obvious overestimations of approximately 3.7 μg/L with RMSRD of 14.7% at moment T2 and of approximately 3.0 μg/L with RMSRD of 18.1% at moment T4. These overestimations may be due to the overestimations of Kd caused by a biased lidar ratio and multiple scattering effects on lidar retrieval. An underestimated lidar ratio, along with spot divergence that exceeds the received field of view with depth, may both contribute to an overestimation of Kd [49,52]. In contrast, a slight underestimation of approximately 2.0 μg/L with RMSRD of 6.1% occurred at moment T6. This deviation may be generated by the increased phytoplankton content and relatively stable inorganic matter at the depth of ~2.5 m, which slightly deviated from our hypothesis [16].

Figure 3.
Comparison of Chl-a profiles from ZJU-MFRL and in situ measurements at different sampling times. The blue error bars represented the lidar-retrieved Chl-a, and the red dotted line represented the in situ-measured Chl-a. The different sampling points were labeled as T1–T8, and their sampling times were also labeled, respectively.
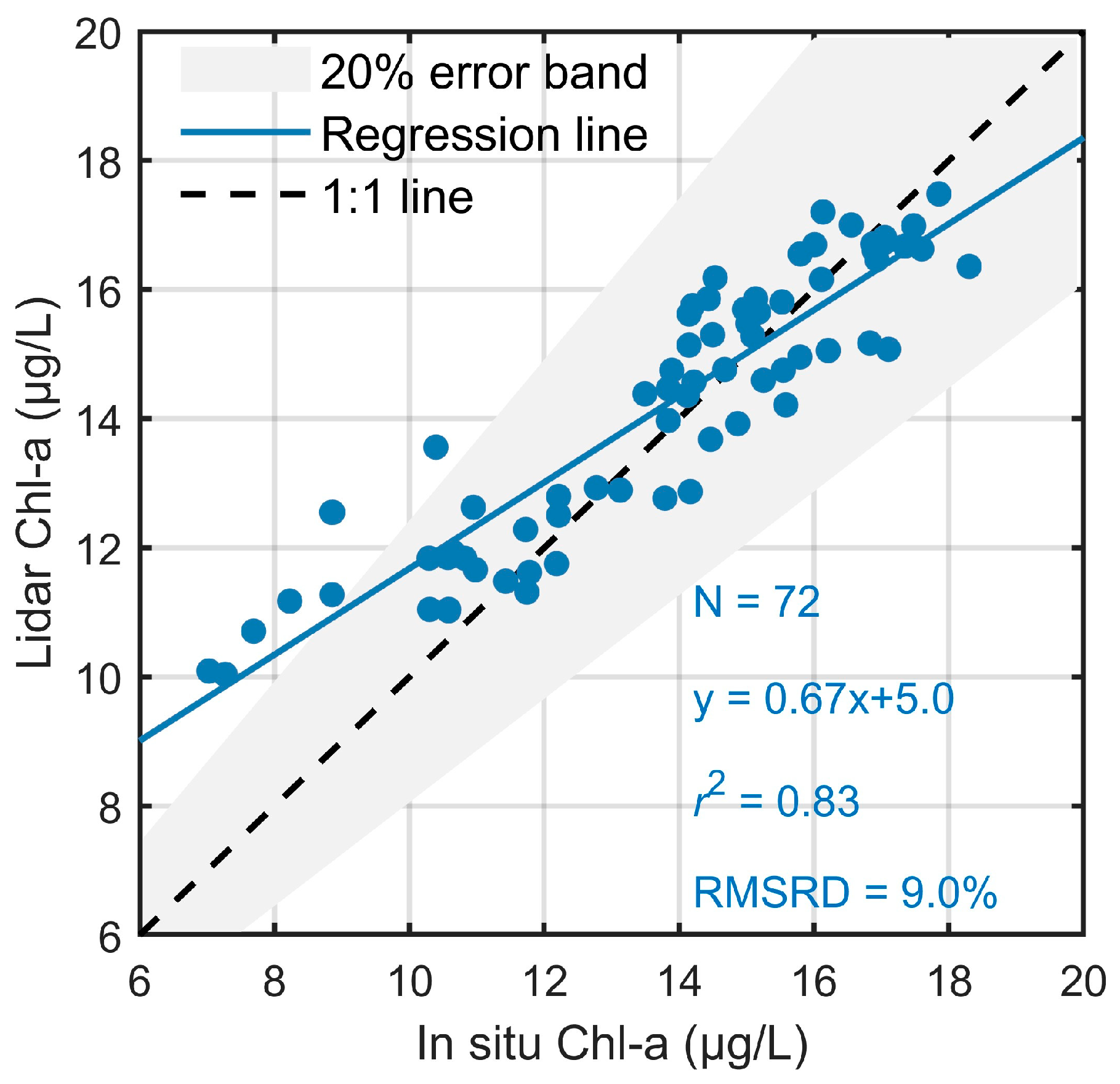
Furthermore, the consistency between Chl-a concentration from ZJU-MFRL and in situ measurement was analyzed in Figure 4. There was a total of 72 sampling points verified with 0.83 and RMSRD of 9.0%, and most retrieved results were within the 20% error band, indicating the dispersion of the two groups of data was rather small. One should be noted that some biases were a bit obvious when the in situ values were relatively low (~10 μg/L), which may indicate that the relatively stable inorganic matter would have effects on the derived results at lower Chl-a concentrations under the retrieval method in this paper [9,16]. The slope and the ordinate at the origin from the regression relationship also showed that Chl-a was slightly overestimated or underestimated by MFRL accordingly when it was relatively low or high, which was consistent with the analysis about Figure 3. Overall, the detection results from ZJU-MFRL remarkably demonstrated that MFRL could restore the original spatiotemporal distribution of Chl-a concentrations.

Figure 4.
Validation results for lidar-retrieved and in situ Chl-a concentration. The gray background represented 20% error band, the blue solid line was the regression line, and the black dotted line indicated a 1:1 line.
