Weather Radars Reveal Environmental Conditions for High Altitude Insect Movement Through the Aerosphere
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Used in This Study
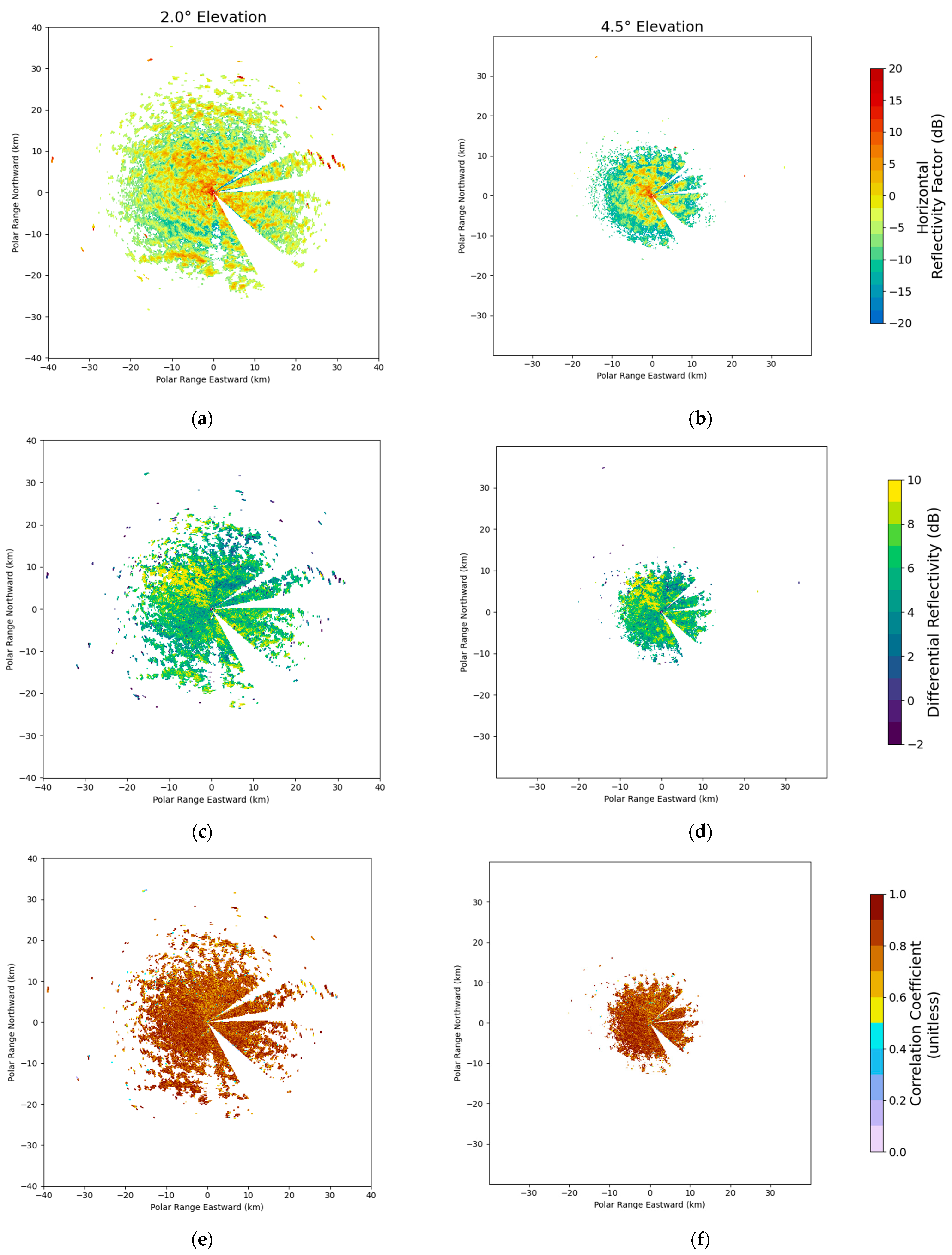
2.1.1. The NXPol-1 Weather Surveillance Radar
2.1.2. The ECMWF Operational Forecast Model
2.1.3. Centre for Ecology and Hydrology Land Cover Maps
2.2. Methodology
2.2.1. Overview
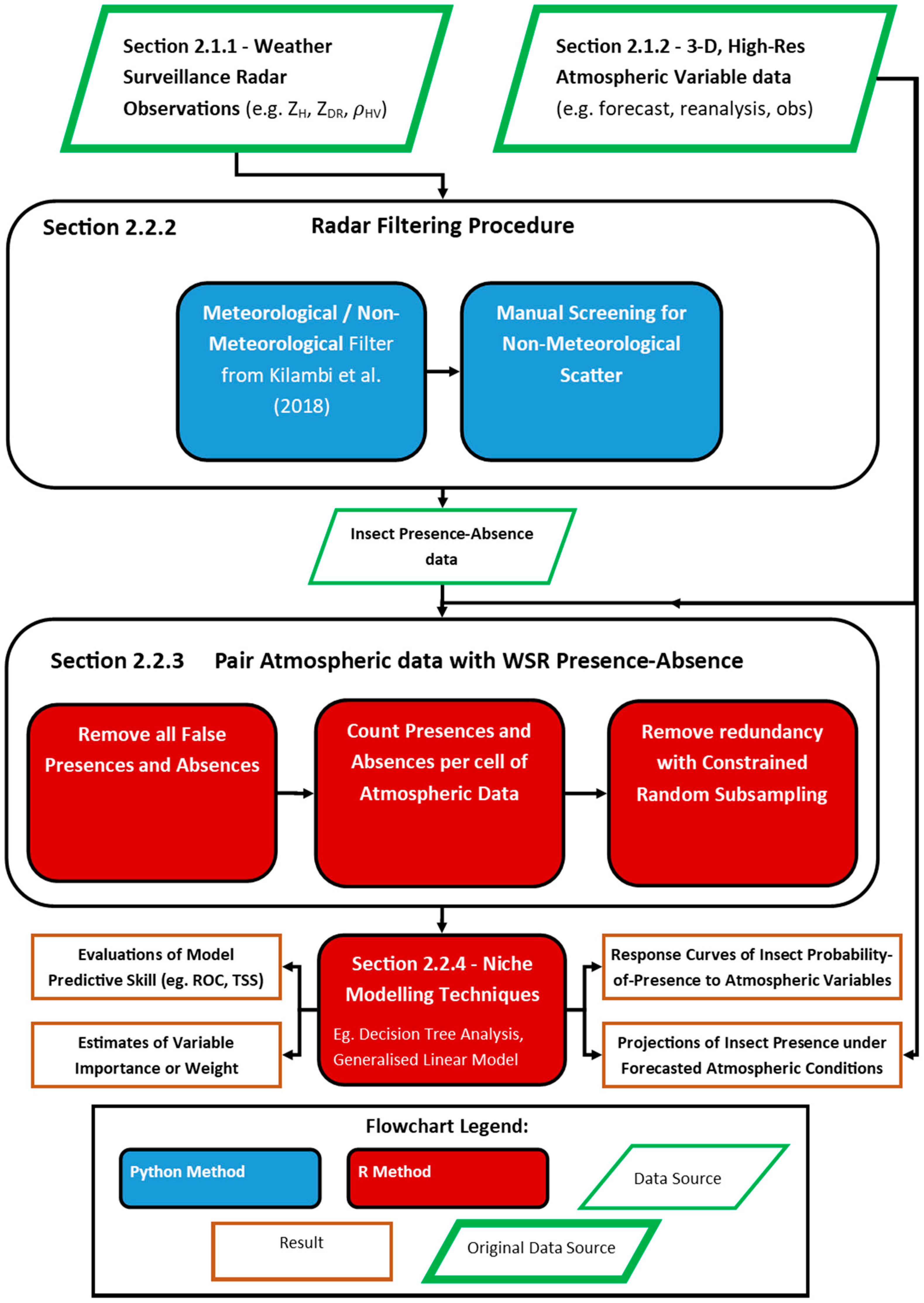
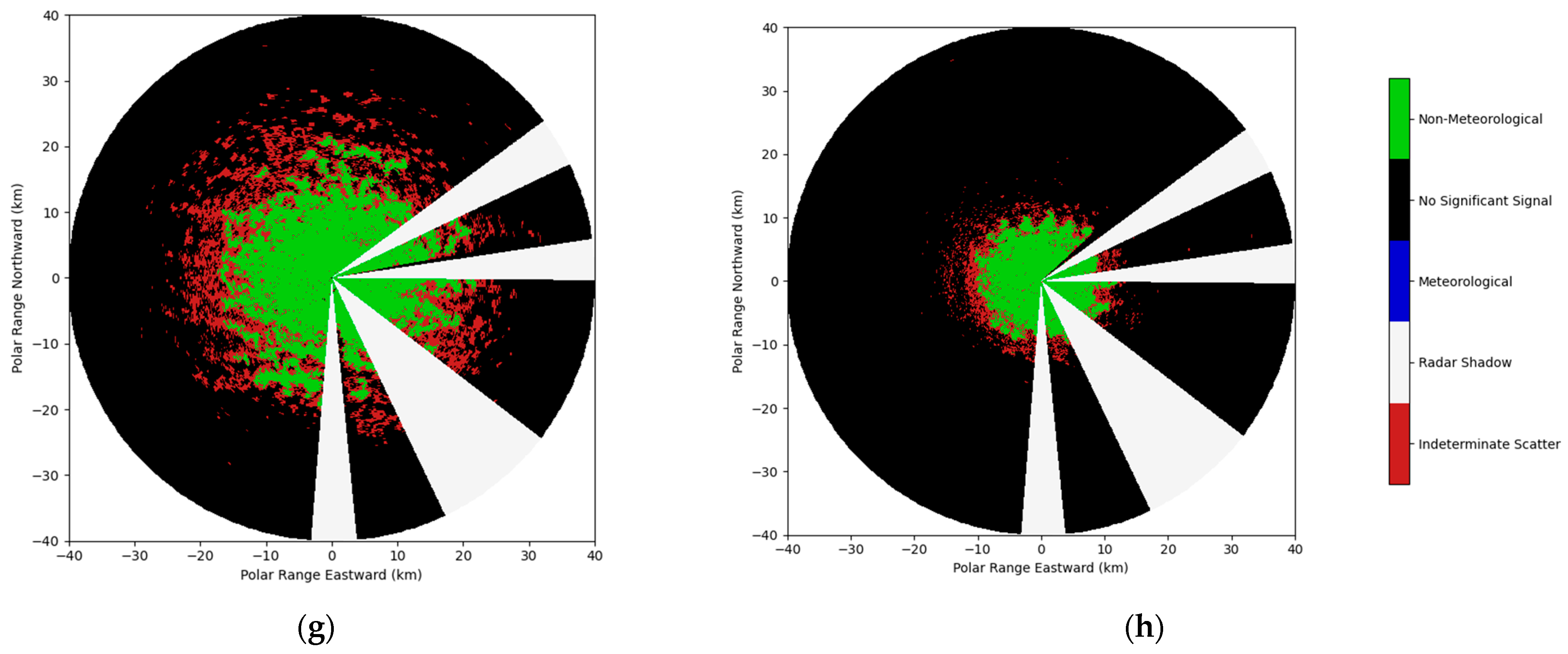
2.2.2. WSR Filtering Procedure
2.2.3. Pairing of WSR and Atmospheric Data
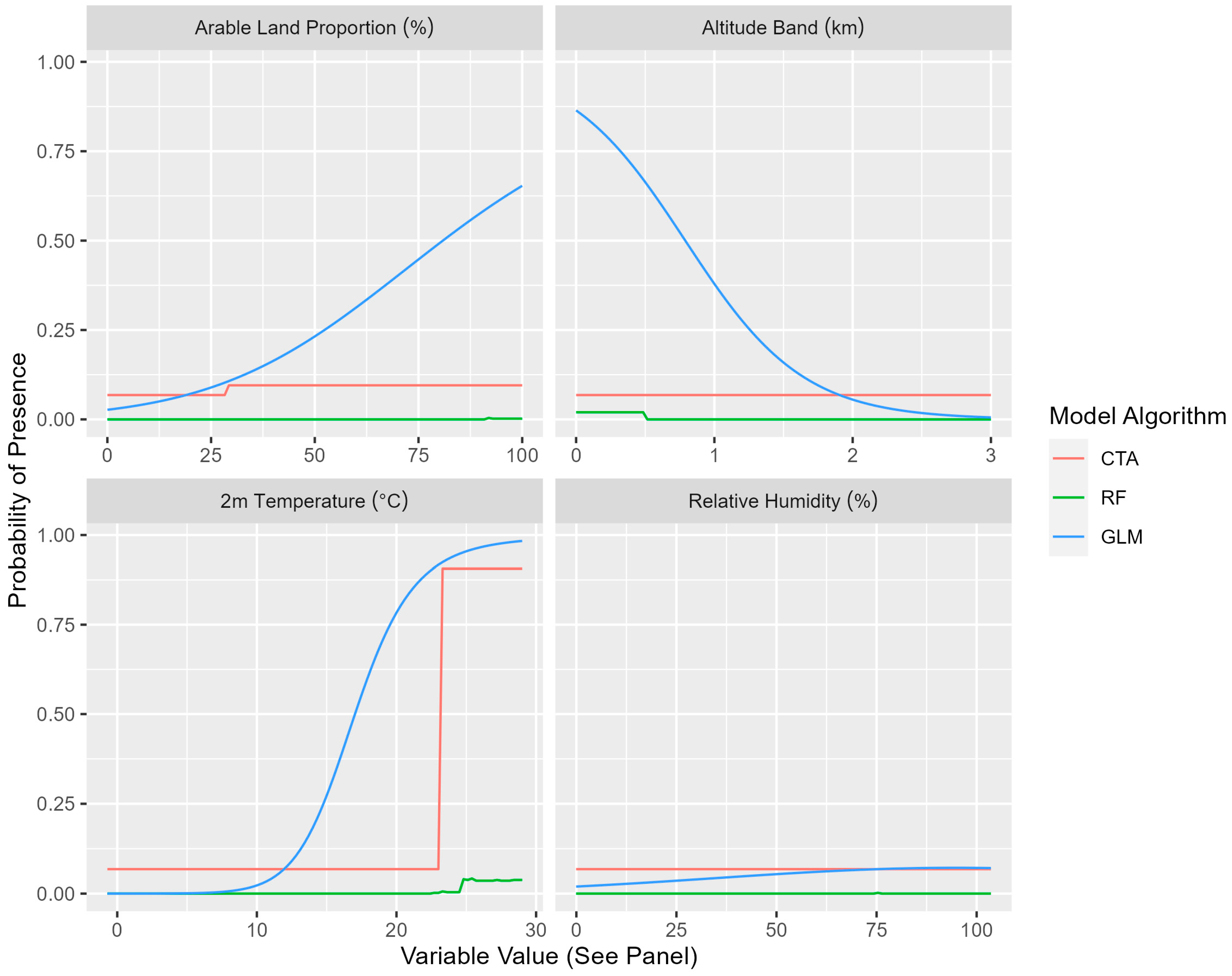
2.2.4. Niche Modelling with Biomod2
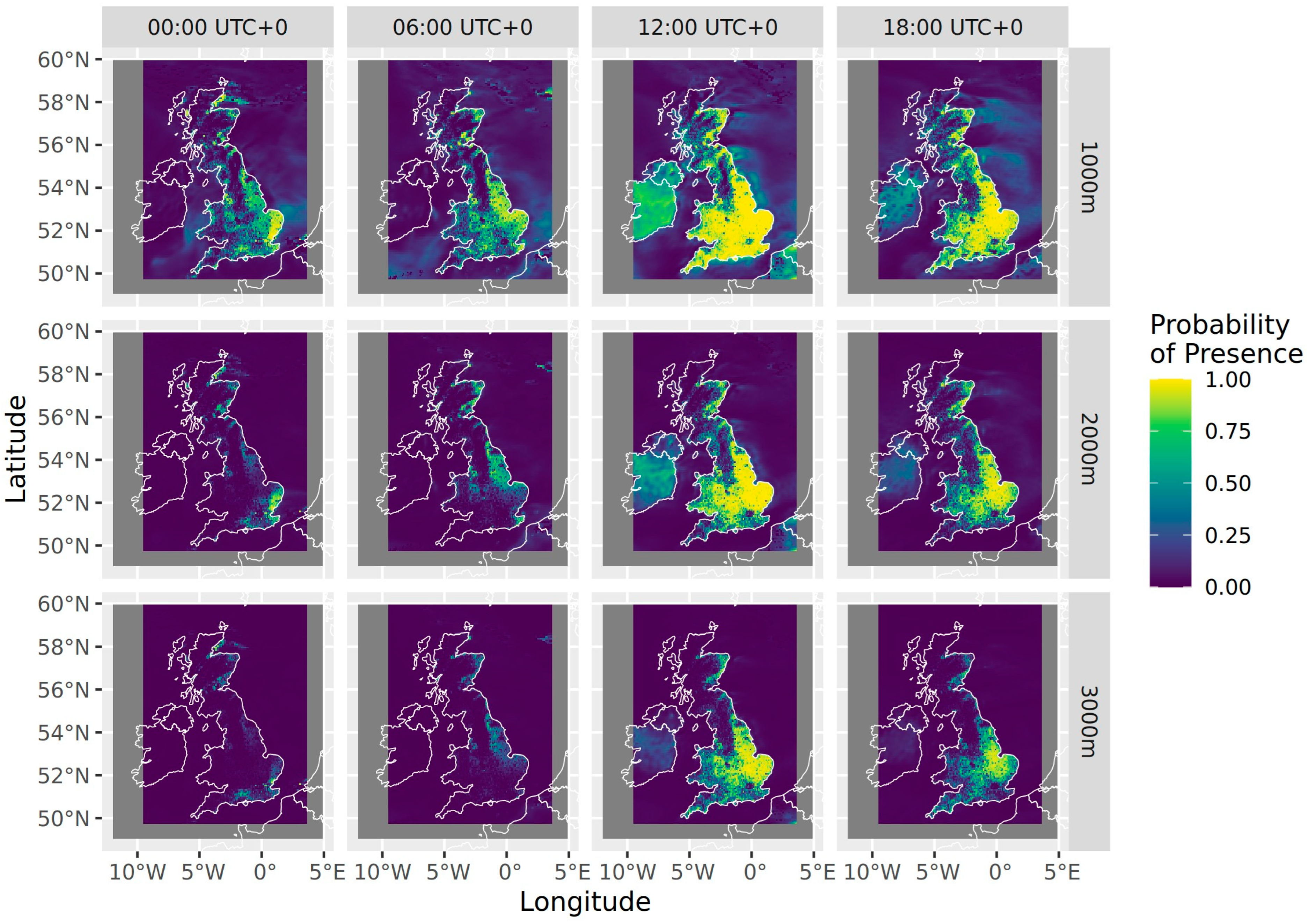
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Altitude | Temperature | Relative Humidity | Zonal Windspeed | Meridional Windspeed | Atmospheric Divergence | Potential Vorticity | Relative Vorticity | Vertical Velocity | 10 m Wind Gust | 10 m Zonal Windspeed | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | 1.000 | −0.640 | −0.440 | 0.210 | −0.072 | −0.023 | 0.073 | 0.010 | 0.009 | 0.017 | 0.018 |
| Temperature | −0.640 | 1.000 | 0.280 | 0.150 | 0.330 | −0.019 | −0.096 | −0.064 | −0.099 | 0.007 | 0.130 |
| Relative Humidity | −0.440 | 0.280 | 1.000 | 0.013 | 0.080 | 0.046 | −0.240 | −0.061 | −0.033 | 0.059 | 0.099 |
| Zonal Windspeed | 0.210 | 0.150 | 0.013 | 1.000 | 0.078 | −0.043 | 0.049 | 0.031 | −0.056 | 0.120 | 0.680 |
| Meridional Windspeed | −0.072 | 0.330 | 0.080 | 0.078 | 1.000 | −0.011 | −0.120 | −0.065 | −0.130 | −0.019 | −0.220 |
| Atmospheric Divergence | −0.023 | −0.019 | 0.046 | −0.043 | −0.011 | 1.000 | −0.048 | −0.047 | −0.018 | −0.025 | −0.040 |
| Potential Vorticity | 0.073 | −0.096 | −0.240 | 0.049 | −0.120 | −0.048 | 1.000 | 0.660 | 0.020 | 0.071 | 0.058 |
| Relative Vorticity | 0.010 | −0.064 | −0.061 | 0.031 | −0.065 | −0.047 | 0.660 | 1.000 | −0.028 | 0.077 | 0.048 |
| Vertical Velocity | 0.009 | −0.099 | −0.033 | −0.056 | −0.130 | −0.018 | 0.020 | −0.028 | 1.000 | −0.006 | 0.002 |
| 10 m Wind Gust | 0.017 | 0.007 | 0.059 | 0.120 | −0.019 | −0.025 | 0.071 | 0.077 | −0.006 | 1.000 | 0.140 |
| 10 m Zonal Windspeed | 0.018 | 0.130 | 0.099 | 0.680 | −0.220 | −0.040 | 0.058 | 0.048 | 0.002 | 0.140 | 1.000 |
| 10 m Meridional Windspeed | 0.001 | 0.250 | 0.047 | 0.390 | 0.490 | −0.024 | −0.068 | −0.110 | −0.055 | −0.130 | 0.200 |
| 2 Temperature | 0.009 | 0.520 | 0.220 | 0.350 | 0.250 | −0.018 | −0.036 | 0.053 | −0.092 | 0.160 | 0.280 |
| Convective Rain Rate | 0.004 | −0.001 | 0.120 | 0.089 | −0.015 | −0.002 | 0.019 | 0.049 | −0.058 | 0.130 | 0.120 |
| Large Scale Rain Rate | 0.028 | 0.011 | 0.200 | 0.170 | −0.024 | −0.010 | 0.032 | 0.037 | −0.046 | 0.210 | 0.170 |
| Skin Temperature | 0.013 | 0.440 | 0.190 | 0.290 | 0.190 | −0.013 | −0.020 | 0.059 | −0.080 | 0.210 | 0.250 |
| Broadleaf Wood Proportion | −0.100 | 0.043 | 0.064 | 0.002 | 0.004 | −0.012 | −0.016 | −0.009 | 0.021 | −0.100 | −0.016 |
| Urban Suburban Proportion | −0.051 | 0.005 | 0.039 | 0.029 | 0.004 | 0.001 | −0.001 | 0.011 | −0.010 | −0.120 | −0.009 |
| Coniferous Wood Proportion | −0.095 | 0.061 | 0.049 | −0.024 | 0.002 | −0.010 | −0.010 | 0.007 | 0.009 | −0.038 | −0.013 |
| Arable Land Proportion | −0.081 | 0.008 | 0.053 | 0.010 | −0.002 | −0.011 | 0.001 | −0.008 | 0.027 | −0.110 | 0.000 |
| Grassland Proportion | −0.024 | 0.016 | 0.010 | −0.004 | 0.007 | −0.009 | −0.004 | 0.004 | 0.005 | −0.019 | −0.007 |
| 10 m Meridional Windspeed | 2 m Temperature | Convective Rain Rate | Large Scale Rain Rate | Skin Temperature | Broadleaf Wood Proportion | Urban Suburban Proportion | Coniferous Wood Proportion | Arable Land Proportion | Grassland Proportion | ||
| Altitude | 0.001 | 0.009 | 0.004 | 0.028 | 0.013 | −0.100 | −0.051 | −0.095 | −0.081 | −0.024 | |
| Temperature | 0.250 | 0.520 | −0.001 | 0.011 | 0.440 | 0.043 | 0.005 | 0.061 | 0.008 | 0.016 | |
| Relative Humidity | 0.047 | 0.220 | 0.120 | 0.200 | 0.190 | 0.064 | 0.039 | 0.049 | 0.053 | 0.010 | |
| Zonal Windspeed | 0.390 | 0.350 | 0.089 | 0.170 | 0.290 | 0.002 | 0.029 | −0.024 | 0.010 | −0.004 | |
| Meridional Windspeed | 0.490 | 0.250 | −0.015 | −0.024 | 0.190 | 0.004 | 0.004 | 0.002 | −0.002 | 0.007 | |
| Atmospheric Divergence | −0.024 | −0.018 | −0.002 | −0.010 | −0.013 | −0.012 | 0.001 | −0.010 | −0.011 | −0.009 | |
| Potential Vorticity | −0.068 | −0.036 | 0.019 | 0.032 | −0.020 | −0.016 | −0.001 | −0.010 | 0.001 | −0.004 | |
| Relative Vorticity | −0.110 | 0.053 | 0.049 | 0.037 | 0.059 | −0.009 | 0.011 | 0.007 | −0.008 | 0.004 | |
| Vertical Velocity | −0.055 | −0.092 | −0.058 | −0.046 | −0.080 | 0.021 | −0.010 | 0.009 | 0.027 | 0.005 | |
| 10 m Wind Gust | −0.130 | 0.160 | 0.130 | 0.210 | 0.210 | −0.100 | −0.120 | −0.038 | −0.110 | −0.019 | |
| 10 m Zonal Windspeed | 0.200 | 0.280 | 0.120 | 0.170 | 0.250 | −0.016 | −0.009 | −0.013 | 0.000 | −0.007 | |
| 10 m Meridional Windspeed | 1.000 | 0.220 | −0.012 | 0.059 | 0.140 | 0.058 | 0.077 | −0.002 | 0.072 | 0.013 | |
| 2 m Temperature | 0.220 | 1.000 | 0.170 | 0.110 | 0.960 | −0.130 | −0.100 | −0.060 | −0.140 | −0.044 | |
