Review of Satellite Remote Sensing and Unoccupied Aircraft Systems for Counting Wildlife on Land
Abstract
:1. Introduction
2. Materials and Methods
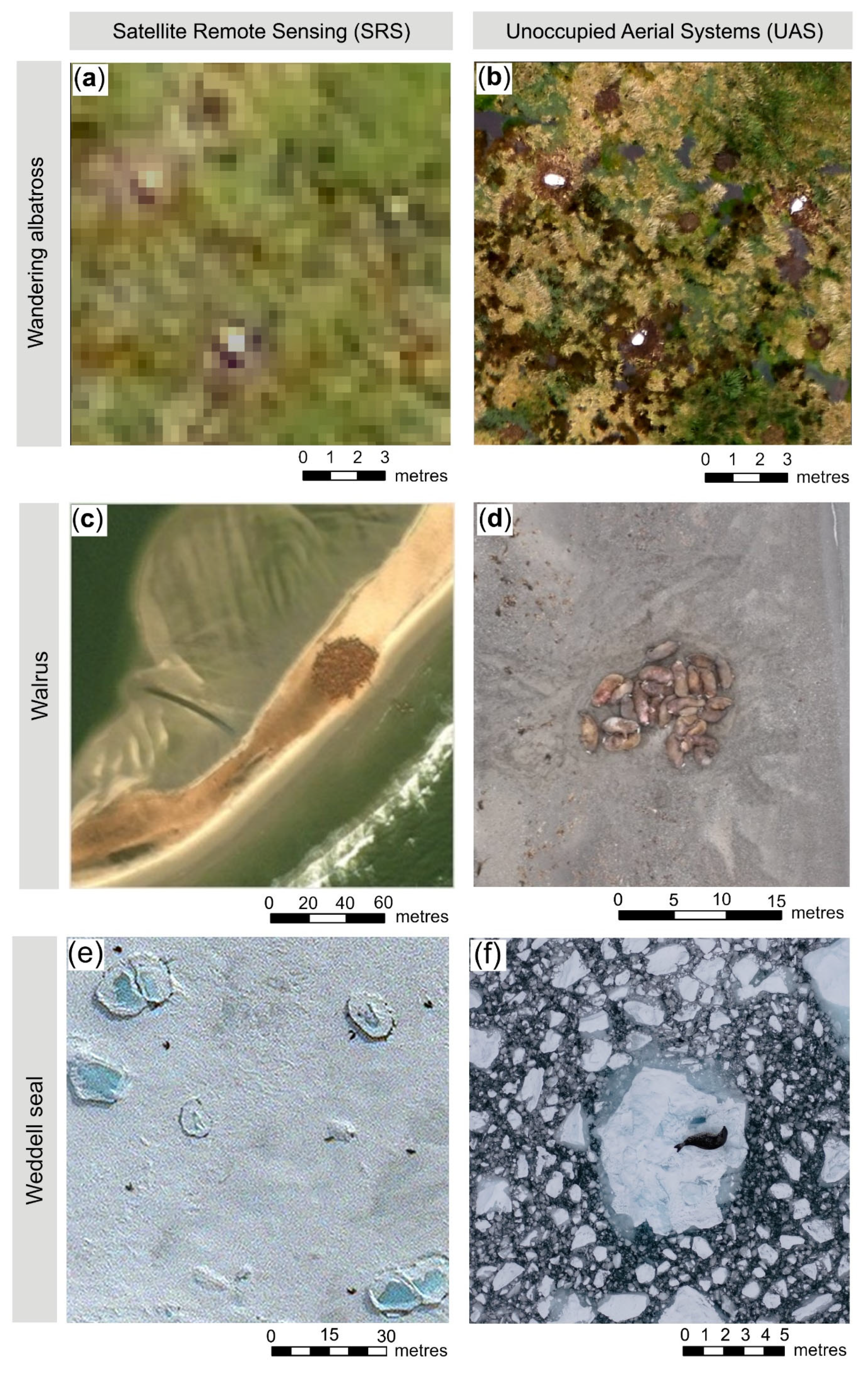
2.1. Detectability
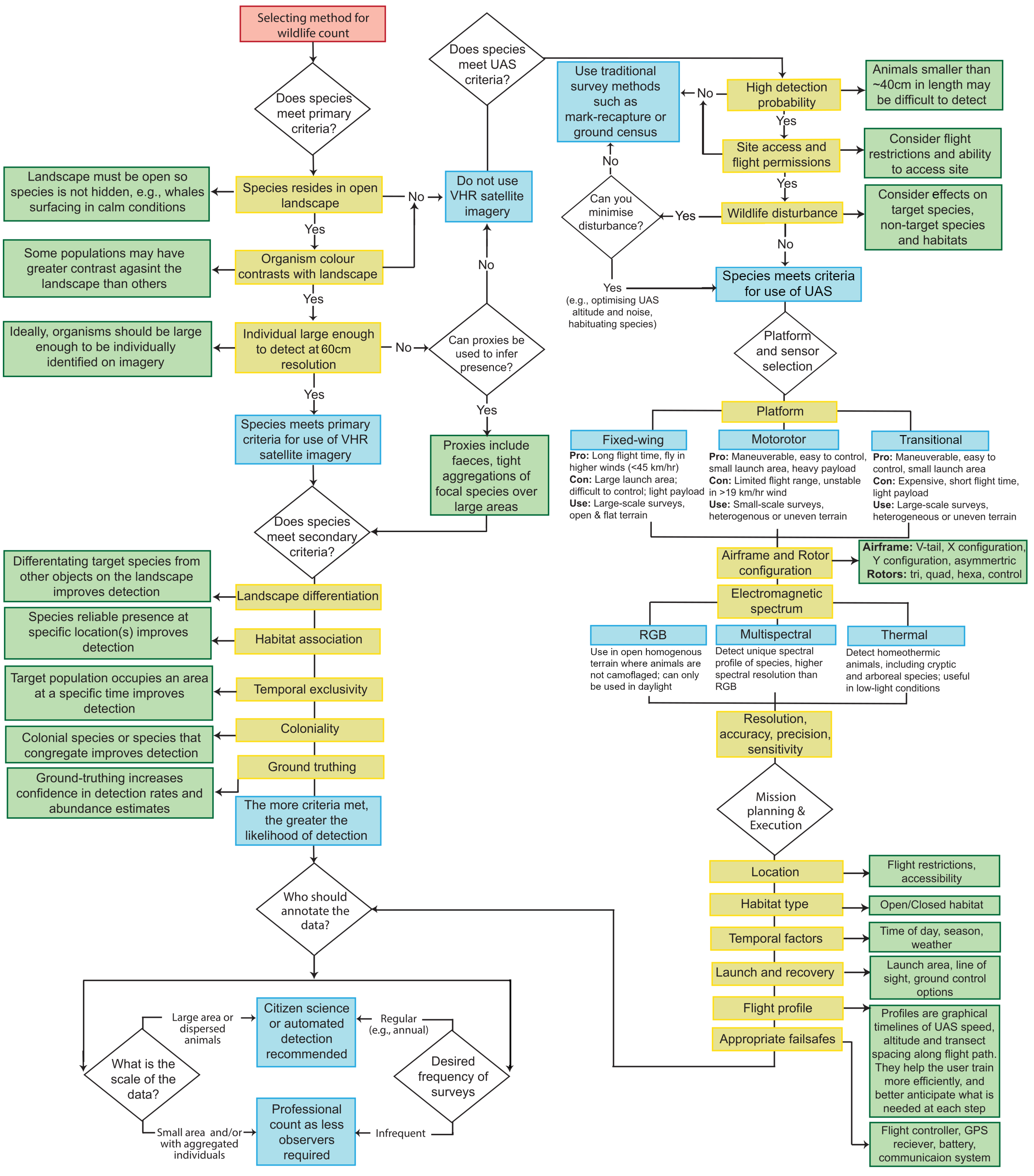
2.2. Species Differentiation
2.3. Indirect Assessment of Population Size
2.4. Use of Spectral Imagery
2.5. Use of Thermal Imagery
2.6. Correction Factors: From Counts to Population Sizes and Trends
3. Availability of Imagery and Processing
3.1. Satellite Imagery
3.1.1. Availability and Cost
3.1.2. Preprocessing and Accuracy
3.2. UAS Imagery
3.2.1. Operational Frameworks
3.2.2. Image Acquisition
3.2.3. Platforms, Sensors, and Data Management
3.3. Spectral Imagery
4. Annotating and Analysing Remote-Sensing Data
4.1. Selection of Observers
4.2. Annotation Methodologies
4.3. Annotation Software
4.4. Challenges
4.5. Citizen Science and Crowdsourcing
4.6. Automated Methods
5. Recommendations and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geller, G.N.; Halpin, P.N.; Helmuth, B.; Hestir, E.L.; Skidmore, A.; Abrams, M.J.; Aguirre, N.; Blair, M.; Botha, E.; Colloff, M. Remote Sensing for Biodiversity. In The GEO Handbook on Biodiversity Observation Networks; Springer: Cham, Switzerland, 2017; pp. 187–210. [Google Scholar]
- Herbreteau, V.; Salem, G.; Souris, M.; Hugot, J.-P.; Gonzalez, J.-P. Thirty years of use and improvement of remote sensing, applied to epidemiology: From early promises to lasting frustration. Health Place 2007, 13, 400–403. [Google Scholar] [CrossRef]
- Chabot, D.; Hodgson, A.J.; Hodgson, J.C.; Anderson, K. ‘Drone’: Technically correct, popularly accepted, socially acceptable. Drone Syst. Appl. 2022, 10, 399–405. [Google Scholar] [CrossRef]
- LaRue, M.A.; Knight, J. Applications of very high-resolution imagery in the study and conservation of large predators in the Southern Ocean. Conserv. Biol. 2014, 28, 1731–1735. [Google Scholar] [CrossRef]
- Wang, D.; Shao, Q.; Yue, H. Surveying wild animals from satellites, manned aircraft and Unmanned Aerial Systems (UASs): A review. Remote Sens. 2019, 11, 1308. [Google Scholar] [CrossRef]
- Keane, J.F.; Carr, S.S. A brief history of early unmanned aircraft. Johns Hopkins APL Tech. Dig. 2013, 32, 558–571. [Google Scholar]
- United States Geological Survey (USGS). Landsat Missions. Available online: https://www.usgs.gov/landsat-missions/landsat-satellite-missions (accessed on 3 January 2024).