3.2. Diel Vertical Variations of Inland Chl-a Concentration
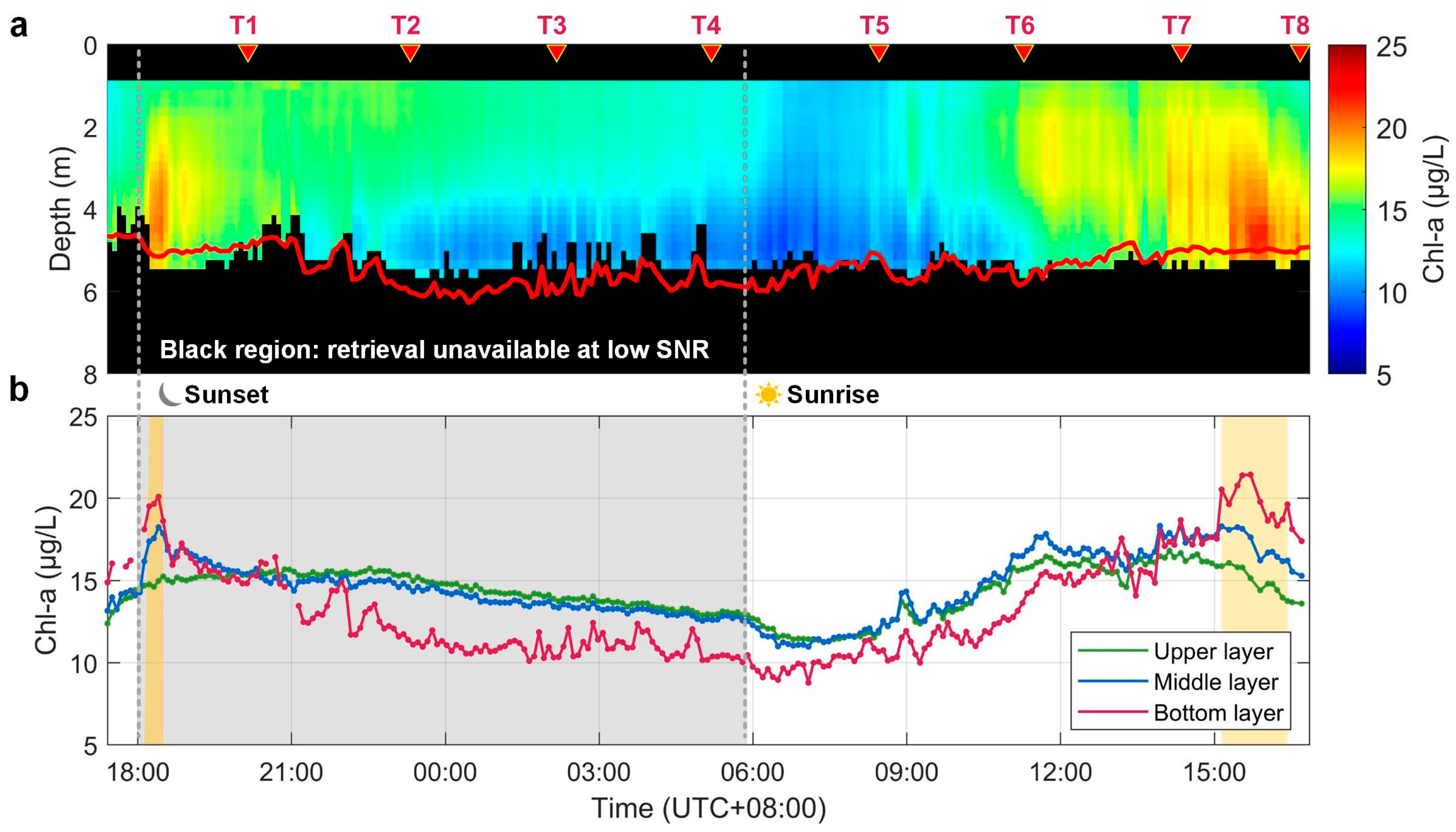
The diel continuous measurement with ZJU-MFRL was carried out from 23 September to 24 September 2021, and the depth-resolved Chl-a concentrations retrieved from MFRL data are shown in Figure 5a. Sunset and sunrise were at 17:59 on 23 September and 05:53 on 24 September, respectively. The black regions were not considered due to the low SNR. Though the maximum penetration depth for MFRL was approximately 5.5 m, the detection optical depth was almost up to three optical depths (red line in Figure 5a), beyond which the lidar signal was generally not usable due to low SNR, indicating that the penetration depth basically reached the detection limit of lidars [29]. The relatively shallow penetration performance here was the result of the relatively high Chl-a concentration and relatively low water transparency at Jiekou station [12,16]. During the experiment period, Chl-a concentrations had noticeable variations on both temporal and spatial scales. The subsurface phytoplankton layer was first observed just after sunset, with a depth of ~4 m and a magnitude of ~20 μg/L. As the night deepened, the vertical distribution of Chl-a gradually became relatively homogeneous in the upper water column and developed towards the form that decreased with depth. This state remained until just before sunrise the next day, when Chl-a concentration fluctuated around 13 μg/L. As the sun rose, Chl-a concentration at all depths showed an obvious decline, and by around 08:00 the values of Chl-a had dropped to ~11 μg/L. Soon after, Chl-a concentrations gradually rebounded with a relatively uniform vertical distribution. Then, the second observed subsurface phytoplankton layer occurred after 15:00, and it lasted for more than an hour with a depth of ~4.5 m and a magnitude of ~22 μg/L. At the end of the experiment, there was a sporadic period of subsurface phytoplankton layer with Chl-a concentrations becoming lower gradually.

Figure 5.
(a) The diel distribution of Chl-a profiles in Jiekou station with three optical depths in the red line. (b) Chl-a concentration in the detected upper (0.7–1 m), middle (2–3 m), and bottom layers (4–5 m) observed by ZJU-MFRL. The gray background represents nighttime, and the yellow background indicates the presence of SPL.
Lidar-retrieved Chl-a concentrations at different depths were also plotted according to the timeline in Figure 5b. Chl-a concentrations from the detected upper (0.7–1 m), the middle (2–3 m), and the bottom (4–5 m) layer were depicted to clearly observe the subtle and rapid vertical variations of Chl-a, respectively. The values and variations of Chl-a from the upper and middle layers were almost consistent throughout the experiment, except for the periods in which subsurface phytoplankton emerged. However, when compared to the bottom layer, these variations were not exactly the same. Chl-a concentrations from the bottom layer showed greater fluctuation than those from the upper water column during the detection period, which was relatively higher when the subsurface phytoplankton layer occurred and relatively lower at almost other times.
