Assessing Forest Species Diversity in Ghana’s Tropical Forest Using PlanetScope Data
Abstract
:1. Introduction
2. Materials and Methods
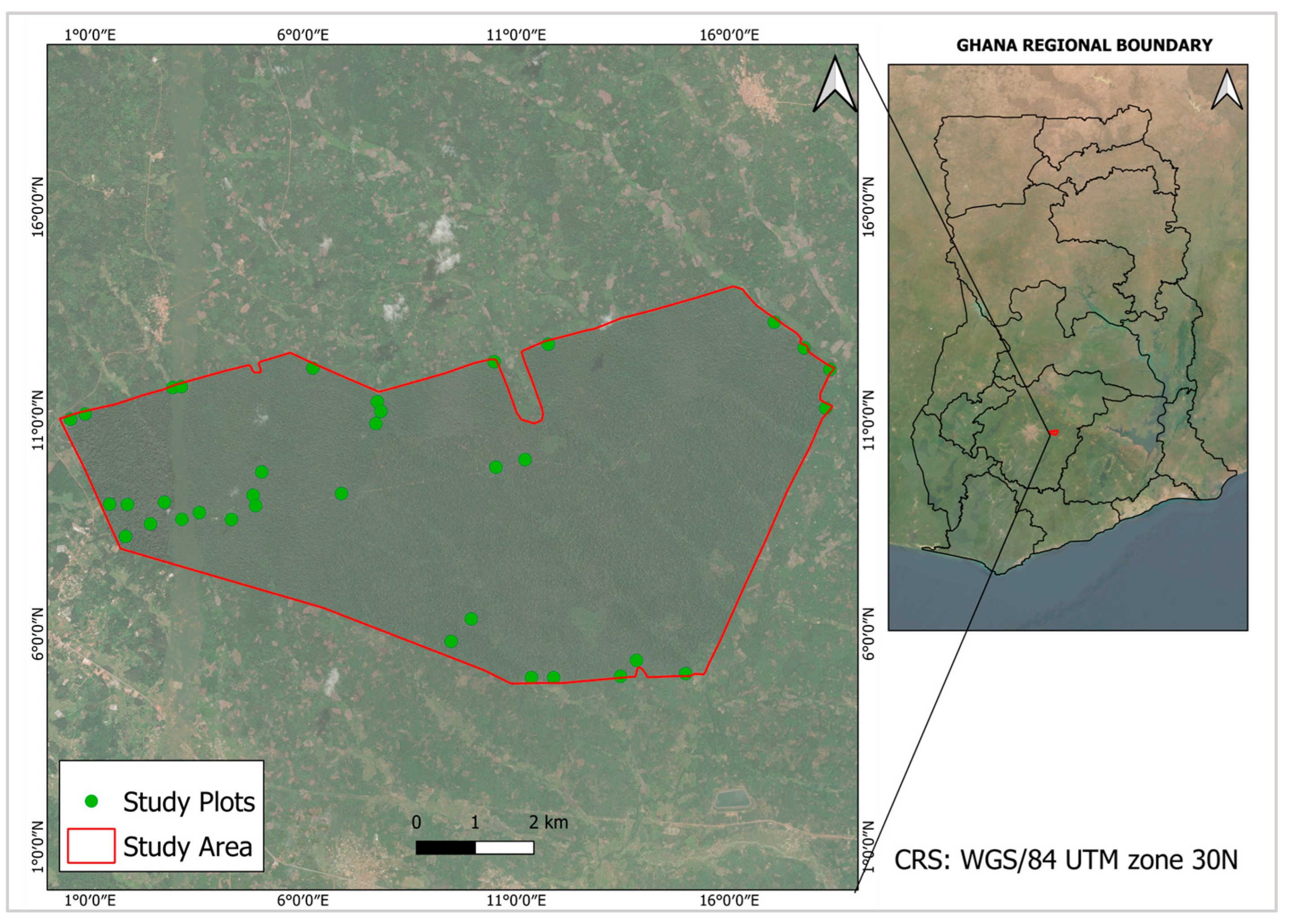
2.1. Study Area
2.2. Remote Sensing Data Acquisition and Preprocessing
2.3. Tree Species Data
2.3.1. Field Sampling Protocol and Tree Species Data Collection
2.3.2. Estimating the Diversity of Tree Species
2.4. Relationship between Tree Species Diversity Indices and Remotely Sensed Data
3. Results
3.1. Correlation between Diversity Indices and Remotely Sensed Data
3.2. Stepwise Linear Regression for Species Diversity Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamfeldt, L.; Snäll, T.; Bagchi, R.; Jonsson, M.; Gustafsson, L.; Kjellander, P.; Ruiz-Jaen, M.C.; Fröberg, M.; Stendahl, J.; Philipson, C.D.; et al. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 2013, 4, 1340. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Saarela, S.; Armston, J.; Ståhl, G.; Dubayah, R. Remote Sensing of Environment Forest biomass estimation over three distinct forest types using TanDEM-X InSAR data and simulated GEDI lidar data. Remote Sens. Environ. 2019, 232, 111283. [Google Scholar] [CrossRef]
- Madonsela, S.; Azong, M.; Ramoelo, A.; Mutanga, O. Remote sensing of species diversity using Landsat 8 spectral variables. ISPRS J. Photogramm. Remote Sens. 2017, 133, 116–127. [Google Scholar] [CrossRef]
- Kumar, P.; Dobriyal, M.; Kale, A.; Pandey, A.K.; Tomar, R.S.; Thounaojam, E. Calculating forest species diversity with information-theory based indices using sentinel-2A sensor’s of Mahavir Swami Wildlife Sanctuary. PLoS ONE 2022, 17, e0268018. [Google Scholar] [CrossRef] [PubMed]
- Tindan, P.D. Savanna primary livelihoods at the edge of land degradation: Linkages and impacts in Ghana. Int. J. Innov. Appl. Stud. 2015, 10, 119–131. [Google Scholar]
- Behera, S.R.; Dash, D.P. The effect of urbanization, energy consumption, and foreign direct investment on the carbon dioxide emission in the SSEA (South and Southeast Asian) region. Renew. Sustain. Energy Rev. 2017, 70, 96–106. [Google Scholar] [CrossRef]
- Vaglio Laurin, G.; Puletti, N.; Hawthorne, W.; Liesenberg, V.; Corona, P.; Papale, D.; Chen, Q.; Valentini, R. Discrimination of tropical forest types, dominant species, and mapping of functional guilds by hyperspectral and simulated multispectral Sentinel-2 data. Remote Sens. Environ. 2016, 176, 163–176. [Google Scholar] [CrossRef]
- Buckland, S.T.; Magurran, A.E.; Green, R.E.; Fewster, R.M. Monitoring change in biodiversity through composite indices. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 243–254. [Google Scholar] [CrossRef]