| Convective Rain Rate | −0.012 | 0.170 | 1.000 | 0.290 | 0.190 | −0.023 | −0.018 | −0.015 | −0.025 | −0.013 | |
| Large Scale Rain Rate | 0.059 | 0.110 | 0.290 | 1.000 | 0.110 | −0.068 | −0.053 | −0.038 | −0.069 | −0.010 | |
| Skin Temperature | 0.140 | 0.960 | 0.190 | 0.110 | 1.000 | −0.170 | −0.140 | −0.081 | −0.180 | −0.054 | |
| Broadleaf Wood Proportion | 0.058 | −0.130 | −0.023 | −0.068 | −0.170 | 1.000 | 0.440 | 0.480 | 0.340 | 0.073 | |
| Urban Suburban Proportion | 0.077 | −0.100 | −0.018 | −0.053 | −0.140 | 0.440 | 1.000 | 0.120 | 0.230 | 0.039 | |
| Coniferous Wood Proportion | −0.002 | −0.060 | −0.015 | −0.038 | −0.081 | 0.480 | 0.120 | 1.000 | 0.090 | 0.090 | |
| Arable Land Proportion | 0.072 | −0.140 | −0.025 | −0.069 | −0.180 | 0.340 | 0.230 | 0.090 | 1.000 | 0.095 | |
| Grassland Proportion | 0.013 | −0.044 | −0.013 | −0.010 | −0.054 | 0.073 | 0.039 | 0.090 | 0.095 | 1.000 |
| Altitude | Temperature | Relative Humidity | Zonal Windspeed | Meridional Windspeed | Atmospheric Divergence | Potential Vorticity | Relative Vorticity | Vertical Velocity | 10 m Wind Gust | 10 m Zonal Windspeed | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Altitude | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.060 | 0.090 | 0.000 | 0.000 |
| Temperature | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.180 | 0.000 |
| Relative Humidity | 0.000 | 0.000 | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Zonal Windspeed | 0.000 | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Meridional Windspeed | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.050 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Atmospheric Divergence | 0.000 | 0.000 | 0.000 | 0.000 | 0.050 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Potential Vorticity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Relative Vorticity | 0.060 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Vertical Velocity | 0.090 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.300 | 0.720 |
| 10 m Wind Gust | 0.000 | 0.180 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.300 | 0.000 | 0.000 |
| 10 m Zonal Windspeed | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.720 | 0.000 | 0.000 |
| 10 m Meridional Windspeed | 0.810 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2 Temperature | 0.090 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Convective Rain Rate | 0.450 | 0.830 | 0.000 | 0.000 | 0.010 | 0.710 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Large-Scale Rain Rate | 0.000 | 0.040 | 0.000 | 0.000 | 0.000 | 0.070 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Skin Temperature | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Broadleaf Wood Proportion | 0.000 | 0.000 | 0.000 | 0.660 | 0.470 | 0.030 | 0.000 | 0.090 | 0.000 | 0.000 | 0.000 |
| Urban Suburban Proportion | 0.000 | 0.400 | 0.000 | 0.000 | 0.460 | 0.910 | 0.860 | 0.050 | 0.060 | 0.000 | 0.110 |
| Coniferous Wood Proportion | 0.000 | 0.000 | 0.000 | 0.000 | 0.750 | 0.080 | 0.060 | 0.180 | 0.120 | 0.000 | 0.010 |
| Arable Land Proportion | 0.000 | 0.130 | 0.000 | 0.070 | 0.770 | 0.040 | 0.870 | 0.170 | 0.000 | 0.000 | 0.990 |
| Grassland Proportion | 0.000 | 0.000 | 0.070 | 0.450 | 0.240 | 0.110 | 0.500 | 0.450 | 0.340 | 0.000 | 0.220 |
| 10 m Meridional Windspeed | 2 m Temperature | Convective Rain Rate | Large-Scale Rain Rate | Skin Temperature | Broadleaf Wood Proportion | Urban Suburban Proportion | Coniferous Wood Proportion | Arable Land Proportion | Grassland Proportion | ||
| Altitude | 0.810 | 0.090 | 0.450 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Temperature | 0.000 | 0.000 | 0.830 | 0.040 | 0.000 | 0.000 | 0.400 | 0.000 | 0.130 | 0.000 | |
| Relative Humidity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.070 | |
| Zonal Windspeed | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.660 | 0.000 | 0.000 | 0.070 | 0.450 | |
| Meridional Windspeed | 0.000 | 0.000 | 0.010 | 0.000 | 0.000 | 0.470 | 0.460 | 0.750 | 0.770 | 0.240 | |
| Atmospheric Divergence | 0.000 | 0.000 | 0.710 | 0.070 | 0.020 | 0.030 | 0.910 | 0.080 | 0.040 | 0.110 | |
| Potential Vorticity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.860 | 0.060 | 0.870 | 0.500 | |
| Relative Vorticity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.090 | 0.050 | 0.180 | 0.170 | 0.450 | |
| Vertical Velocity | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.060 | 0.120 | 0.000 | 0.340 | |
| 10 m Wind Gust | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| 10 m Zonal Windspeed | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.110 | 0.010 | 0.990 | 0.220 | |
| 10 m Meridional Windspeed | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 | 0.000 | 0.000 | 0.710 | 0.000 | 0.020 | |
| 2 m Temperature | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Convective Rain Rate | 0.030 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.010 | 0.000 | 0.010 | |
| Large-Scale Rain Rate | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.070 | |