3.3. The Relationships between Vertical Variations of Phytoplankton and Water Column Temperature
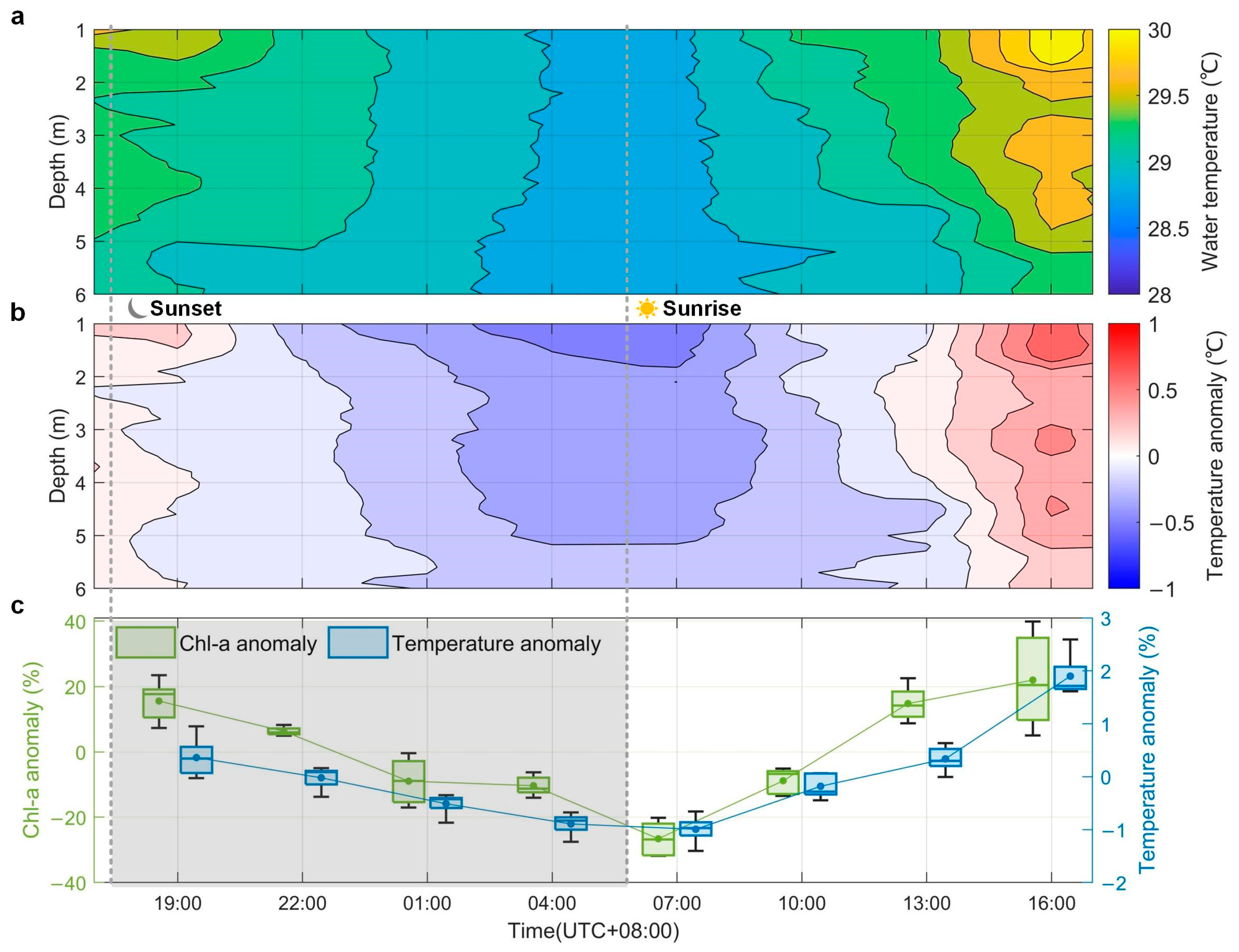
Light is essential for photosynthesis, which is present in periodic seasonal and diel cycles. Meanwhile, light is also responsible for the rise in water temperature, affecting the growth and division of phytoplankton [1,53]. To further understand the potential causes of the diel vertical variations of phytoplankton, water temperature together with its anomaly were displayed in Figure 6a,b. The water temperature, measured every three hours, was subtracted by its mean values at each depth to obtain the anomaly. Just after the sunset, the water temperature decreased with depth in the upper layer. Yet, there was an obvious uplift at ~4 m between 18:00 and 19:00, 23 September. The first observed subsurface phytoplankton layers in Figure 5 might be closely related to the temperature anomaly, as shown in Figure 6b. Thereafter, as the night fell, the water temperature dropped over time and depth in the absence of solar radiation. The downward trends of Chl-a concentration in the upper, middle, and bottom layers were almost consistent with it at this time. In addition, the relatively lower values of Chl-a in the bottom layer correspond to the relatively lower water temperature. Although the sun rose at 05:53 on 24 September, the water temperature did not rise immediately, and the negative temperature anomaly was at its lowest until around 08:00. At the same time, Chl-a concentration at all depths reached the lowest values after sunrise. Then, with the strengthening of the solar radiation, the water temperature together with Chl-a concentration increased from the water surface. Afterward, at around 15:00, the water temperature from 3 m to 5 m started to be a bit higher than before, with obvious positive temperature anomalies appearing. It was at the same time that the second subsurface phytoplankton layer occurred.

Figure 6.
Distribution of (a) water temperature, and (b) water temperature anomaly measured by buoy YSI-EXO2. (c) Quartile diagram of anomalies of Chl-a concentration and water temperature at different depths as a function of sampling moment. Their mean values were connected by lines, respectively. The gray background represents nighttime.
Anomaly of Chl-a concentration was also calculated together with that of water temperature in Figure 6c to look for the variations in both over time. For each sampling moment, the two anomalies showed the same variation trend. At ~19:00 on September 23, the Chl-a concentration anomaly was relatively high (15.6%) and the temperature anomaly also displayed a relatively high level (0.4%). The two anomalies fell together over time until around 07:00 on September 24, with a Chl-a concentration anomaly of −26.6% and a water temperature anomaly of −1.0%. Subsequently, as illustrated in Figure 5b and Figure 6b, anomalies were both rising until the evening came, with the growth in Chl-a concentration (22.0%) and the increase of water temperature (1.9%) at ~16:00. It was interesting to find that the longest box chart of Chl-a concentration anomaly appeared around 16:00, when the second subsurface phytoplankton layer formed.