- Tanaka, S.; Sugimura, T. Cover: A new frontier of remote sensing from IKONOS images. Int. J. Remote Sens. 2001, 22, 1–5. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Ripple, W.J. Automated wildlife counts from remotely sensed imagery. Wildl. Soc. Bull. 2003, 31, 362–371. [Google Scholar]
- Burn, D.M.; Cody, M.B. Use of Satellite Imagery to Estimate Walrus Abundance at Round Island, Alaska. In Proceedings of the 16th Biennial Conference on the Biology of Marine Mammals, San Diego, CA, USA, 12–16 December 2005; pp. 12–16. [Google Scholar]
- Sasamal, S.K.; Chaudhury, S.B.; Samal, R.N.; Pattanaik, A.K. QuickBird spots flamingos off Nalabana Island, Chilika Lake, India. Int. J. Remote Sens. 2008, 29, 4865–4870. [Google Scholar] [CrossRef]
- Hodgson, J.C.; Mott, R.; Baylis, S.M.; Pham, T.T.; Wotherspoon, S.; Kilpatrick, A.D.; Raja Segaran, R.; Reid, I.; Terauds, A.; Koh, L.P. Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 2018, 9, 1160–1167. [Google Scholar] [CrossRef]
- Clarke, P.J.; Cubaynes, H.C.; Stockin, K.A.; Olavarría, C.; de Vos, A.; Fretwell, P.T.; Jackson, J.A. Cetacean strandings from space: Challenges and opportunities of very high resolution satellites for the remote monitoring of cetacean mass strandings. Front. Mar. Sci. 2021, 8, 650735. [Google Scholar] [CrossRef]
- Duporge, I.; Isupova, O.; Reece, S.; Macdonald, D.W.; Wang, T. Using very-high-resolution satellite imagery and deep learning to detect and count African elephants in heterogeneous landscapes. Remote Sens. Ecol. Conserv. 2020, 7, 369–381. [Google Scholar] [CrossRef]
- Fretwell, P.T.; Trathan, P.N. Discovery of new colonies by Sentinel2 reveals good and bad news for emperor penguins. Remote Sens. Ecol. Conserv. 2021, 7, 139–153. [Google Scholar] [CrossRef]
- Hayes, M.C.; Gray, P.C.; Harris, G.; Sedgwick, W.C.; Crawford, V.D.; Chazal, N.; Crofts, S.; Johnston, D.W. Drones and deep learning produce accurate and efficient monitoring of large-scale seabird colonies. Ornithol. Appl. 2021, 123, duab022. [Google Scholar] [CrossRef]
- Corcoran, E.; Denman, S.; Hanger, J.; Wilson, B.; Hamilton, G. Automated detection of koalas using low-level aerial surveillance and machine learning. Sci. Rep. 2019, 9, 3208. [Google Scholar] [CrossRef] [PubMed]
- Koski, W.R.; Abgrall, P.; Yazvenko, S.B. An inventory and evaluation of unmanned aerial systems for offshore surveys of marine mammals. J. Cetacean Res. Manag. 2010, 11, 239–247. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UAVs) the future of wildlife monitoring? A review of accomplishments and challenges. Mammal Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Christiansen, F.; Dujon, A.M.; Sprogis, K.R.; Arnould, J.P.Y.; Bejder, L. Noninvasive unmanned aerial vehicle provides estimates of the energetic cost of reproduction in humpback whales. Ecosphere 2016, 7, e01468. [Google Scholar] [CrossRef]