| Skin Temperature | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Broadleaf Wood Proportion | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Urban Suburban Proportion | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Coniferous Wood Proportion | 0.710 | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Arable Land Proportion | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Grassland Proportion | 0.020 | 0.000 | 0.010 | 0.070 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| ANOVA Statistic | F-Value | p-Value | ||||
|---|---|---|---|---|---|---|
| Model Type | Combined | Aerial | Terrestrial | Combined | Aerial | Terrestrial |
| Variable Name | 10,604.9 | 2295.61 | 5993.0 | <2e−16 | <2e−16 | <2e−16 |
| Subsampling Factor | 311.9 | 78.86 | 268.2 | <2e−16 | <2e−16 | <2e−16 |
| Algorithm | 15,168.6 | 382.29 | 3604.5 | <2e−16 | <2e−16 | <2e−16 |
| Environment Variable Types | Aerial | Aerial + Terrestrial | Terrestrial | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Algorithm | Subsampling Fraction | 0.001 | 0.005 | 0.01 | 0.001 | 0.005 | 0.01 | 0.001 | 0.005 | 0.01 | |
| Evaluation Metric | |||||||||||
| CTA | ROC | 0.628 | 0.798 | 0.767 | 0.720 | 0.871 | 0.804 | 0.744 | 0.819 | 0.811 | |
| TSS | 0.277 | 0.602 | 0.531 | 0.467 | 0.760 | 0.612 | 0.518 | 0.612 | 0.627 | ||
| GLM | ROC | 0.701 | 0.900 | 0.845 | 0.642 | 0.871 | 0.863 | 0.699 | 0.836 | 0.820 | |
| TSS | 0.526 | 0.719 | 0.598 | 0.293 | 0.696 | 0.667 | 0.430 | 0.599 | 0.550 | ||
| RF | ROC | 0.739 | 0.861 | 0.825 | 0.884 | 0.950 | 0.927 | 0.839 | 0.897 | 0.907 | |
| TSS | 0.544 | 0.664 | 0.553 | 0.783 | 0.833 | 0.745 | 0.700 | 0.722 | 0.709 | ||
| Explanatory Variable | Mean Variable Importance | Mean CTA Variable Importance | Mean GLM Variable Importance | Mean RF Variable Importance |
|---|---|---|---|---|
| Arable Land Proportion | 0.468 | 0.697 | 0.445 | 0.262 |
| Altitude Band (+500 m) | 0.257 | 0.317 | 0.327 | 0.129 |
| 2 m Temperature | 0.195 | 0.131 | 0.379 | 0.073 |
| Temperature | 0.155 | 0.204 | 0.150 | 0.112 |
| Relative Humidity | 0.152 | 0.188 | 0.182 | 0.086 |
| Skin Temperature | 0.117 | 0.079 | 0.186 | 0.087 |
| Zonal Wind | 0.060 | 0.046 | 0.103 | 0.031 |
| Coniferous Wood Proportion | 0.046 | 0.042 | 0.034 | 0.062 |
| 10 m Zonal Wind | 0.046 | 0.008 | 0.116 | 0.014 |
| Urban Suburban Land Proportion | 0.045 | 0.027 | 0.089 | 0.019 |
| Broadleaf Wood Proportion | 0.042 | 0.020 | 0.086 | 0.020 |
| Instantaneous 10 m Wind Gust | 0.040 | 0.009 | 0.096 | 0.016 |
| Large-Scale Rain Rate | 0.038 | 0.022 | 0.079 | 0.015 |
| Time (h) | 0.037 | 0.027 | 0.060 | 0.024 |
| Meridional Wind | 0.033 | 0.018 | 0.049 | 0.032 |
| 10 m Meridional Wind | 0.032 | 0.008 | 0.065 | 0.021 |
| Vertical Velocity | 0.031 | 0.014 | 0.058 | 0.020 |
| Grassland Proportion | 0.029 | 0.007 | 0.071 | 0.010 |
| Atmospheric Divergence | 0.025 | 0.008 | 0.055 | 0.013 |
| Potential Vorticity | 0.021 | 0.009 | 0.018 | 0.035 |
| Relative Vorticity | 0.020 | 0.010 | 0.030 | 0.021 |
| Convective Rain Rate | 0.013 | 0.000 | 0.025 | 0.013 |
References
- Crespo-Pérez, V.; Kazakou, E.; Roubik, D.W.; Cárdenas, R.E. The importance of insects on land and in water: A tropical view. Curr. Opin. Insect Sci. 2020, 40, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Auger-Rozenberg, M.A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Verma, R.C.; Waseem, A.M.; Sharma, N.; Bharathi, K.; Singh, S.; Anto Rashwin, A.; Pandey, S.K.; Singh, B.V. The Role of Insects in Ecosystems, an in-depth Review of Entomological Research. Int. J. Environ. Clim. Chang. 2023, 13, 4340–4348. [Google Scholar] [CrossRef]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.I.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Doak, D.F.; Morris, W.F. Demographic compensation and tipping points in climate-induced range shifts. Nature 2010, 467, 959–962. [Google Scholar] [CrossRef]
- Tomiolo, S.; Ward, D. Species migrations and range shifts: A synthesis of causes and consequences. Perspect. Plant Ecol. Evol. Syst. 2018, 33, 62–77. [Google Scholar] [CrossRef]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2022, 242, 108426. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef]
- Boulanger, Y.; Fabry, F.; Kilambi, A.; Pureswaran, D.S.; Sturtevant, B.R.; Saint-Amant, R. The use of weather surveillance radar and high-resolution three dimensional weather data to monitor a spruce budworm mass exodus flight. Agric. For. Meteorol. 2017, 234–235, 127–135. [Google Scholar] [CrossRef]
- Florio, J.; Verú, L.M.; Dao, A.; Yaro, A.S.; Diallo, M.; Sanogo, Z.L.; Samaké, D.; Huestis, D.L.; Yossi, O.; Talamas, E.; et al. Diversity, dynamics, direction, and magnitude of high-altitude migrating insects in the Sahel. Sci. Rep. 2020, 10, 20523. [Google Scholar] [CrossRef]
- Gandiaga, F.; James, P.M.A. Quantifying long-distance dispersal of an outbreaking insect species using trap capture data and phenology. For. Ecol. Manag. 2023, 544, 121187. [Google Scholar] [CrossRef]