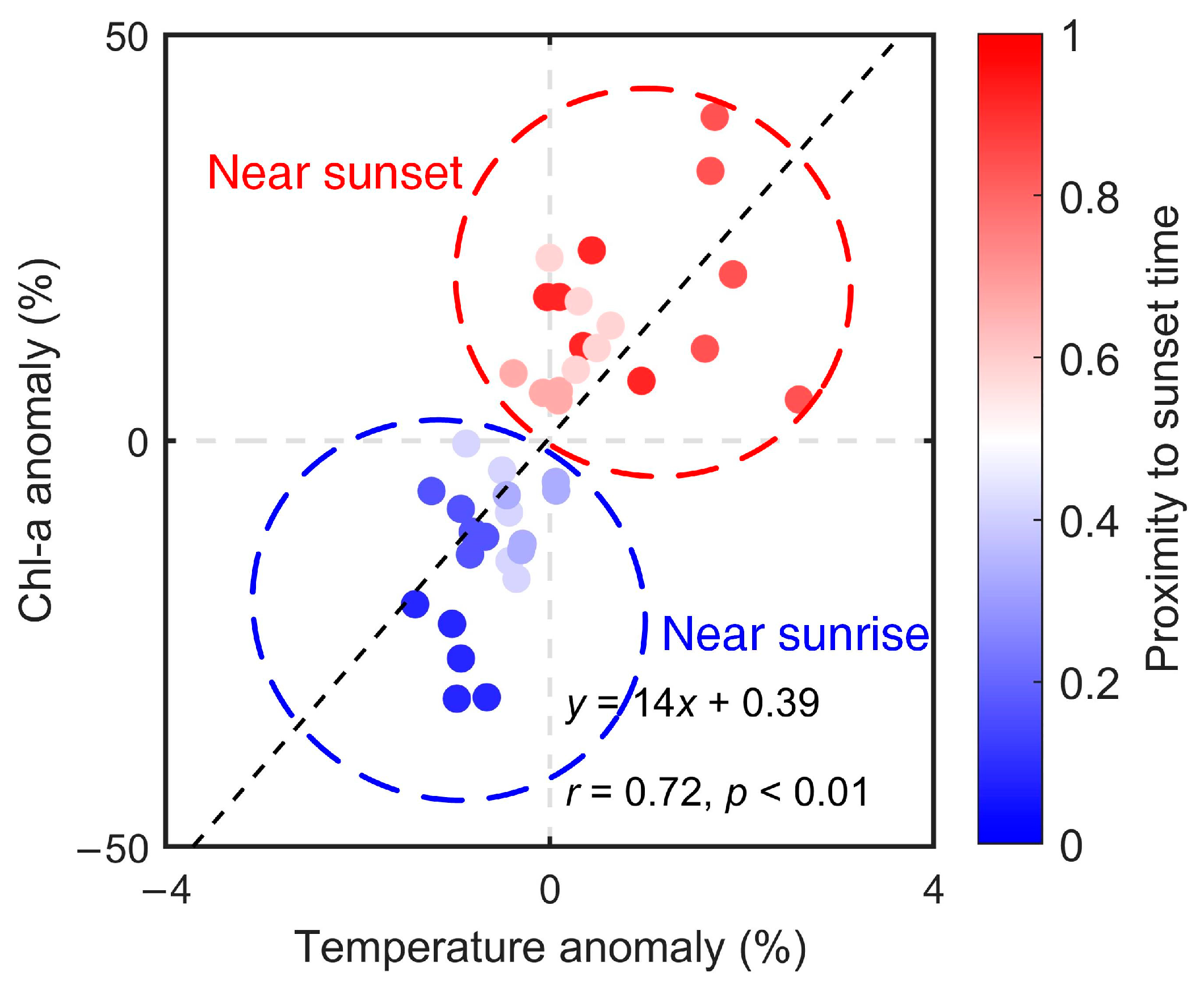
The relationship between the anomalies of Chl-a concentration and water temperature was further analyzed in Figure 7. In the general case, the diel variation of Chl-a concentration was positively correlated with that of water temperature. The results showed that their variations were highly correlated (r = 0.72, p < 0.01), demonstrating that diel variations of Chl-a were possible outcomes of water temperature changes due to solar radiation. Meanwhile, it was distinctly observed that the closer to sunrise, the greater the negative anomalies, and the closer to sunset, the greater the positive anomalies.

Figure 7.
Relationship between Chl-a concentration anomaly and water temperature anomaly. The value “1” in the color bar means sunset time and the value “0” means sunrise time. Shades of red (blue) points indicate their proximity to sunset (sunrise) time.
4. Discussion
Lidar detection of phytoplankton has flourished in recent years. However, in stark contrast to the relatively broad applications in oceans [49,51,54,55], there were only a handful of applications of lidar in inland waters in the past decades, especially quantitative detection of Chl-a concentration. Chen et al. utilized shipborne lidar to detect subsurface phytoplankton layers in artificial reservoirs [31]. They conducted a qualitative comparison with in situ results, demonstrating the feasibility of using lidar to detect phytoplankton layers in inland waters. Moore et al. reported a cyanobacteria bloom in a natural lake and analyzed the extensive relationship between vertical phytoplankton populations and cross-polarized channel signal strength measured by airborne polarization lidar qualitatively [12]. This pioneering work demonstrated the ability of lidar to characterize the various forms of vertical Chl-a profiles in inland waters. Furthermore, it has been proven that the Mie-fluorescence-Raman lidar has the potential to observe vertical Chl-a concentration distributions quantitatively in inland waters in our previous study [16]. Herein, we modified the bio-optical model proposed by Morel and Maritorena on the basis that the vertical variations of the optical properties can respond to the vertical variations of Chl-a [12,31,47]. By obtaining the contribution ratio from Raman normalized fluorescence signals, we corrected for the impacts of nonalgal factors within an acceptable range of error. Then, the feasibility of MFRL was further proven. The correlation of Chl-a concentration between ZJU-MFRL and in situ measurements in this paper was a bit lower than that of 0.92 from the previous four-month shipboard experiment, which may be due to the relatively concentrated distribution of the data, spreading over 10 μg/L to 18 μg/L (Figure 4). Nevertheless, the RMSRD of 9.0% here was relatively better than that of 16.6%, which may be a result of our hypothesis being more in line with the water bio-optical properties in this experiment. Although there were some relatively obvious overestimations at moments T2 and T4 in Figure 3, the vertical trends of Chl-a for lidar and in situ instruments were consistent, which indicated our analysis and conclusions did not deviate from the right direction. The penetration depth of lidar technology is also a notable problem. Due to the SNR of lidar systems, the detection depth of lidar is usually within three optical depths [29], ranging from tens of meters in the relatively clean ocean to several meters in rather turbid lakes [12,16,49]. At such penetration depths, lidars are sometimes difficult to make complete observations of biogeochemical phenomena, such as SPL, in water columns. This poses a challenge to the evolution of future lidar transmission and detection systems. Anyway, the combined utilization of Mie, fluorescence, and Raman channel signals complements their weakness in Chl-a concentration detection in inland water, enabling the quantitative diel vertical measurements and further cooperation with other synchronous technologies [29,56,57,58]. Meanwhile, MFRL also has the advantages compared with other different platform-based or complex design lidars due to its high spatial and temporal resolution, easy deployment, and lower cost [23,26,59,60,61].
The underlying pattern in the diel vertical variations of Chl-a concentration is another focus in this paper. Different phytoplankton species exhibit varied responses to diel changes [19,53,62]. In the general case, the phytoplankton community in the water column is of great diversity, making it difficult to estimate the response of all phytoplankton to diel variations in a given area based on a single population alone. Previous studies have shown that for specific phytoplankton populations, such as certain cyanobacteria and diatoms, Chl-a reaches its minimum near sunrise and its maximum near sunset [13,53]. The results presented in this paper are consistent with this trend, aiding in assessing regional phytoplankton compositions and understanding the ecological implications of diel phytoplankton variations. Therein, light is the fundamental factor driving this phenomenon. On one hand, light allows phytoplankton to photosynthesize during daytime, promoting biomass accumulation and phytoplankton growth and further boosting the accumulation of Chl-a [21,63]. As shown in Figure 7, Chl-a concentration is obviously regulated by the alternation of day and night. On the other hand, solar radiation causes variations in water temperature. Due to the dynamics of the water column, the water body would exhibit mixing, thermal stratification, or temperature anomalies at certain depths. Proper water temperature is conducive to phytoplankton growth and can accelerate the phytoplankton growth rate [5,64]. In this study, the significant fluctuation in bottom Chl-a concentration observed clearly indicates that this is an effect of the corresponding water temperature (Figure 5b and Figure 6b). That is, the Chl-a concentration at different depths changes positively with water temperature. Thermal stratification is also intimately linked with the formation of subsurface phytoplankton layers [20]. For subtropical monomictic reservoirs like Xinanjiang Reservoir, the thermal stratification of the upper water column is gradually eroded in autumn, turning into a mixing distribution [65,66,67]. Likewise, the vertical distributions of phytoplankton tend to be uniform in the upper water column in most cases in this season, accompanying some subsurface phytoplankton layers. The distribution and anomalies of water temperature provided a sound explanation for the lidar-observed diel vertical variations of Chl-a concentration. Therefrom, we can see that lidar captured the high spatiotemporal resolution changes in great detail, which could provide new insights into bio-optical vertical structures in inland waters. This dataset would complement and promote studies in diel variations of phytoplankton in inland waters.