- Krause, D.J.; Hinke, J.T.; Perryman, W.L.; Goebel, M.E.; LeRoi, D.J. An accurate and adaptable photogrammetric approach for estimating the mass and body condition of pinnipeds using an unmanned aerial system. PLoS ONE 2017, 12, e0187465. [Google Scholar] [CrossRef]
- Fiori, L.; Doshi, A.; Martinez, E.; Orams, M.B.; Bollard-Breen, B. The use of unmanned aerial systems in marine mammal research. Remote Sens. 2017, 9, 543. [Google Scholar] [CrossRef]
- Pasetto, D.; Arenas-Castro, S.; Bustamante, J.; Casagrandi, R.; Chrysoulakis, N.; Cord, A.F.; Dittrich, A.; Domingo-Marimon, C.; El Serafy, G.; Karnieli, A. Integration of satellite remote sensing data in ecosystem modelling at local scales: Practices and trends. Methods Ecol. Evol. 2018, 9, 1810–1821. [Google Scholar] [CrossRef]
- Wich, S.A.; Piel, A.K. Conservation Technology; Oxford University Press: Oxford, UK, 2021; ISBN 0192590847. [Google Scholar]
- Stapleton, S.; LaRue, M.A.; Lecomte, N.; Atkinson, S.; Garshelis, D.; Porter, C.; Atwood, T. Polar bears from space: Assessing satellite imagery as a tool to track Arctic wildlife. PLoS ONE 2014, 9, e101513. [Google Scholar] [CrossRef]
- Hollings, T.; Burgman, M.; van Andel, M.; Gilbert, M.; Robinson, T.; Robinson, A. How do you find the green sheep? A critical review of the use of remotely sensed imagery to detect and count animals. Methods Ecol. Evol. 2018, 9, 881–892. [Google Scholar] [CrossRef]
- LaRue, M.A.; Stapleton, S.; Anderson, M. Feasibility of using high-resolution satellite imagery to assess vertebrate wildlife populations. Conserv. Biol. 2017, 31, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Pettorelli, N.; Laurance, W.F.; O’Brien, T.G.; Wegmann, M.; Nagendra, H.; Turner, W. Satellite remote sensing for applied ecologists: Opportunities and challenges. J. Appl. Ecol. 2014, 51, 839–848. [Google Scholar] [CrossRef]
- Abileah, R. Marine mammal census using space satellite imagery. U.S. Navy J. Underw. Acoust. 2002, 52, 709–723. [Google Scholar]
- Brown, J.; Qiao, Y.; Clark, C.; Lomax, S.; Rafique, K.; Sukkarieh, S. Automated aerial animal detection when spatial resolution conditions are varied. Comput. Electron. Agric. 2022, 193, 106689. [Google Scholar] [CrossRef]
- Rush, G.P.; Clarke, L.E.; Stone, M.; Wood, M.J. Can drones count gulls? Minimal disturbance and semiautomated image processing with an unmanned aerial vehicle for colony-nesting seabirds. Ecol. Evol. 2018, 8, 12322–12334. [Google Scholar] [CrossRef] [PubMed]