- Vavassori, L.; Saddler, A.; Müller, P. Active dispersal of Aedes albopictus: A mark-release-recapture study using self-marking units. Parasites Vectors 2019, 12, 583. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Hu, C.; Wang, R.; Li, S.; Wu, D.; Ma, S. Quantifying insect migration across Bohai strait using weather radar. J. Eng. 2019, 2019, 6095–6098. [Google Scholar] [CrossRef]
- Hawkes, W.L.S.; Walliker, E.; Gao, B.; Forster, O.; Lacey, K.; Doyle, T.; Massy, R.; Roberts, N.W.; Reynolds, D.R.; Özden, Ö.; et al. Huge spring migrations of insects from the Middle East to Europe: Quantifying the migratory assemblage and ecosystem services. Ecography 2022, 2022, e06288. [Google Scholar] [CrossRef]
- Knoblauch, A.; Thoma, M.; Menz, M.H.M. Autumn southward migration of dragonflies along the Baltic coast and the influence of weather on flight behaviour. Anim. Behav. 2021, 176, 99–109. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Mouritsen, H.; Hill, J.K.; Riley, J.R.; Sivell, D.; Smith, A.D.; Woiwod, I.P. Wind Selection and Drift Compensation Optimize Migratory Pathways in a High-Flying Moth. Curr. Biol. 2008, 18, 514–518. [Google Scholar] [CrossRef]
- Menz, M.H.M.; Scacco, M.; Bürki-Spycher, H.-M.; Williams, H.J.; Reynolds, D.R.; Chapman, J.W.; Wikelski, M. Individual Tracking Reveals Long-Distance Flight-Path Control in a Nocturnally Migrating Moth. Science. 2022, 377, pp. 764–768. [CrossRef]
- Bell, J.R.; Shephard, G. How aphids fly: Take-off, free flight and implications for short and long distance migration. Agric. For. Entomol. 2024, 1–10. [Google Scholar] [CrossRef]
- Nieminen, M.; Leskinen, M.; Helenius, J. Doppler radar detection of exceptional mass-migration of aphids into Finland. Int. J. Biometeorol. 2000, 44, 172–181. [Google Scholar] [CrossRef]
- Parry, H.R. Cereal aphid movement: General principles and simulation modelling. Mov. Ecol. 2013, 1, 14. [Google Scholar] [CrossRef]
- Crossley, M.S.; Lagos-Kutz, D.; Davis, T.S.; Eigenbrode, S.D.; Hartman, G.L.; Voegtlin, D.J.; Snyder, W.E. Precipitation change accentuates or reverses temperature effects on aphid dispersal. Ecol. Appl. 2022, 32, e2593. [Google Scholar] [CrossRef]
- Drake, V.A.; Reynolds, D.R. Radar Entomology: Observing Insect Flight and Migration; CABI Publishing: Wallingford, UK, 2010. [Google Scholar]
- Morsello, S.C.; Groves, R.L.; Nault, B.A.; Kennedy, G.G. Temperature and Precipitation Affect Seasonal Patterns of Dispersing Tobacco Thrips, Frankliniella fusca, and Onion Thrips, Thrips tabaci (Thysanoptera: Thripidae) Caught on Sticky Traps. Environ. Entomol. 2008, 37, 79–86. [Google Scholar] [CrossRef]
- Pasek, J.E. 30. Influence of Wind and Windbreaks on Local Dispersal of Insects. Agric. Ecosyst. Environ. 1988, 22, 539–554. [Google Scholar] [CrossRef]
- Reynolds, A.M.; Reynolds, D.R. Aphid aerial density profiles are consistent with turbulent advection amplifying flight behaviours: Abandoning the epithet “passive”. Proc. R. Soc. B Biol. Sci. 2009, 276, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Blazquez-Cabrera, S.; Bodin, Ö.; Saura, S. Indicators of the impacts of habitat loss on connectivity and related conservation priorities: Do they change when habitat patches are defined at different scales? Ecol. Indic. 2014, 45, 704–716. [Google Scholar] [CrossRef]
- Mancini, F.; Hodgson, J.A.; Isaac, N.J.B. Co-Designing an Indicator of Habitat Connectivity for England. Front. Ecol. Evol. 2022, 10, 892987. [Google Scholar] [CrossRef]
- Platts, P.J.; Mason, S.C.; Palmer, G.; Hill, J.K.; Oliver, T.H.; Powney, G.D.; Fox, R.; Thomas, C.D. Habitat availability explains variation in climate-driven range shifts across multiple taxonomic groups. Sci. Rep. 2019, 9, 15039. [Google Scholar] [CrossRef]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130554. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Nathan, R.; Perry, G.; Richardson, D.M. The importance of long-distance dispersal in biodiversity conservation. Divers. Distrib. 2005, 11, 173–181. [Google Scholar] [CrossRef]
- Alzate, A.; Onstein, R.E. Understanding the relationship between dispersal and range size. Ecol. Lett. 2022, 25, 2303–2323. [Google Scholar] [CrossRef]
- Liu, B.R. Biphasic range expansions with short- and long-distance dispersal. Theor. Ecol. 2021, 14, 409–427. [Google Scholar] [CrossRef]
- Marco, D.E.; Montemurro, M.A.; Cannas, S.A. Comparing short and long-distance dispersal: Modelling and field case studies. Ecography 2011, 34, 671–682. Available online: https://www.jstor.org/stable/41239430 (accessed on 4 November 2024). [CrossRef]
- Mitchell, Z. Dragonfly Locomotion: Ecology, Form and Function. Ph.D. Thesis, University of Leeds, Leeds, UK, 2018. [Google Scholar]
- Flockhart, D.T.T.; Fitzgerald, B.; Brower, L.P.; Derbyshire, R.; Altizer, S.; Hobson, K.A.; Wassenaar, L.I.; Norris, D.R. Migration distance as a selective episode for wing morphology in a migratory insect. Mov. Ecol. 2017, 5, 7. [Google Scholar] [CrossRef]