5. Conclusions
In this paper, we report the first MFRL observation about diel vertical distributions of Chl-a profiles in inland water. A more particular knowledge of the performance of MFRL was acquired in this diel observation. Although some biases were present in the MFRL-detected Chl-a profiles compared to the in situ data due to some assumptions adopted in this study, the strong agreement with r2 of 0.83 and RMSRD of 9.0% between the lidar-retrieved and in situ measured Chl-a concentration verified the feasibility of the MFRL bio-optical retrieval method, and the diel vertical Chl-a profiles observation was realized at approximate cost. This result indicated that Mie–fluorescence–Raman lidars can monitor the diel vertical distribution of phytoplankton exclusively and continuously in inland waters. Passive remote sensing data can provide large-scale and high-frequency views for near-surface environmental changes. However, it cannot offer adequate vertical information at night, leading to incomplete cognition of diel phytoplankton variations [15,26]. Buoy detection in inland waters can obtain accurate Chl-a profiles and other synchronous environmental information. Yet, the observation interval is generally recorded in hours, which may ignore the dynamic variations occurring in a short period [21,38,68]. The continuous monitoring ability and high time resolution of MFRL can complement these shortcomings and provide new insights into diel bio-optical vertical structure observations. More experiments will be carried out in more water bodies to further expand the applications of this technology in the coming works.
This study also described the variations of phytoplankton under the day-night alternation. Despite the upper water column tending towards mixing in autumn, an obvious subsurface phytoplankton layer together with maximum Chl-a values occurred during the experiment period with a depth of ~4 m near sunset. Moreover, the minimal Chl-a values were observed nearly two hours after sunrise, indicating changes in water temperature due to solar radiation affected phytoplankton growth rates. The water temperature variations also agreed well with the Chl-a concentration variations. These results revealed that light availability and water temperature were powerful driving factors for the vertical diel variations of Chl-a. Light and water temperature influence photosynthesis in periodic seasonal and diel cycles. Although seasonality of light and water temperature is typically resolved in ocean biogeochemical/ecosystem models because of its significance for seasonal succession and biogeography of phytoplankton, the diel cycle is often not explicitly addressed. The mechanisms of phytoplankton competition [1], energy storage [6], and physiological behaviors [13] are not yet well understood. Mie–fluorescence–Raman lidars create a new approach to observing diel heterogeneous Chl-a distribution. More detailed research will be conducted in the future in combination with other advanced techniques, contributing to clarifying the subtle temporal variations.
Author Contributions
Conceptualization, H.Z., Y.Z. and D.L.; methodology, H.Z., Y.H. and P.X.; software, Y.C., H.W. and C.J.; validation, H.Z.; formal analysis, P.X., L.L. and W.L.; investigation, Y.C., H.W. and L.W. (Lingyun Wu); data curation, L.L. and W.L.; writing—original draft preparation, H.Z.; writing—review and editing, Y.Z., L.W. (Lan Wu), W.S., C.L. and D.L.; visualization, Q.G. and M.Y.; supervision, Y.Z. and D.L.; project administration, D.L.; funding acquisition, Y.Z. and D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Key Research and Development Program of China (2022YFB3901704); National Natural Science Foundation of China (NSFC) (62205289; 61835001); Fundamental Research Funds for the Central Universities (226-2023-00138); Zhejiang Provincial Natural Science Foundation of China (LQ24F050002; LQ23F050011); Project of Hangzhou Institute of Environmental Protection Science; Ningbo Natural Science Foundation (2022J153); Donghai Laboratory Preresearch Project (DH2022ZY0003); the Fundamental Research Funds for the Central Universities (2023FZZX06-03); State Key Laboratory of Extreme Photonics and Instrumentation Innovation Program (EPI2023ZD01).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors thank the scientists at the Chun’an Branch of Ecological Protection Bureau, Hangzhou for providing buoy data and analyzing in situ data. The authors also thank Zhiying Yue at Institute of Hydrobiology, Chinese Academy of Sciences for her professional guidance on water sampling.
Conflicts of Interest
We declare no conflicts of interest.
Appendix A
Discrete measurements of Chl-a concentrations were determined through water samplings through a laboratory spectrophotometer (UV-1800, SHIMADZU Co., Ltd., Kyoto, Japan) at wavelengths of 630 nm, 647 nm, 664 nm, and 750 nm through absorption photometry. The specific measurements were described in the main text. Here, the spectrophotometer-measured results were displayed in Table A1.

Table A1.
Chl-a concentrations from different depths measured at two sampling moments by UV-1800 in the laboratory.
Table A1.
Chl-a concentrations from different depths measured at two sampling moments by UV-1800 in the laboratory.
| Water Depth (m) | Sampling Moment 1 | Sampling Moment 2 |
|---|---|---|
| 20:02, 23 September 2021 | 08:17, 24 September 2021 | |
| Chl-a (μg/L) | Chl-a (μg/L) | |
| 1 | 16.85 | 11.75 |
| 5 | 16.51 | 11.23 |
| 7 | 9.92 | 9.65 |
Afterwards, the ChlF profiles detected by YSI-EXO2 were calibrated by the spectrophotometer-measured Chl-a concentrations, as shown in Figure A1. The correlation between the two measured results was 0.87.

Figure A1.
Calibration result between ChlF measured by YSI-EXO2 and Chl-a measured by UV-1800 in the laboratory.
Figure A1.
Calibration result between ChlF measured by YSI-EXO2 and Chl-a measured by UV-1800 in the laboratory.
Appendix B
The notations, along with their definitions and dimensions, used throughout this manuscript are detailed in Table A2. This table provides a comprehensive reference for understanding the various symbols and terms employed in the study.

Table A2.
Used notations, definitions and dimension in the manuscript.
Table A2.