- Soininen, J.; Passy, S.; Hillebrand, H. The relationship between species richness and evenness: A meta-analysis of studies across aquatic ecosystems. Oecologia 2012, 169, 803–809. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.Y.H.; Reich, P.B. Forest productivity increases with evenness, species richness and trait variation: A global meta-analysis. J. Ecol. 2012, 100, 742–749. [Google Scholar] [CrossRef]
- Crowder, D.W.; Northfield, T.D.; Gomulkiewicz, R.; Snyder, W.E. Conserving and promoting evenness: Organic farming and fire-based wildland management as case studies. Ecology 2012, 93, 2001–2007. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Wang, Y.T.; Jost, L. Entropy and the species accumulation curve: A novel entropy estimator via discovery rates of new species. Methods Ecol. Evol. 2013, 4, 1091–1100. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Chalcraft, D.R.; Bowles, C.M.; Willig, M.R. Relationships among indices suggest that richness is an incomplete surrogate for grassland biodiversity. Ecology 2005, 86, 1178–1184. [Google Scholar] [CrossRef]
- Hakkenberg, C.R.; Song, C.; Peet, R.K.; White, P.S. Forest structure as a predictor of tree species diversity in the North Carolina Piedmont. J. Veg. Sci. 2016, 27, 1151–1163. [Google Scholar] [CrossRef]
- Tuomisto, H. A consistent terminology for quantifying species diversity? Yes, it does exist. Oecologia 2010, 164, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Stachow, U.; Takacs, D.A.; Brubaker, H.W.; Amy, R.; Meaney, J.J.; Neil, J.A.S.O.; Onsi, D.E.; Corzilius, D.B.; Dumas, A.R.; et al. Conserving Biological Diversity in Most biological diversity exists in Agricultural/Forestry Systems. Bioscience 1992, 42, 354–362. [Google Scholar] [CrossRef]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Appiah, M. Tree population inventory, diversity and degradation analysis of a tropical dry deciduous forest in Afram Plains, Ghana. For. Ecol. Manag. 2013, 295, 145–154. [Google Scholar] [CrossRef]
- Chrysafis, I.; Korakis, G.; Kyriazopoulos, A.P.; Mallinis, G. Predicting tree species diversity using geodiversity and sentinel-2 multi-seasonal spectral information. Sustainability 2020, 12, 9250. [Google Scholar] [CrossRef]
- Nagendra, H.; Lucas, R.; Honrado, J.P.; Jongman, R.H.G.; Tarantino, C.; Adamo, M.; Mairota, P. Remote sensing for conservation monitoring: Assessing protected areas, habitat extent, habitat condition, species diversity, and threats. Ecol. Indic. 2013, 33, 45–59. [Google Scholar] [CrossRef]