- Gao, B.; Hedlund, J.; Reynolds, D.R.; Zhai, B.; Hu, G.; Chapman, J.W. The ‘migratory connectivity’ concept, and its applicability to insect migrants. Mov. Ecol. 2020, 8, 48. [Google Scholar] [CrossRef]
- Jordano, P. What is long-distance dispersal? And a taxonomy of dispersal events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Chilson, P.B.; Bridge, E.; Frick, W.F.; Chapman, J.W.; Kelly, J.F. Radar aeroecology: Exploring the movements of aerial fauna through radio-wave remote sensing. Biol. Lett. 2012, 8, 698–701. [Google Scholar] [CrossRef]
- Diehl, R.H. The airspace is habitat. Trends Ecol. Evol. 2013, 28, 377–379. [Google Scholar] [CrossRef]
- Braderic, M. On the Radar: Weather, Bird Migration and Aeroconservation over the North Sea. Ph.D. Thesis, Institute for Biodiversity and Ecosystem Dynamics, Amsterdam, The Netherlands, 2022. [Google Scholar]
- Horton, K.G.; Van Doren, B.M.; Stepanian, P.M.; Farnsworth, A.; Kelly, J.F. Where in the air? Aerial habitat use of nocturnally migrating birds. Biol. Lett. 2016, 12, 20160591. [Google Scholar] [CrossRef]
- Aralimarad, P.; Reynolds, A.M.; Lim, K.S.; Reynolds, D.R.; Chapman, J.W. Flight altitude selection increases orientation performance in high-flying nocturnal insect migrants. Anim. Behav. 2011, 82, 1221–1225. [Google Scholar] [CrossRef]
- Mateos-Rodríguez, M.; Liechti, F. How do diurnal long-distance migrants select flight altitude in relation to wind? Behav. Ecol. 2012, 23, 403–409. [Google Scholar] [CrossRef]
- Wood, C.R.; Reynolds, D.R.; Wells, P.M.; Barlow, J.F.; Woiwod, I.P.; Chapman, J.W. Flight periodicity and the vertical distribution of high-altitude moth migration over southern Britain. Bull. Entomol. Res. 2009, 99, 525–535. [Google Scholar] [CrossRef]
- Srygley, R.B.; Dudley, R. Optimal strategies for insects migrating in the flight boundary layer: Mechanisms and consequences. Integr. Comp. Biol. 2008, 48, 119–133. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Smith, A.D.; Chapman, J.W. A radar study of emigratory flight and layer formation by insects at dawn over southern Britain. Bull. Entomol. Res. 2008, 98, 35–52. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Stepanian, P.M.; Reynolds, D.R.; Reynolds, A.M. The movement of small insects in the convective boundary layer: Linking patterns to processes. Sci. Rep. 2017, 7, 5438. [Google Scholar] [CrossRef]
- Neely, R.; Bennett, L.; Blyth, A.; Collier, C.; Dufton, D.; Groves, J.; Walker, D.; Walden, C.; Bradford, J.; Brooks, B.; et al. The NCAS mobile dual-polarisation Doppler X-band weather radar (NXPol). Atmos. Meas. Tech. 2018, 11, 6481–6494. [Google Scholar] [CrossRef]
- Anjita, N.A.; Indu, J. Leveraging weather radars for desert locust monitoring. Remote Sens. Appl. Soc. Environ. 2023, 31, 100983. [Google Scholar] [CrossRef]
- Shamoun-Baranes, J.; Bauer, S.; Chapman, J.W.; Desmet, P.; Dokter, A.M.; Farnsworth, A.; van Gasteren, H.; Haest, B.; Koistinen, J.; Kranstauber, B.; et al. Meteorological Data Policies Needed to Support Biodiversity Monitoring with Weather Radar. Bull. Am. Meteorol. Soc. 2022, 103, E1234–E1242. [Google Scholar] [CrossRef]
- Tielens, E.K.; Cimprich, P.M.; Clark, B.A.; Dipilla, A.M.; Kelly, J.F.; Mirkovic, D.; Strand, A.I.; Zhai, M.; Stepanian, P.M. Nocturnal city lighting elicits a macroscale response from an insect outbreak population. Biol. Lett. 2021, 17, 20200808. [Google Scholar] [CrossRef]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D. Vertical-Looking Radar: A New Tool for Monitoring High-Altitude Insect Migration. BioScience 2003, 53, 503–511. [Google Scholar] [CrossRef]
- Stepanian, P.M.; Horton, K.G.; Melnikov, V.M.; Zrnić, D.S.; Gauthreaux, S.A. Dual-polarization radar products for biological applications. Ecosphere 2016, 7, e01539. [Google Scholar] [CrossRef]
- Horn, J.W.; Kunz, T.H. Analyzing NEXRAD doppler radar images to assess nightly dispersal patterns and population trends in Brazilian free-tailed bats (Tadarida brasiliensis). Integr. Comp. Biol. 2007, 48, 24–39. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Volponi, S.N.; Stepanian, P.M.; Reynolds, D.R.; Richter, D.H. Using cloud radar to investigate the effect of rainfall on migratory insect flight. Methods Ecol. Evol. 2022, 14, 655–668. [Google Scholar] [CrossRef]
- Alerstam, T.; Chapman, J.W.; Bäckman, J.; Smith, A.D.; Karlsson, H.; Nilsson, C.; Reynolds, D.R.; Klaassen, R.H.G.; Hill, J.K. Convergent patterns of long-distance nocturnal migration in noctuid moths and passerine birds. Proc. R. Soc. B Biol. Sci. 2011, 278, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Gürbüz, S.Z.; Reynolds, D.R.; Koistinen, J.; Liechti, F.; Leijnse, H.; Shamoun-Baranes, J.; Dokter, A.M.; Kelly, J.; Chapman, J.W. Exploring the skies: Technological challenges in radar aeroecology. In Proceedings of the IEEE National Radar Conference, Arlington, VA, USA, 10–15 May 2015; pp. 817–822. [Google Scholar] [CrossRef]
- Hüppop, O.; Ciach, M.; Diehl, R.; Reynolds, D.R.; Stepanian, P.M.; Menz, M.H.M. Perspectives and challenges for the use of radar in biological conservation. Ecography 2019, 42, 912–930. [Google Scholar] [CrossRef]