- Fretwell, P.T.; LaRue, M.A.; Morin, P.; Kooyman, G.L.; Wienecke, B.; Ratcliffe, N.; Fox, A.J.; Fleming, A.H.; Porter, C.; Trathan, P.N. An emperor penguin population estimate: The first global, synoptic survey of a species from space. PLoS ONE 2012, 7, e33751. [Google Scholar] [CrossRef]
- Fudala, K.; Bialik, R.J. The use of drone-based aerial photogrammetry in population monitoring of Southern Giant Petrels in ASMA 1, King George Island, maritime Antarctica. Glob. Ecol. Conserv. 2022, 33, e01990. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Skidmore, A.K.; de Leeuw, J.; Said, M.Y.; Freer, J. Spotting East African mammals in open savannah from space. PLoS ONE 2015, 9, e115989. [Google Scholar] [CrossRef]
- Corcoran, E.; Winsen, M.; Sudholz, A.; Hamilton, G. Automated detection of wildlife using drones: Synthesis, opportunities and constraints. Methods Ecol. Evol. 2021, 12, 1103–1114. [Google Scholar] [CrossRef]
- McMahon, C.R.; Howe, H.; van den Hoff, J.; Alderman, R.; Brolsma, H.; Hindell, M.A. Satellites, the all-seeing eyes in the sky: Counting elephant seals from space. PLoS ONE 2014, 9, e92613. [Google Scholar] [CrossRef] [PubMed]
- Fudala, K.; Bialik, R.J. Seals from outer space—Population census of southern elephant seals using VHR satellite imagery. Remote Sens. Appl. Soc. Environ. 2022, 28, 100836. [Google Scholar] [CrossRef]
- Mustafa, O.; Braun, C.; Esefeld, J.; Knetsch, S.; Maercker, J.; Pfeifer, C.; Rümmler, M.-C. Detecting Antarctic seals and flying seabirds usign UAV. ISPRS Ann. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, IV-2/W5, 141–148. [Google Scholar] [CrossRef]
- Fischbach, A.S.; Douglas, D.C. Evaluation of satellite imagery for monitoring Pacific walruses at a large coastal haulout. Remote Sens. 2021, 13, 4266. [Google Scholar] [CrossRef]
- LaRue, M.A.; Salas, L.; Nur, N.; DG, A.; Stammerjohn, S.; Barrington, L.; Stamatiou, K.; Pennycook, J.; Dozier, M.; Saints, J.; et al. Physical and ecological factors explain the distribution of Ross Sea Weddell seals during the breeding season. Mar. Ecol. Prog. Ser. 2019, 612, 193–208. [Google Scholar] [CrossRef]
- Wege, M.; Salas, L.; LaRue, M.A. Ice matters: Life-history strategies of two Antarctic seals dictate climate change eventualities in the Weddell Sea. Glob. Chang. Biol. 2021, 27, 6252–6262. [Google Scholar] [CrossRef]
- Barber-Meyer, S.M.; Kooyman, G.L.; Ponganis, P.J. Estimating the relative abundance of Emperor penguins at inaccessible colonies using satellite imagery. Polar Biol. 2007, 30, 1565–1570. [Google Scholar] [CrossRef]