- Huesca, M.; Merino-de-miguel, S.; Eklundh, L.; Litago, J.; Cicuéndez, V.; Rodríguez-rastrero, M.; Ustin, S.L.; Palacios-orueta, A. Ecosystem functional assessment based on the “optical type” concept and self-similarity patterns: An application using MODIS-NDVI time series autocorrelation. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 132–148. [Google Scholar] [CrossRef]
- Astola, H.; Häme, T.; Sirro, L.; Molinier, M.; Kilpi, J. Comparison of Sentinel-2 and Landsat 8 imagery for forest variable prediction in boreal region. Remote Sens. Environ. 2019, 223, 257–273. [Google Scholar] [CrossRef]
- Fagua, J.C.; Jantz, P.; Rodriguez-buritica, S.; Duncanson, L.; Goetz, S.J. Integrating LiDAR, Multispectral and SAR Data to Estimate and Map Canopy Height in Tropical Forests. Remote Sens. 2019, 11, 2697. [Google Scholar] [CrossRef]
- Mura, M.; Bottalico, F.; Giannetti, F.; Bertani, R.; Mancini, M.; Orlandini, S.; Travaglini, D.; Chirici, G. Exploiting the capabilities of the Sentinel-2 multi spectral instrument for predicting growing stock volume in forest ecosystems. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 126–134. [Google Scholar] [CrossRef]
- Riihimäki, H.; Luoto, M.; Heiskanen, J. Remote Sensing of Environment Estimating fractional cover of tundra vegetation at multiple scales using unmanned aerial systems and optical satellite data. Remote Sens. Environ. 2019, 224, 119–132. [Google Scholar] [CrossRef]
- Csillik, O.; Kumar, P.; Mascaro, J.; Shea, T.O.; Asner, G.P. Monitoring tropical forest carbon stocks and emissions using Planet satellite data. Sci. Rep. 2019, 9, 17831. [Google Scholar] [CrossRef] [PubMed]
- Baloloy, A.B.; Blanco, A.C.; Candido, C.G.; Argamosa, R.J.L.; Dumalag, J.B.L.C.; Dimapilis, L.L.C.; Paringit, E.C. Estimation of mangrove forest aboveground biomass using multispectral bands, vegetation indices and biophysical variables derived from optical satellite imageries: Rapideye, planetscope and sentinel-2. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, 4, 29–36. [Google Scholar] [CrossRef]
- Mulatu, K.A.; Decuyper, M.; Brede, B.; Kooistra, L.; Reiche, J.; Mora, B.; Herold, M. Linking Terrestrial LiDAR Scanner and Conventional Forest Structure Measurements with Multi-Modal Satellite Data. Forests 2019, 10, 291. [Google Scholar] [CrossRef]
- Viña, A.; Gitelson, A.A.; Nguy-robertson, A.L.; Peng, Y. Remote Sensing of Environment Comparison of different vegetation indices for the remote assessment of green leaf area index of crops. Remote Sens. Environ. 2011, 115, 3468–3478. [Google Scholar] [CrossRef]