Used notations, definitions and dimension in the manuscript.
| Notations | Definition | Dimension |
|---|---|---|
| klidar | Lidar attenuation coefficient | m−1 |
| Kd | Diffusion attenuation coefficient | m−1 |
| Kd,bio | Diffusion attenuation coefficient due to biogenic components | m−1 |
| Kd,w | Diffusion attenuation coefficient due to water | m−1 |
| Kd,x | Diffusion attenuation coefficient due to inorganic components | m−1 |
| Kd,p | Diffusion attenuation coefficient due to biogenic and inorganic components | m−1 |
| χ(λ) | A linear factor that relates Chl to Kd,bio | N/A |
| e(λ) | An exponential factor that relates Chl to Kd,bio | N/A |
| D(z) | The range-corrected Mie signal | W·m2 |
| z | Water depth | m |
| zc | Boundary depth in the retrieval process | m |
| R | Lidar ratios of the suspended matter | sr |
| Rw | Lidar ratios of the water molecules | sr |
| RS | The ratio between the lidar ratios of the suspended matter and water molecules | N/A |
| Chla | Chl-a concentration | μg/L |
| F{*} | The bio-optical model that transfers the attenuation to the Chl-a | N/A |
| N | The total number of sampling points | N/A |
| xi | The lidar-measured Chl-a concentration | μg/L |
| The in situ measured Chl-a concentration | μg/L |
References
- Tsakalakis, I.; Follows, M.J.; Dutkiewicz, S.; Follett, C.L.; Vallino, J.J. Diel light cycles affect phytoplankton competition in the global ocean. Glob. Ecol. Biogeogr. 2022, 31, 1838–1849. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Cao, Z.; Lai, L.; Geng, J.; Yu, K.; Zhan, P.; Liu, Z. Effects of satellite temporal resolutions on the remote derivation of trends in phytoplankton blooms in inland waters. ISPRS J. Photogramm. Remote Sens. 2022, 191, 188–202. [Google Scholar] [CrossRef]
- Leach, T.H.; Beisner, B.E.; Carey, C.C.; Pernica, P.; Rose, K.C.; Huot, Y.; Brentrup, J.A.; Domaizon, I.; Grossart, H.-P.; Ibelings, B.W.; et al. Patterns and drivers of deep chlorophyll maxima structure in 100 lakes: The relative importance of light and thermal stratification. Limnol. Oceanogr. 2018, 63, 628–646. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Hu, Y.; Hostetler, C.A.; Dall’Olmo, G.; Rodier, S.D.; Hair, J.W.; Trepte, C.R. Space-based lidar measurements of global ocean carbon stocks. Geophys. Res. Lett. 2013, 40, 4355–4360. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Becker, K.W.; Collins, J.R.; Durham, B.P.; Groussman, R.D.; White, A.E.; Fredricks, H.F.; Ossolinski, J.E.; Repeta, D.J.; Carini, P.; Armbrust, E.V.; et al. Daily changes in phytoplankton lipidomes reveal mechanisms of energy storage in the open ocean. Nat. Commun. 2018, 9, 5179. [Google Scholar] [CrossRef]
- Luo, J.; Hu, Z.; Chen, X.; Li, X.; Liu, L.; Yang, M.; Miao, H.; Chu, Y.; Xu, P.; Wang, F. Chlorophyll maxima layer in a large subtropical reservoir (Xinanjiang Reservoir): Spatial development process and limitation by CO2 and phosphorus. Water Res. 2022, 222, 118912. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Morel, A.; Hooker, S.B. Vertical distribution of phytoplankton communities in open ocean: An assessment based on surface chlorophyll. J. Geophys. Res. Ocean. 2006, 111, C08005. [Google Scholar] [CrossRef]
- Schulien, J.A.; Behrenfeld, M.J.; Hair, J.W.; Hostetler, C.A.; Twardowski, M.S. Vertically-resolved phytoplankton carbon and net primary production from a high spectral resolution lidar. Opt. Express 2017, 25, 13577–13587. [Google Scholar] [CrossRef]
- Xue, K.; Zhang, Y.; Ma, R.; Duan, H. An approach to correct the effects of phytoplankton vertical nonuniform distribution on remote sensing reflectance of cyanobacterial bloom waters. Limnol. Oceanogr. Methods 2017, 15, 302–319. [Google Scholar] [CrossRef]
- Cullen, J.J. Subsurface chlorophyll maximum layers: Enduring enigma or mystery solved? Ann. Rev. Mar. Sci. 2015, 7, 207–239. [Google Scholar] [CrossRef]
- Moore, T.S.; Churnside, J.H.; Sullivan, J.M.; Twardowski, M.S.; Nayak, A.R.; McFarland, M.N.; Stockley, N.D.; Gould, R.W.; Johengen, T.H.; Ruberg, S.A. Vertical distributions of blooming cyanobacteria populations in a freshwater lake from LIDAR observations. Remote Sens. Environ. 2019, 225, 347–367. [Google Scholar] [CrossRef]
- Baetge, N.; Halsey, K.H.; Graff, J.R.; Ver Wey, B.; Westberry, T.K.; Appel, A.E.; Bourdin, G.; Demeaux, C.B.; Boss, E.; Behrenfeld, M.J. Physiological and interspecific factors determine diel changes in phytoplankton bio-optical properties. Limnol. Oceanogr. 2024, 69, 390–407. [Google Scholar] [CrossRef]
- Tsakalakis, I.; Pahlow, M.; Oschlies, A.; Blasius, B.; Ryabov, A.B. Diel light cycle as a key factor for modelling phytoplankton biogeography and diversity. Ecol. Model. 2018, 384, 241–248. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Gaube, P.; Della Penna, A.; O’Malley, R.T.; Burt, W.J.; Hu, Y.; Bontempi, P.S.; Steinberg, D.K.; Boss, E.S.; Siegel, D.A.; et al. Global satellite-observed daily vertical migrations of ocean animals. Nature 2019, 576, 257–261. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Y.; Wu, H.; Kutser, T.; Han, Y.; Ma, R.; Yao, Z.; Zhao, H.; Xu, P.; Jiang, C.; et al. Potential of Mie-fluorescence-Raman lidar to profile chlorophyll a concentration in inland waters. Environ. Sci. Technol. 2023, 57, 14226–14236. [Google Scholar] [CrossRef]
- Archibald, K.M.; Siegel, D.A.; Doney, S.C. Modeling the impact of zooplankton diel vertical migration on the carbon export flux of the biological pump. Glob. Biogeochem. Cycles 2019, 33, 181–199. [Google Scholar] [CrossRef]
- Brentrup, J.A.; Williamson, C.E.; Colom-Montero, W.; Eckert, W.; de Eyto, E.; Grossart, H.-P.; Huot, Y.; Isles, P.D.F.; Knoll, L.B.; Leach, T.H.; et al. The potential of high-frequency profiling to assess vertical and seasonal patterns of phytoplankton dynamics in lakes: An extension of the Plankton Ecology Group (PEG) model. Inland Waters 2016, 6, 565–580. [Google Scholar] [CrossRef]