- Wu, Q.L.; Westbrook, J.K.; Hu, G.; Lu, M.H.; Liu, W.C.; Sword, G.A.; Zhai, B.P. Multiscale analyses on a massive immigration process of Sogatella furcifera (Horváth) in south-central China: Influences of synoptic-scale meteorological conditions and topography. Int. J. Biometeorol. 2018, 62, 1389–1406. [Google Scholar] [CrossRef]
- Chilson, P.B.; Frick, W.F.; Kelly, J.F.; Liechti, F. Aeroecology; Springer Link: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Kelly, J.F.; Stepanian, P.M. Radar aeroecology. Remote Sens. 2020, 12, 1768. [Google Scholar] [CrossRef]
- Kunz, T.H.; Gauthreaux, S.A.; Hristov, N.I.; Horn, J.W.; Jones, G.; Kalko, E.K.V.; Larkin, R.P.; McCracken, G.F.; Swartz, S.M.; Srygley, R.B.; et al. Aeroecology: Probing and modeling the aerosphere. Integr. Comp. Biol. 2008, 48, 1–11. [Google Scholar] [CrossRef]
- Aubry, S.; Labaune, C.; Magnin, F.; Roche, P.; Kiss, L. Active and passive dispersal of an invading land snail in Mediterranean France. J. Anim. Ecol. 2006, 75, 802–813. [Google Scholar] [CrossRef]
- Drew, C.A.; Eggleston, D.B. Currents, landscape structure, and recruitment success along a passive-active dispersal gradient. Landsc. Ecol. 2006, 21, 917–931. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Huang, L.; Dong, Y.; Huang, W.; Ma, H.; Zhang, H.; Zhang, X.; Chen, X.; Xu, Y. Migration risk of fall armyworm (Spodoptera frugiperda) from North Africa to Southern Europe. Front. Plant Sci. 2023, 14, 1141470. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Huang, L.; Xu, C.F.; Li, J.H.; Wang, F.Y.; Cheng, W.; Gao, B.Y.; Chapman, J.W.; Hu, G. Flight Capability and the Low Temperature Threshold of a Chinese Field Population of the Fall Armyworm Spodoptera frugiperda. Insects 2022, 13, 422. [Google Scholar] [CrossRef]
- Wu, M.F.; Qi, G.J.; Chen, H.; Ma, J.; Liu, J.; Jiang, Y.Y.; Lee, G.S.; Otuka, A.; Hu, G. Overseas immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), invading Korea and Japan in 2019. Insect Sci. 2022, 29, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.A.; Randle, Z.; Shortall, C.R.; Oliver, T.H. Where and why are species’ range shifts hampered by unsuitable landscapes? Glob. Chang. Biol. 2022, 28, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, M.A.; Weiskopf, S.R.; Bertrand, R.; Carter, S.L.; Comte, L.; Eaton, M.J.; Johnson, C.G.; Lenoir, J.; Lynch, A.J.; Miller, B.W.; et al. Climate change and the global redistribution of biodiversity: Substantial variation in empirical support for expected range shifts. Environ. Evid. 2023, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Drury, D.W.; Whitesell, M.E.; Wade, M.J. The effects of temperature, relative humidity, light, and resource quality on flight initiation in the red flour beetle, Tribolium castaneum. Entomol. Exp. Appl. 2016, 158, 269–274. [Google Scholar] [CrossRef]
- Hart, A.G.; Hesselberg, T.; Nesbit, R.; Goodenough, A.E. The spatial distribution and environmental triggers of ant mating flights: Using citizen-science data to reveal national patterns. Ecography 2018, 41, 877–888. [Google Scholar] [CrossRef]
- Venter, G.J.; Boikanyo, S.N.B.; De Beer, C.J. The influence of temperature and humidity on the flight activity of Culicoides imicola both under laboratory and field conditions. Parasites Vectors 2019, 12, 4. [Google Scholar] [CrossRef]
- ECMWF. IFS Documentation CY48R1; ECMWF: Reading, UK, 2023. [Google Scholar]
- Morton, R.D.; Marston, C.G.; O’Neil, A.W.; Rowland, C.S. Land Cover Map 2017 (1km Classified Pixels, GB); UK Centre for Ecology & Hydrology: Lancaster, UK, 2020. [Google Scholar]
- Boomsma, J.J.; Leusink, A. Weather Conditions during Nuptial Flights of Four European Ant Species. Oecologia 1981, 50, 236–241. [Google Scholar] [CrossRef]
- Staab, M.; Kleineidam, C. Initiation of swarming behavior and synchronization of mating flights in the leaf-cutting ant Atta vollenweideri FOREL, 1893 (Hymenoptera: Formicidae). Myrmecol. News 2013, 19, 93–102. [Google Scholar]
- Knop, E.; Grimm, M.L.; Korner-Nievergelt, F.; Schmid, B.; Liechti, F. Patterns of high-flying insect abundance are shaped by landscape type and abiotic conditions. Sci. Rep. 2023, 13, 15114. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System. QGIS Association, 2014. Available online: https://www.qgis.org (accessed on 4 November 2024).
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R. biomod2: Ensemble Platform for Species Distribution Modeling (R 4.2-2). 2023. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 4 November 2024).
- Kilambi, A.; Fabry, F.; Meunier, V. A simple and effective method for separating meteorological from nonmeteorological targets using dual-polarization data. J. Atmos. Ocean. Technol. 2018, 35, 1415–1424. [Google Scholar] [CrossRef]
- Helmus, J.J.; Collis, S.M. The Python ARM Radar Toolkit (Py-ART), a Library for Working with Weather Radar Data in the Python Programming Language. J. Open Res. Softw. 2016, 4, e25. [Google Scholar] [CrossRef]