- Hughes, B.J.; Martin, G.R.; Reynolds, S.J. The use of Google EarthTM satellite imagery to detect the nests of masked boobies Sula dactylatra. Wildl. Biol. 2011, 17, 210–216. [Google Scholar] [CrossRef]
- Swinbourne, M.J.; Taggart, D.A.; Swinbourne, A.M.; Lewis, M.; Ostendorf, B. Using satellite imagery to assess the distribution and abundance of Southern hairy-nosed wombats (Lasiorhinus latifrons). Remote Sens. Environ. 2018, 211, 196–203. [Google Scholar] [CrossRef]
- Velasco, M. A Quickbird’s-Eye View on Marmots. MSc Thesis, University of Twente, Enschede, The Netherlands, 2009. [Google Scholar]
- Turner, W.; Spector, S.; Gardiner, N.; Fladeland, M.; Sterling, E.; Steininger, M. Remote sensing for biodiversity science and conservation. Trends Ecol. Evol. 2003, 18, 306–314. [Google Scholar] [CrossRef]
- Maini, A.K.; Agrawal, V. Satellite Technology: Principles and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pettorelli, N.; Vik, J.O.; Mysterud, A.; Gaillard, J.-M.; Tucker, C.J.; Stenseth, N.C. Using the satellite-derived NDVI to assess ecological responses to environmental change. Trends Ecol. Evol. 2005, 20, 503–510. [Google Scholar] [CrossRef]
- Rees, W.G.; Brown, J.A.; Fretwell, P.T.; Trathan, P.N. What colour is penguin guano? Antarct. Sci. 2017, 29, 417–425. [Google Scholar] [CrossRef]
- Paterson, W.; Sparling, C.E.; Thompson, D.; Pomeroy, P.P.; Currie, J.I.; McCafferty, D.J. Seals like it hot: Changes in surface temperature of harbour seals (Phoca vitulina) from late pregnancy to moult. J. Therm. Biol. 2012, 37, 454–461. [Google Scholar] [CrossRef]
- Schwaller, M.R.; Benninghoff, W.S.; Olson, C.E. Prospects for satellite remote sensing of Adelie penguin rookeries. Int. J. Remote Sens. 1984, 5, 849–853. [Google Scholar] [CrossRef]
- Schwaller, M.R.; Lynch, H.J.; Tarroux, A.; Prehn, B. A continent-wide search for Antarctic petrel breeding sites with satellite remote sensing. Remote Sens. Environ. 2018, 210, 444–451. [Google Scholar] [CrossRef]
- Lynch, H.J. Satellite remote sensing for wildlife research in the polar regions. Mar. Technol. Soc. J. 2023, 57, 43–50. [Google Scholar] [CrossRef]
- Sellés-Ríos, B.; Flatt, E.; Ortiz-García, J.; García-Colomé, J.; Latour, O.; Whitworth, A. Warm beach, warmer turtles: Using drone-mounted thermal infrared sensors to monitor sea turtle nesting activity. Front. Conserv. Sci. 2022, 3, 954791. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Lee, W.Y.; Park, M.; Hyun, C.-U. Detection of two Arctic birds in Greenland and an endangered bird in Korea using RGB and thermal cameras with an unmanned aerial vehicle (UAV). PLoS ONE 2019, 14, e0222088. [Google Scholar] [CrossRef]