- Peng, Y.; Fan, M.; Song, J.; Cui, T.; Li, R. Assessment of plant species diversity based on hyperspectral indices at a fine scale. Sci. Rep. 2018, 8, 4776. [Google Scholar] [CrossRef] [PubMed]
- Baffour-Ata, F.; Antwi-Agyei, P.; Nkiaka, E. Climate variability, land cover changes and livelihoods of communities on the fringes of bobiri forest reserve, Ghana. Forests 2021, 12, 278. [Google Scholar] [CrossRef]
- Djagbletey, G. Impact of Selective Logging on Plant Diversity, Natural Recovery and Vegetation Carbon Stock: The Case of Bobiri Forest Reserve. Ph.D. Thesis, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana, 2014. [Google Scholar]
- Addo-Fordjour, P.; Anning, A.K.; Larbi, J.A.; Akyeampong, S. Liana species richness, abundance and relationship with trees in the Bobiri forest reserve, Ghana: Impact of management systems. For. Ecol. Manag. 2009, 257, 1822–1828. [Google Scholar] [CrossRef]
- Planet Labs. Planet Imagery Product Specifications. Web Document. 2019. Available online: https://assets.planet.com/docs/combined-imagery-product-spec-april-2019.pdf (accessed on 6 September 2023).
- Wang, J.; Yang, D.; Detto, M.; Nelson, B.W.; Chen, M.; Guan, K.; Wu, S.; Yan, Z.; Wu, J. Multi-scale integration of satellite remote sensing improves characterization of dry-season green-up in an Amazon tropical evergreen forest. Remote Sens. Environ. 2020, 246, 111865. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol. Model. 2000, 128, 127–147. [Google Scholar] [CrossRef]
- Mapfumo, R.B.; Murwira, A.; Masocha, M.; Andriani, R. The relationship between satellite-derived indices and species diversity across African savanna ecosystems. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 306–317. [Google Scholar] [CrossRef]
- Mutowo, G.; Murwira, A. Relationship between remotely sensed variables and tree species diversity in savanna woodlands of Southern Africa. Int. J. Remote Sens. 2012, 33, 6378–6402. [Google Scholar] [CrossRef]
- Oli, B.N.; Subedi, M.R. Effects of management activities on vegetation diversity, dispersion pattern and stand structure of community-managed forest (Shorea robusta) in Nepal. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2015, 11, 96–105. [Google Scholar] [CrossRef]
- Paudel, S.; Sah, J.P. Effects of Different Management Practices on Stand Composition and Species Diversity in Subtropical Forests in Nepal: Implications of Community Participation in Biodiversity Conservation. J. Sustain. For. 2015, 34, 738–760. [Google Scholar] [CrossRef]
- WFO. Wold Flora Online. Published on the Internet. 2021. Available online: https://about.worldfloraonline.org. (accessed on 6 September 2023).