- Minaudo, C.; Odermatt, D.; Bouffard, D.; Rahaghi, A.I.; Lavanchy, S.; Wuest, A. The imprint of primary production on high-frequency profiles of lake optical properties. Environ. Sci. Technol. 2021, 55, 14234–14244. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Shi, K.; Melack, J.; Zhang, Y.; Zhou, Y.; Zhu, M.; Zhu, G.; Wu, Z.; Liu, M. Spatial variations of subsurface chlorophyll maxima during thermal stratification in a large, deep subtropical reservoir. J. Geophys. Res. Biogeosci. 2020, 125, e2019JG005480. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Deng, J.; Liu, M.; Zhou, Y.; Zhang, Y.; Shi, K.; Jiang, C. High-resolution temporal detection of cyanobacterial blooms in a deep and oligotrophic lake by high-frequency buoy data. Environ. Res. 2022, 203, 111848. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 2015, 157, 1–8. [Google Scholar] [CrossRef]
- Hostetler, C.A.; Behrenfeld, M.J.; Hu, Y.; Hair, J.W.; Schulien, J.A. Spaceborne lidar in the study of marine systems. Annu. Rev. Mar. Sci. 2018, 10, 121–147. [Google Scholar] [CrossRef]
- Kutser, T.; Metsamaa, L.; Dekker, A.G. Influence of the vertical distribution of cyanobacteria in the water column on the remote sensing signal. Estuar. Coast. Shelf Sci. 2008, 78, 649–654. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, H.; Hu, E.; Gao, Y.; Zhou, Y.; Liu, D. Estimation of the seawater lidar ratio by MODIS: Spatial–temporal characteristics and ecological significance. Remote Sens. 2023, 15, 3328. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Hu, Y.; O’Malley, R.T.; Boss, E.S.; Hostetler, C.A.; Siegel, D.A.; Sarmiento, J.L.; Schulien, J.; Hair, J.W.; Lu, X.; et al. Annual boom–bust cycles of polar phytoplankton biomass revealed by space-based lidar. Nat. Geosci. 2016, 10, 118–122. [Google Scholar] [CrossRef]
- Ke, J.; Sun, Y.; Dong, C.; Zhang, X.; Wang, Z.; Lyu, L.; Zhu, W.; Ansmann, A.; Su, L.; Bu, L.; et al. Development of China’s first space-borne aerosol-cloud high-spectral-resolution lidar: Retrieval algorithm and airborne demonstration. PhotoniX 2022, 3, 17. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, K.; Shen, X.; Wang, Y.; Li, J.; Li, C.; Mao, J.; Malinka, A.; Zhao, C.; Russell, L.M.; et al. Dual-field-of-view high-spectral-resolution lidar: Simultaneous profiling of aerosol and water cloud to study aerosol-cloud interaction. Proc. Natl. Acad. Sci. USA 2022, 119, e2110756119. [Google Scholar] [CrossRef]
- Jamet, C.; Ibrahim, A.; Ahmad, Z.; Angelini, F.; Babin, M.; Behrenfeld, M.J.; Boss, E.; Cairns, B.; Churnside, J.; Chowdhary, J.; et al. Going beyond standard ocean color observations: Lidar and polarimetry. Front. Mar. Sci. 2019, 6, 251. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Y.; Zhao, H.; Lee, Z.; Boss, E.; Stachlewska, I.; Dionisi, D.; Jamet, C.; Girolamo, P.D.; Malinka, A.; et al. Comprehensive, continuous, and vertical measurements of seawater constituents with triple-field-of-view high-spectral-resolution lidar. Research 2023, 6, 0201. [Google Scholar] [CrossRef]
- Chen, P.; Mao, Z.; Zhang, Z.; Liu, H.; Pan, D. Detecting subsurface phytoplankton layer in Qiandao Lake using shipborne lidar. Opt. Express 2020, 28, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Cui, X.; Chen, W.; Liu, C.; Bai, J.; Zhang, Y.; Zhou, Y.; Liu, Z.; Xu, P.; Che, H.; et al. A semianalytic Monte Carlo radiative transfer model for polarized oceanic lidar: Experiment-based comparisons and multiple scattering effects analyses. J. Quant. Spectrosc. Radiat. Transf. 2019, 237, 106638. [Google Scholar] [CrossRef]
- Xu, P.; Liu, D.; Shen, Y.; Chen, Y.; Zhang, H.; Ye, Z.; Jiang, C.; Zhou, Y.; Liu, Q.; Liu, C. Design and validation of a shipborne multiple-field-of-view lidar for upper ocean remote sensing. J. Quant. Spectrosc. Radiat. Transf. 2020, 254, 107201. [Google Scholar] [CrossRef]
- Jiang, B.; Zhu, S.; Ren, L.; Shi, L.; Zhang, X. Simultaneous ultraviolet, visible, and near-infrared continuous-wave lasing in a rare-earth-doped microcavity. Adv. Photonics 2022, 4, 046003. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Li, X.; Wang, X.; Zhao, S.; Liu, Q.; Zhao, C. Second harmonic generation of laser beams in transverse mode locking states. Adv. Photonics 2022, 4, 026002. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Huang, F. All-silicon photovoltaic detectors with deep ultraviolet selectivity. PhotoniX 2020, 1, 15. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Z. Silicon-based optoelectronics for general-purpose matrix computation: A review. Adv. Photonics 2022, 4, 044001. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Shi, K.; Zhu, G.; Wu, Z.; Liu, M.; Zhang, Y. Thermal stratification dynamics in a large and deep subtropical reservoir revealed by high-frequency buoy data. Sci. Total Environ. 2019, 651, 614–624. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Z.; Qiao, H.; Liu, F. Assessment of eutrophication and water quality in the estuarine area of Lake Wuli, Lake Taihu, China. Sci. Total Environ. 2019, 650, 1392–1402. [Google Scholar] [CrossRef]
- Bertone, E.; Burford, M.A.; Hamilton, D.P. Fluorescence probes for real-time remote cyanobacteria monitoring: A review of challenges and opportunities. Water Res. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- Bertone, E.; Chuang, A.; Burford, M.A.; Hamilton, D.P. In-situ fluorescence monitoring of cyanobacteria: Laboratory-based quantification of species-specific measurement accuracy. Harmful Algae 2019, 87, 101625. [Google Scholar] [CrossRef] [PubMed]
- Hemsley, V.S.; Smyth, T.J.; Martin, A.P.; Frajka-Williams, E.; Thompson, A.F.; Damerell, G.; Painter, S.C. Estimating oceanic primary production using vertical irradiance and chlorophyll profiles from ocean gliders in the North Atlantic. Environ. Sci. Technol. 2015, 49, 11612–11621. [Google Scholar] [CrossRef] [PubMed]
- GB 17378.7-2007; The Specification for Marine Monitoring—Part 7: Ecological Survey for Offshore Pollution and Biological Monitoring. National Standards of People’s Republic of China: Beijing, China, 2007.