- Rivas, A.; Chamoso, P.; González-Briones, A.; Corchado, J.M. Detection of cattle using drones and convolutional neural networks. Sensors 2018, 18, 2048. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Oguma, H.; Tamura, A.; Nakamura, R.; Matsunaga, T. Animal detection using thermal images and its required observation conditions. Remote Sens. 2018, 10, 1050. [Google Scholar] [CrossRef]
- Lhoest, S.; Linchant, J.; Quevauvillers, S.; Vermeulen, C.; Lejeune, P. How many hippos (HOMHIP): Algorithm for automatic counts of animals with infra-red thermal imagery from UAV. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, XL-3/W3, 355–362. [Google Scholar] [CrossRef]
- Gooday, O.J.; Key, N.; Goldstien, S.; Zawar-Reza, P. An assessment of thermal-image acquisition with an unmanned aerial vehicle (UAV) for direct counts of coastal marine mammals ashore. J. Unmanned Veh. Syst. 2018, 6, 100–108. [Google Scholar] [CrossRef]
- Kellenberger, B.; Marcos, D.; Tuia, D. Detecting mammals in UAV images: Best practices to address a substantially imbalanced dataset with deep learning. Remote Sens. Environ. 2018, 216, 139–153. [Google Scholar] [CrossRef]
- Chrétien, L.-P.; Théau, J.; Ménard, P. Visible and thermal infrared remote sensing for the detection of white-tailed deer using an unmanned aerial system. Wildl. Soc. Bull. 2016, 40, 181–191. [Google Scholar] [CrossRef]
- Larsen, G.D.; Seymour, A.C.; Richmond, E.L.; Divine, L.M.; Moreland, E.E.; Newton, E.; London, J.M.; Johnston, D.W. Drones reveal spatial patterning of sympatric Alaskan pinniped species and drivers of their local distributions. Drone Syst. Appl. 2022, 10, 235–255. [Google Scholar] [CrossRef]
- Lee, S.; Song, Y.; Kil, S.-H. Feasibility analyses of real-time detection of wildlife using UAV-derived thermal and RGB images. Remote Sens. 2021, 13, 2169. [Google Scholar] [CrossRef]
- López, A.; Jurado, J.M.; Ogayar, C.J.; Feito, F.R. A framework for registering UAV-based imagery for crop-tracking in Precision Agriculture. Int. J. Appl. Earth Obs. Geoinf. 2021, 97, 102274. [Google Scholar] [CrossRef]
- Wolfaardt, A.; Phillips, R. Guideline Census Methodologies for Albatrosses and Petrels; Agreement on the Conservation of Albatrosses and Petrels: Hobart, TAS, Australia, 2020; pp. 1–12. Available online: https://www.acap.aq/resources/acap-conservation-guidelines (accessed on 15 November 2023).