- Madonsela, S.; Cho, M.A.; Ramoelo, A.; Mutanga, O.; Naidoo, L. Estimating tree species diversity in the savannah using NDVI and woody canopy cover. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 106–115. [Google Scholar] [CrossRef]
- Oldeland, J.; Wesuls, D.; Rocchini, D.; Schmidt, M.; Jürgens, N. Does using species abundance data improve estimates of species diversity from remotely sensed spectral heterogeneity? Ecol. Indic. 2010, 10, 390–396. [Google Scholar] [CrossRef]
- Rouse, R.W.H.; Haas, J.A.W.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA Special Publication: College Station, TX, USA, 1974. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and Photographic Infrared Linear Combinations for Monitoring Vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huete, A.; Justice, C.; Van Leeuwen, W. Modis Vegetation Index (mod 13). In Algorithm Theoretical Basis Document Version 3; NASA: Washington, DC, USA, 1999; pp. 295–309. [Google Scholar]
- Modzelewska, A.; Stereńczak, K.; Mierczyk, M.; Maciuk, S.; Bałazy, R.; Zawiła-Niedźwiecki, T. Sensitivity of vegetation indices in relation to parameters of Norway spruce stands. Folia For. Pol. Ser. A 2017, 59, 85–98. [Google Scholar] [CrossRef]
- Simpson, E. Measurment of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C. A Mathematical Theory of Communication By c. E. Shannon Ixtroduction. Bell Syst. Tech. J. 1948, 27, 519–520. [Google Scholar] [CrossRef]
- Carlo, H.R.; Herman, P.; Soetaery, K. Indices of diversity and evenness. Oceanis 1998, 24, 61–87. [Google Scholar]
- Mensah, I.; Ernest, A. Community Participation in Ecotourism: The Case of Bobiri Forest Reserve and Butterfly Sanctuary in Ashanti Region of Ghana. Am. J. Tour. Manag. 2013, 2013, 34–42. [Google Scholar]
- Loranty, M.M.; Davydov, S.P.; Kropp, H.; Alexander, H.D.; Mack, M.C.; Natali, S.M.; Zimov, N.S. Vegetation Indices Do Not Capture Forest Cover Variation in Upland Siberian Larch Forests. Remote Sens. 2018, 10, 1686. [Google Scholar] [CrossRef]
- Pickering, J.; Tyukavina, A.; Khan, A.; Potapov, P.; Adusei, B.; Hansen, M.C. Using Multi-Resolution Satellite Data to Quantify Land Dynamics: Applications of PlanetScope Imagery for Cropland and Tree-Cover Loss Area Estimation. Remote Sens. 2021, 4, 2191. [Google Scholar] [CrossRef]
- John, A.; Ong, J.; Theobald, E.J.; Olden, J.D.; Tan, A.; Hillerislambers, J. Detecting Montane Flowering Phenology with CubeSat Imagery. Remote Sens. 2020, 12, 2894. [Google Scholar] [CrossRef]
- Cheng, Y.; Vrieling, A.; Fava, F.; Meroni, M.; Marshall, M.; Gachoki, S. Remote Sensing of Environment Phenology of short vegetation cycles in a Kenyan rangeland from PlanetScope and Sentinel-2. Remote Sens. Environ. 2020, 248, 112004. [Google Scholar] [CrossRef]
- Badourdine, C. Exploring the link between spectral variance and upper canopy taxonomic diversity in a tropical forest: Influence of spectral processing and feature selection. Remote Sens. Ecol. Conserv. 2022, 9, 235–250. [Google Scholar] [CrossRef]
- Aklilu Tesfaye, A.; Gessesse Awoke, B. Evaluation of the saturation property of vegetation indices derived from sentinel-2 in mixed crop-forest ecosystem. Spat. Inf. Res. 2021, 29, 109–121. [Google Scholar] [CrossRef]
- Conti, L.; Malavasi, M.; Galland, T.; Komárek, J.; Lagner, O.; Carmona, C.P.; de Bello, F.; Rocchini, D.; Šímová, P. The relationship between species and spectral diversity in grassland communities is mediated by their vertical complexity. Appl. Veg. Sci. 2021, 24, 1–8. [Google Scholar] [CrossRef]