- Lukach, M.; Dally, T.; Evans, W.; Hassall, C.; Duncan, E.J.; Bennett, L.; Addison, F.I.; Kunin, W.E.; Chapman, J.W.; Neely, R.R. The development of an unsupervised hierarchical clustering analysis of dual-polarization weather surveillance radar observations to assess nocturnal insect abundance and diversity. Remote Sens. Ecol. Conserv. 2022, 8, 698–716. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hanczar, B.; Hua, J.; Sima, C.; Weinstein, J.; Bittner, M.; Dougherty, E.R. Small-sample precision of ROC-related estimates. Bioinformatics 2010, 26, 822–830. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis (4.3.3); Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 4 November 2024).
- R Core Team. R: A Language and Environment for Statistical Computing (4.2.2); R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Sillero, N.; Arenas-Castro, S.; Enriquez-Urzelai, U.; Vale, C.G.; Sousa-Guedes, D.; Martínez-Freiría, F.; Real, R.; Barbosa, A.M. Want to model a species niche? A step-by-step guideline on correlative ecological niche modelling. Ecol. Model. 2021, 456, 109671. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2021, 35, 213–226. [Google Scholar] [CrossRef]
- Kautz, M.; Imron, M.A.; Dworschak, K.; Schopf, R. Dispersal variability and associated population-level consequences in tree-killing bark beetles. Mov. Ecol. 2015, 4, 9. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.-H.; Grant, W.E.; Brewer, M.J.; Elliott, N.C.; Westbrook, J.K. Modeling the dispersal of wind-borne pests: Sensitivity of infestation forecasts to uncertainty in parameterization of long-distance airborne dispersal. Agric. For. Meteorol. 2021, 301–302, 108357. [Google Scholar] [CrossRef]
- Mungee, M.; Lukach, M.; Shortall, C.; Bell, J.R.; Duncan, E.J.; Addison, F.; Brown, L.; Kunin, W.E.; Hassall, C.; Neely, R.R., III. Weather radar reveal spatio-temporal trends in aerial arthropod abundance at a national scale. 2024. in review. [Google Scholar]
- Van Doren, B.M.; Horton, K.G. A continental system for forecasting bird migration. Science 2018, 361, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.M.; Pitman, G.M.; Flockhart, D.T.T.; Norris, D.R. Radio-tracking reveals how wind and temperature influence the pace of daytime insect migration. Biol. Lett. 2019, 15, 20190327. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, I.; Schou, W.C.; Richardson, B.; Ross, S.D.; Withers, T.M.; Schmale Iii, D.G.; Strand, T.M. In the wind: Invasive species travel along predictable atmospheric pathways. Ecol. Appl. 2023, 33, e2806. [Google Scholar] [CrossRef]
- Chen, Y.; Aukema, B.H.; Seybold, S.J. The effects of weather on the flight of an invasive bark beetle, Pityophthorus juglandis. Insects 2020, 11, 156. [Google Scholar] [CrossRef]
- Waldock, J.; Chandra, N.L.; Lelieveld, J.; Proestos, Y.; Michael, E.; Christophides, G.; Parham, P.E. The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathog. Glob. Health 2013, 107, 224–241. [Google Scholar] [CrossRef]
- Martini, X.; Stelinski, L.L. Influence of abiotic factors on flight initiation by Asian citrus psyllid (Hemiptera: Liviidae). Environ. Entomol. 2017, 46, 369–375. [Google Scholar] [CrossRef]
- Benton, T.G.; Bryant, D.M.; Cole, L.; Crick, H.Q.P. Linking agricultural practice to insect and bird populations: A historical study over three decades. J. Appl. Ecol. 2002, 39, 673–687. [Google Scholar] [CrossRef]
- Outhwaite, C.L.; McCann, P.; Newbold, T. Agriculture and climate change are reshaping insect biodiversity worldwide. Nature 2022, 605, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ziesche, T.M.; Ordon, F.; Schliephake, E.; Will, T. Long-term data in agricultural landscapes indicate that insect decline promotes pests well adapted to environmental changes. J. Pest Sci. 2023, 97, 1281–1297. [Google Scholar] [CrossRef]
- Blumgart, D.; Botham, M.S.; Menéndez, R.; Bell, J.R. Moth declines are most severe in broadleaf woodlands despite a net gain in habitat availability. Insect Conserv. Divers. 2022, 15, 496–509. [Google Scholar] [CrossRef]
- Jackson, D.L. Guidance on the Interpretation of the Biodiversity Broad Habitat Classification (Terrestrial and Freshwater Types): Definitions and the Relationship with Other Classifications; Joint Nature Conservation Committee: Peterborough, UK, 2000. [Google Scholar]
- Henrys, P.A.; Jarvis, S.G. Integration of ground survey and remote sensing derived data: Producing robust indicators of habitat extent and condition. Ecol. Evol. 2019, 9, 8104–8112. [Google Scholar] [CrossRef] [PubMed]
- Jatau, P.; Melnikov, V.; Yu, T.-Y. Detecting Birds and Insects in the Atmosphere Using Machine Learning on NEXRAD Radar Echoes. Environ. Sci. Proc. 2021, 8, 48. [Google Scholar] [CrossRef]
- Nussbaumer, R.; Schmid, B.; Bauer, S.; Liechti, F. A gaussian mixture model to separate birds and insects in single-polarization weather radar data. Remote Sens. 2021, 13, 1989. [Google Scholar] [CrossRef]
- Zaugg, S.; Saporta, G.; van Loon, E.; Schmaljohann, H.; Liechti, F. Automatic identification of bird targets with radar via patterns produced by wing flapping. J. R. Soc. Interface 2008, 5, 1041–1053. [Google Scholar] [CrossRef]