- Hammond, P.S.; Francis, T.B.; Heinemann, D.; Long, K.J.; Moore, J.E.; Punt, A.E.; Reeves, R.R.; Sepúlveda, M.; Sigurðsson, G.M.; Siple, M.C.; et al. Estimating the abundance of marine mammal populations. Front. Mar. Sci. 2021, 8, 735770. [Google Scholar] [CrossRef]
- Poncet, S.; Wolfaardt, A.C.; Barbraud, C.; Reyes-Arriagada, R.; Black, A.; Powell, R.B.; Phillips, R.A. The distribution, abundance, status and global importance of giant petrels (Macronectes giganteus and M. halli) breeding at South Georgia. Polar Biol. 2020, 43, 17–34. [Google Scholar] [CrossRef]
- Robertson, G.; Moreno, C.A.; Lawton, K.; Kirkwood, R.; Valencia, J. Comparison of census methods for black-browed albatrosses breeding at the Ildefonso Archipelago, Chile. Polar Biol. 2008, 31, 153–162. [Google Scholar] [CrossRef]
- Planet Education and Research Program. Available online: https://www.planet.com/markets/education-and-research/ (accessed on 3 January 2024).
- Witharana, C.; LaRue, M.A.; Lynch, H.J. Benchmarking of data fusion algorithms in support of earth observation based Antarctic wildlife monitoring. ISPRS J. Photogramm. Remote Sens. 2016, 113, 124–143. [Google Scholar] [CrossRef]
- Charry, B.; Tissier, E.; Iacozza, J.; Marcoux, M.; Watt, C.A. Mapping Arctic cetaceans from space: A case study for beluga and narwhal. PLoS ONE 2021, 16, e0254380. [Google Scholar] [CrossRef]
- Fretwell, P.T.; Scofield, P.; Phillips, R.A. Using super-high resolution satellite imagery to census threatened albatrosses. Ibis 2017, 159, 481–490. [Google Scholar] [CrossRef]
- Bowler, E.; Fretwell, P.T.; French, G.; Mackiewicz, M. Using deep learning to count albatrosses from space: Assessing results in light of ground truth uncertainty. Remote Sens. 2020, 12, 2026. [Google Scholar] [CrossRef]
- Millner, N.; Cunliffe, A.M.; Mulero-Pázmány, M.; Newport, B.; Sandbrook, C.; Wich, S. Exploring the opportunities and risks of aerial monitoring for biodiversity conservation. Glob. Soc. Chall. J. 2023, 2, 2–23. [Google Scholar] [CrossRef]
- Nowlin, M.B.; Roady, S.E.; Newton, E.; Johnston, D.W. Applying unoccupied aircraft systems to study human behavior in marine science and conservation programs. Front. Mar. Sci. 2019, 6, 567. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Werden, L.K.; Tanner, J.C.; Vincent, J.B.; Callahan, P.; Iaizzo, P.A.; Laske, T.G.; Garshelis, D.L. Bears habituate to the repeated exposure of a novel stimulus, unmanned aircraft systems. Conserv. Physiol. 2019, 7, coy067. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef] [PubMed]
- Westoby, M.J.; Brasington, J.; Glasser, N.F.; Hambrey, M.J.; Reynolds, J.M. “Structure-from-Motion” photogrammetry: A low-cost, effective tool for geoscience applications. Geomorphology 2012, 179, 300–314. [Google Scholar] [CrossRef]
- Lyons, M.B.; Brandis, K.J.; Murray, N.J.; Wilshire, J.H.; McCann, J.A.; Kingsford, R.T.; Callaghan, C.T. Monitoring large and complex wildlife aggregations with drones. Methods Ecol. Evol. 2019, 10, 1024–1035. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Advanced Distance Sampling; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Hodgson, A.; Kelly, N.; Peel, D. Unmanned Aerial Vehicles (UAVs) for Surveying Marine Fauna: A Dugong Case Study. PLoS ONE 2013, 8, e79556. [Google Scholar] [CrossRef]
- Fewster, R.M.; Southwell, C.; Borchers, D.L.; Buckland, S.T.; Pople, A.R. The influence of animal mobility on the assumption of uniform distances in aerial line-transect surveys. Wildl. Res. 2008, 35, 275–288. [Google Scholar] [CrossRef]