- Gyamfi-Ampadu, E.; Gebreslasie, M.; Mendoza-Ponce, A. Evaluating multi-sensors spectral and spatial resolutions for tree species diversity prediction. Remote Sens. 2021, 13, 1033. [Google Scholar] [CrossRef]
- Imran, H.A.; Gianelle, D.; Scotton, M.; Rocchini, D.; Dalponte, M.; Macolino, S.; Sakowska, K.; Pornaro, C.; Vescovo, L. Potential and Limitations of Grasslands α -Diversity Prediction Using Fine-Scale Hyperspectral Imagery. Remote Sens. 2021, 13, 2649. [Google Scholar] [CrossRef]
- Kulawardhana, R.W. Remote sensing of vegetation: Principles, techniques and applications. By Hamlyn G. Jones and Robin a Vaughan. J. Veg. Sci. 2011, 22, 1151–1153. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Huang, Y.; Reddy, K.N.; Lee, M.A.; Fletcher, R.S.; Thomson, S.J. Early detection of crop injury from herbicide glyphosate by leaf biochemical parameter inversion. Int. J. Appl. Earth Obs. Geoinf. 2014, 31, 78–85. [Google Scholar] [CrossRef]
- Cabacinha, C.D. Relationships between floristic diversity and vegetation indices, forest structure and landscape metrics of fragments in Brazilian Cerrado. For. Ecol. Manag. 2009, 257, 2157–2165. [Google Scholar] [CrossRef]
- Foody, G.M.; Cutler, M.E.; Mcmorrow, J.; Pelz, D.; Tangki, H.; Boyd, D.S.; Douglas, I.A.N. Mapping the biomass of Bornean tropical rain forest from remotely sensed data. Glob. Ecol. Biogeogr. 2001, 10, 379–387. [Google Scholar] [CrossRef]
- Arekhi, M.; Yılmaz, O.Y.; Yılmaz, H.; Akyüz, Y.F. Can tree species diversity be assessed with Landsat data in a temperate forest? Environ. Monit. Assess. 2017, 189, 586. [Google Scholar] [CrossRef]
- Fajji, N.G.; Palamuleni, L.G.; Mlambo, V. Evaluating derived vegetation indices and cover fraction to estimate rangeland aboveground biomass in semi-arid environments. S. Afr. J. Geomat. 2017, 6, 333–348. [Google Scholar] [CrossRef]
- Gaitán, J.J.; Bran, D.; Oliva, G.; Ciari, G.; Nakamatsu, V.; Salomone, J.; Ferrante, D.; Buono, G.; Massara, V.; Humano, G.; et al. Evaluating the performance of multiple remote sensing indices to predict the spatial variability of ecosystem structure and functioning in Patagonian steppes. Ecol. Indic. 2013, 34, 181–191. [Google Scholar] [CrossRef]
- Rampheri, M.; Dube, T.; Dhau, I. Use of remotely sensed data to estimate tree species diversity as an indicator of biodiversity in Blouberg Nature Reserve, South Africa. Geocarto Int. 2022, 37, 526–542. [Google Scholar] [CrossRef]
- Rejauar, R.; Hassan Abu, H.I. NDVI Derived Sugarcane Area Identification and Crop Condition Assessment. Plan Plus 2004, 1, 2. [Google Scholar]
- Möckel, T.; Dalmayne, J.; Schmid, B.C.; Prentice, H.C.; Hall, K. Airborne hyperspectral data predict fine-scale plant species diversity in grazed dry grasslands. Remote Sens. 2016, 8, 133. [Google Scholar] [CrossRef]
- Li, W.; Shi, M.; Huang, Y.; Chen, K.; Sun, H.; Chen, J. Climatic change can influence species diversity patterns and potential habitats of salicaceae plants in China. Forests 2019, 10, 220. [Google Scholar] [CrossRef]
- Shoko, C.; Mutanga, O.; Dube, T. Remotely sensed C3 and C4 grass species aboveground biomass variability in response to seasonal climate and topography. Afr. J. Ecol. 2019, 57, 477–489. [Google Scholar] [CrossRef]