- Baldini, L.; Gorgucci, E. Identification of the Melting Layer Through Dual-Polarization Radar Measurements at Vertical Incidence. J. Atmos. Ocean. Technol. 2006, 23, 829–839. [Google Scholar] [CrossRef]
- Ryzhkov, A.; Krause, J. New Polarimetric Radar Algorithm for Melting-Layer Detection and Determination of Its Height. J. Atmos. Ocean. Technol. 2022, 39, 529–543. [Google Scholar] [CrossRef]
- Rocchini, D.; Tordoni, E.; Marchetto, E.; Marcantonio, M.; Barbosa, A.M.; Bazzichetto, M.; Beierkuhnlein, C.; Castelnuovo, E.; Gatti, R.C.; Chiarucci, A.; et al. A quixotic view of spatial bias in modelling the distribution of species and their diversity. npj Biodivers. 2023, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Drake, V.A.; Taylor, J.R.; Warrant, E. Insect target classes discerned from entomological radar data. Remote Sens. 2020, 12, 673. [Google Scholar] [CrossRef]
- Bell, J.R.; Aralimarad, P.; Lim, K.-S.; Chapman, J.W. Predicting Insect Migration Density and Speed in the Daytime Convective Boundary Layer. PLoS ONE 2013, 8, e54202. [Google Scholar] [CrossRef]
- Drake, V.A.; Wang, H.K.; Harman, I.T. Insect monitoring radar: Remote and network operation. Comput. Electron. Agric. 2002, 35, 77–94. [Google Scholar] [CrossRef]
- Adonyeva, N.V.; Menshanov, P.N.; Gruntenko, N. A link between atmospheric pressure and fertility of drosophila laboratory strains. Insects 2021, 12, 947. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, A.A.; Lazzari, C.; Diotaiuti, L.; Lorenzo, M.G. The effect of relative humidity on the behaviour and development of Triatoma brasiliensis. Physiol. Entomol. 2002, 27, 142–147. [Google Scholar] [CrossRef]
- Norhisham, A.R.; Abood, F.; Rita, M.; Rehman Hakeem, K. Effect of humidity on egg hatchability and reproductive biology of the bamboo borer (Dinoderus minutus Fabricius). SpringerPlus 2013, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Solbreck, C.; Knape, J.; Förare, J. Role of weather and other factors in the dynamics of a low-density insect population. Ecol. Evol. 2022, 12, e9261. [Google Scholar] [CrossRef]
- Bennett, L. NCAS Mobile X-Band Radar Scan Data from 1st November 2016 to 4th June 2018 Deployed on Long-Term Observations at the Chilbolton Facility for Atmospheric and Radio Research (CFARR), Hampshire, UK; Centre for Environmental Data Analysis: Didcot, UK, 2020. [Google Scholar] [CrossRef]



| Variable Category | Variable Type | Variable Full Name | Units |
|---|---|---|---|
| Aerial (Pressure Levels) | Wind | Zonal Wind | m s−1 |
| Meridional Wind | m s−1 | ||
| Vertical Velocity | Pa s−1 | ||
| Stability and Flow | Divergence | s−1 | |
| Relative Vorticity | s−1 | ||
| Potential Vorticity | K° m−2 kg−1 s−1 | ||
| Temperature | Temperature | C° | |
| Precipitation | Relative Humidity | % | |
| Geometry | Altitude Band | m (±500 m) | |
| Time | Hours | ||
| Terrestrial (Surface) | Wind | 10 m U-component of Wind | m s−1 |
| 10 m V-component of Wind | m s−1 | ||
| Instantaneous 10 m Wind Gust | m s−1 | ||
| Temperature | 2 m Temperature | C° | |
| Skin Temperature | C° | ||
| Precipitation | Convective Rain Rate | Kg m−2 s−1 | |
| Large Scale Rain Rate | Kg m−2 s−1 | ||
| Geometry | Time | Hours | |
| Land Cover Type | Broadleaf Woodland | % | |
| Coniferous Woodland | % | ||
| Arable | % | ||
| Grassland | % | ||
| Urban–Suburban | % |
| Signal Type | Presence/Absence Code | Determinants |
|---|---|---|
| Non-Meteorological (TP) | 1 | DR > −12 & ZH < 35 & SNR > 0.5 |
| No Signal (TA) | 0 | ZH = NA | SNR < 0.5 |
| Weather (FA) | −1 | DR < −12 | ZH > 35 | SNR > 0.5 |
| Beam Blockage (FA) | −2 | 54° < Az < 65° | 82° < Az < 91° | 128° < Az < 155° | 175° < Az <185° | El < 2° |
| Indeterminate Scatter (FA) | −3 | ZH! = NA & ZV = NA |
| Raster Box Designation | Presence/Absence Code | Determinants |
|---|---|---|
| True Presence (TP) | 1 | TP count > 0 |
| True Absence (TA) | 0 | TP count = 0 & TA count > 0 |
| No Data | NA | TP count = 0 & TA count = 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodges, S.; Hassall, C.; Neely, R., III. Weather Radars Reveal Environmental Conditions for High Altitude Insect Movement Through the Aerosphere. Remote Sens. 2024, 16, 4388. https://doi.org/10.3390/rs16234388
Hodges S, Hassall C, Neely R III. Weather Radars Reveal Environmental Conditions for High Altitude Insect Movement Through the Aerosphere. Remote Sensing. 2024; 16(23):4388. https://doi.org/10.3390/rs16234388
Chicago/Turabian StyleHodges, Samuel, Christopher Hassall, and Ryan Neely, III. 2024. "Weather Radars Reveal Environmental Conditions for High Altitude Insect Movement Through the Aerosphere" Remote Sensing 16, no. 23: 4388. https://doi.org/10.3390/rs16234388
APA StyleHodges, S., Hassall, C., & Neely, R., III. (2024). Weather Radars Reveal Environmental Conditions for High Altitude Insect Movement Through the Aerosphere. Remote Sensing, 16(23), 4388. https://doi.org/10.3390/rs16234388