- Mitchell, B.G.; Kahru, M.; Wieland, J.; Stramska, M. Determination of spectral absorption coefficients of particles, dissolved material and phytoplankton for discrete water samples. In Ocean Optics Protocols for Satellite Ocean Color Sensor Validation, NASA/TM-2002-210004/Rev3-Vol2; Fargion, G.S., Mueller, J.L., Eds.; NASA Goddard Space Flight Center: Greenbelt, MD, USA, 2002; Volume 2, pp. 231–257. [Google Scholar]
- Roesler, C.; Uitz, J.; Claustre, H.; Boss, E.; Xing, X.; Organelli, E.; Briggs, N.; Bricaud, A.; Schmechtig, C.; Poteau, A.; et al. Recommendations for obtaining unbiased chlorophyll estimates from in situ chlorophyll fluorometers: A global analysis of WET Labs ECO sensors. Limnol. Oceanogr. Methods 2017, 15, 572–585. [Google Scholar] [CrossRef]
- Fernald, F.G. Analysis of atmospheric lidar observations: Some comments. Appl. Opt. 1984, 23, 652–653. [Google Scholar] [CrossRef]
- Morel, A.; Maritorena, S. Bio-optical properties of oceanic waters: A reappraisal. J. Geophys. Res. Oceans 2001, 106, 7163–7180. [Google Scholar] [CrossRef]
- Gordon, H.R. Interpretation of airborne oceanic lidar: Effects of multiple scattering. Appl. Opt. 1982, 21, 2996–3001. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Zhao, H.; Jamet, C.; Dionisi, D.; Chami, M.; Di Girolamo, P.; Churnside, J.H.; Malinka, A.; Zhao, H.; et al. Shipborne oceanic high-spectral-resolution lidar for accurate estimation of seawater depth-resolved optical properties. Light Sci. Appl. 2022, 11, 261. [Google Scholar] [CrossRef]
- Churnside, J.H.; Marchbanks, R.D. Inversion of oceanographic profiling lidars by a perturbation to a linear regression. Appl. Opt. 2017, 56, 5228–5233. [Google Scholar] [CrossRef]
- Liu, D.; Xu, P.; Zhou, Y.; Chen, W.; Han, B.; Zhu, X.; He, Y.; Mao, Z.; Le, C.; Chen, P.; et al. Lidar remote sensing of seawater optical properties: Experiment and Monte Carlo simulation. IEEE Trans. Geosci. Remote Sens. 2019, 57, 9489–9498. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, X.; Gu, Q.; Zhou, Y.; Zhao, H.; Zhang, H.; Ma, S.; Xu, P.; Frielinghaus, H.; Wu, L.; et al. This is MATE: A Multiple scAttering correcTion rEtrieval algorithm for accurate lidar profiling of seawater optical properties. Remote Sens. Environ. 2024, 307, 114166. [Google Scholar] [CrossRef]
- Kheireddine, M.; Antoine, D. Diel variability of the beam attenuation and backscattering coefficients in the northwestern Mediterranean Sea (BOUSSOLE site). J. Geophys. Res. Oceans 2014, 119, 5465–5482. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, H.; Zheng, D.; Zhao, H.; Zhou, Y.; Liu, D. Characteristics and formation conditions of thin phytoplankton layers in the northern gulf of Mexico revealed by airborne lidar. Remote Sens. 2022, 14, 4179. [Google Scholar] [CrossRef]
- Shen, X.; Kong, W.; Chen, P.; Chen, T.; Huang, G.; Shu, R. A shipborne photon-counting lidar for depth-resolved ocean observation. Remote Sens. 2022, 14, 3351. [Google Scholar] [CrossRef]
- Sun, H.; Wang, S.; Hu, X.; Liu, H.; Zhou, X.; Huang, J.; Cheng, X.; Sun, F.; Liu, Y.; Liu, D. Detection of surface defects and subsurface defects of polished optics with multisensor image fusion. PhotoniX 2022, 3, 6. [Google Scholar] [CrossRef]
- Schulien, J.A.; Della Penna, A.; Gaube, P.; Chase, A.P.; Haëntjens, N.; Graff, J.R.; Hair, J.W.; Hostetler, C.A.; Scarino, A.J.; Boss, E.S.; et al. Shifts in phytoplankton community structure across an anticyclonic eddy revealed from high spectral resolution lidar scattering measurements. Front. Mar. Sci. 2020, 7, 493. [Google Scholar] [CrossRef]
- Chen, S.; Tong, B.; Russell, L.M.; Wei, J.; Guo, J.; Mao, F.; Liu, D.; Huang, Z.; Xie, Y.; Qi, B.; et al. Lidar-based daytime boundary layer height variation and impact on the regional satellite-based PM2.5 estimate. Remote Sens. Environ. 2022, 281, 113224. [Google Scholar] [CrossRef]
- Dionisi, D.; Brando, V.E.; Volpe, G.; Colella, S.; Santoleri, R. Seasonal distributions of ocean particulate optical properties from spaceborne lidar measurements in Mediterranean and Black sea. Remote Sens. Environ. 2020, 247, 111889. [Google Scholar] [CrossRef]
- Lu, X.; Hu, Y.; Yang, Y.; Bontempi, P.; Omar, A.; Baize, R. Antarctic spring ice-edge blooms observed from space by ICESat-2. Remote Sens. Environ. 2020, 245, 111827. [Google Scholar] [CrossRef]
- Liu, R.; Ling, Q.; Zhang, Q.; Zhou, Y.; Le, C.; Chen, Y.; Liu, Q.; Chen, W.; Tang, J.; Liu, D. Detection of chlorophyll a and CDOM absorption coefficient with a dual-wavelength oceanic lidar: Wavelength optimization method. Remote Sens. 2020, 12, 3021. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.M.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.M.; Kassabgy, M.; Huang, S.; Mann, A.J.; Waldmann, J.; et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E. Beam attenuation and chlorophyll concentration as alternative optical indices of phytoplankton biomass. J. Mar. Res. 2006, 64, 431–451. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Halsey, K.H.; Milligan, A.J. Evolved physiological responses of phytoplankton to their integrated growth environment. Philos. Trans. R. Soc. Biol. Sci. 2008, 363, 2687–2703. [Google Scholar] [CrossRef]
- Hamilton, D.P.; O’Brien, K.R.; Burford, M.A.; Brookes, J.D.; McBride, C.G. Vertical distributions of chlorophyll in deep, warm monomictic lakes. Aquat. Sci. 2010, 72, 295–307. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Liu, M.; Yu, Z.; Song, D.; Jeppesen, E.; Zhou, Q. Patterns of thermocline structure and the deep chlorophyll maximum feature in multiple stratified lakes related to environmental drivers. Sci. Total Environ. 2022, 851, 158431. [Google Scholar] [CrossRef] [PubMed]
- Mena, C.; Reglero, P.; Hidalgo, M.; Sintes, E.; Santiago, R.; Martin, M.; Moya, G.; Balbin, R. Phytoplankton community structure Is driven by stratification in the oligotrophic Mediterranean Sea. Front. Microbiol. 2019, 10, 1698. [Google Scholar] [CrossRef]
- Huang, Q.; Li, N.; Li, Y. Long-term trend of heat waves and potential effects on phytoplankton blooms in Lake Qiandaohu, a key drinking water reservoir. Environ. Sci. Pollut. Res. 2021, 28, 68448–68459. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).