- Silva, B.; Álava-Núñez, P.; Strobl, S.; Beck, E.; Bendix, J. Area-wide evapotranspiration monitoring at the crown level of a tropical mountain rain forest. Remote Sens. Environ. 2017, 194, 219–229. [Google Scholar] [CrossRef]
- Naidu, M.T.; Premavani, D.; Suthari, S.; Venkaiah, M. Assessment of tree diversity in tropical deciduous forests of Northcentral Eastern Ghats, India. Geol. Ecol. Landsc. 2018, 2, 216–227. [Google Scholar]
- Panda, P.C.; Mahapatra, A.K.; Acharya, P.K.; Debata, A.K. Plant diversity in tropical deciduous forests of Eastern Ghats, India: A landscape level assessment. Int. J. Biodivers. Conserv. 2013, 5, 625–639. [Google Scholar]



| Dataset | Spatial Resolution (m) | Band Name/Number | Wavelength (nm) | Date of Image Acquisition |
|---|---|---|---|---|
| PlanetScope | 3.0 | Blue | 465–515 | 19 April 2023 |
| GreenI * | 513–549 | |||
| Green | 547–585 | |||
| Yellow * | 600–620 | |||
| Red | 600–620 | |||
| RedEdge * | 697–713 | |||
| NIR | 845–885 |
| Vegetation Index | Equation | Reference |
|---|---|---|
| Normalized difference vegetation index (NDVI) | [46] | |
| Enhanced vegetation index (EVI) | EVI = G × (NIR − RED)/(NRI + C1 × RED − C2 × BLUE + L) | [47] |
| Simple ratio index (SRI) | [48] | |
| Soil-adjusted vegetation index (SAVI) | [49] | |
| Normalized difference red edge index | [50] |
| Species Diversity Index | Equation | Reference |
|---|---|---|
| Species richness | [12] | |
| Simpson index | [12,51] | |
| Shannon index | [12,52] | |
| Species evenness | [53] |
| Family | Scientific Names | Number of Individual Tree Species |
|---|---|---|
| 1. Leguminosae-Papilionoideae | Baphia pubescens | 16 |
| 2. Leguminosae-Caesalpinioideae | Bussea occidentalis | 18 |
| 3. Ulmaceae | Celtis zenkeri | 19 |
| Celtis mildbraedii | 27 | |
| 4. Malvaceae | Cola caricifolia | 17 |
| Cola giganti | 26 | |
| Nesogordonia | 18 | |
| papaverifera | ||
| Pterygota macrocarpa | 20 | |
| Sterculia Oblonga | 20 | |
| Sterculia rhinopetala | 28 | |
| Triplochiton scleroxylon | 27 | |
| 5. Meliaceae | Carapa Procera | 18 |
| 6. Apocynaceae | Funtumia elastica | 18 |
| 7. Simaroubaceae | Hannoa klaineana | 14 |
| 8. Leguminosae | Hymenostegia afzelii | 25 |
| Total | 311 |
| Diversity Index | Regression Equation | R2 | RMSE | AIC |
|---|---|---|---|---|
| S | Species_Ri~−4.23 + 23.45 × band6_mean − 22.18 × band2_mean | 0.47 | 1.00 | 85.15 |
| H′ | Shanon_Div~0.08 + 3.45 × band6_mean − 3.14 × band2_mean | 0.42 | 0.17 | −10.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Njomaba, E.; Ofori, J.N.; Guuroh, R.T.; Aikins, B.E.; Nagbija, R.K.; Surový, P. Assessing Forest Species Diversity in Ghana’s Tropical Forest Using PlanetScope Data. Remote Sens. 2024, 16, 463. https://doi.org/10.3390/rs16030463
Njomaba E, Ofori JN, Guuroh RT, Aikins BE, Nagbija RK, Surový P. Assessing Forest Species Diversity in Ghana’s Tropical Forest Using PlanetScope Data. Remote Sensing. 2024; 16(3):463. https://doi.org/10.3390/rs16030463
Chicago/Turabian StyleNjomaba, Elisha, James Nana Ofori, Reginald Tang Guuroh, Ben Emunah Aikins, Raymond Kwame Nagbija, and Peter Surový. 2024. "Assessing Forest Species Diversity in Ghana’s Tropical Forest Using PlanetScope Data" Remote Sensing 16, no. 3: 463. https://doi.org/10.3390/rs16030463
APA StyleNjomaba, E., Ofori, J. N., Guuroh, R. T., Aikins, B. E., Nagbija, R. K., & Surový, P. (2024). Assessing Forest Species Diversity in Ghana’s Tropical Forest Using PlanetScope Data. Remote Sensing, 16(3), 463. https://doi.org/10.3390/rs16030463