- Jones, G.P. The Feasibility of Using Small Unmanned Aerial Vehicles for Wildlife Research. MSc Thesis, University of Florida, Gainesville, FL, USA, 2003. [Google Scholar]
- Jones, G.P.; Pearlstine, L.G.; Percival, H.F. An assessment of small unmanned aerial vehicles for wildlife research. Wildl. Soc. Bull. 2006, 34, 750–758. [Google Scholar] [CrossRef]
- Koski, W.R.; Allen, T.; Ireland, D.; Buck, G.; Smith, P.R.; Macrander, A.M.; Halick, M.A.; Rushing, C.; Sliwa, D.J.; McDonald, T.L. Evaluation of an unmanned airborne system for monitoring marine mammals. Aquat. Mamm. 2009, 35, 347. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Stolper, R.; van Essen, L.D.; Negro, J.J.; Sassen, T. Remotely piloted aircraft systems as a rhinoceros anti-poaching tool in Africa. PLoS ONE 2014, 9, e83873. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.W. Unoccupied aircraft systems in marine science and conservation. Ann. Rev. Mar. Sci. 2019, 11, 439–463. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.; Barbosa, A.; Mustafa, O.; Peter, H.-U.; Rümmler, M.-C.; Brenning, A. Using fixed-wing UAV for detecting and mapping the distribution and abundance of penguins on the South Shetlands Islands, Antarctica. Drones 2019, 3, 39. [Google Scholar] [CrossRef]
- Wich, S.A.; Koh, L.P. Conservation Drones: Mapping and Monitoring Biodiversity; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Fretwell, P.T.; Phillips, R.A.; Brooke, M.L.; Fleming, A.H.; McArthur, A. Using the unique spectral signature of guano to identify unknown seabird colonies. Remote Sens. Environ. 2015, 156, 448–456. [Google Scholar] [CrossRef]
- LaRue, M.A.; Stapleton, S.; Porter, C.; Atkinson, S.; Atwood, T.; Dyck, M.; Lecomte, N. Testing methods for using high-resolution satellite imagery to monitor polar bear abundance and distribution. Wildl. Soc. Bull. 2015, 39, 772–779. [Google Scholar] [CrossRef]
- Chabot, D.; Stapleton, S.; Francis, C.M. Measuring the spectral signature of polar bears from a drone to improve their detection from space. Biol. Conserv. 2019, 237, 125–132. [Google Scholar] [CrossRef]
- Brodrick, P.G.; Davies, A.B.; Asner, G.P. Uncovering ecological patterns with convolutional neural networks. Trends Ecol. Evol. 2019, 34, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.D.; Chen, D. Segmentation for Object-Based Image Analysis (OBIA): A review of algorithms and challenges from remote sensing perspective. ISPRS J. Photogramm. Remote Sens. 2019, 150, 115–134. [Google Scholar] [CrossRef]
- Cubaynes, H.C.; Fretwell, P.T. Whales from space dataset, an annotated satellite image dataset of whales for training machine learning models. Sci. Data 2022, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Humphries, G.R.W.; Naveen, R.; Schwaller, M.; Che-Castaldo, C.; McDowall, P.; Schrimpf, M.; Lynch, H.J. Mapping Application for Penguin Populations and Projected Dynamics (MAPPPD): Data and tools for dynamic management and decision support. Polar Rec. 2017, 53, 160–166. [Google Scholar] [CrossRef]
- McCarthy, E.D.; Martin, J.M.; Boer, M.M.; Welbergen, J.A. Ground-based counting methods underestimate true numbers of a threatened colonial mammal: An evaluation using drone-based thermal surveys as a reference. Wildl. Res. 2023, 50, 484–493. [Google Scholar] [CrossRef]
- Vermeulen, C.; Lejeune, P.; Lisein, J.; Sawadogo, P.; Bouché, P. Unmanned aerial survey of elephants. PLoS ONE 2013, 8, e54700. [Google Scholar] [CrossRef]
- Beaver, J.T.; Baldwin, R.W.; Messinger, M.; Newbolt, C.H.; Ditchkoff, S.S.; Silman, M.R. Evaluating the use of drones equipped with thermal Sensors as an effective method for estimating wildlife. Wildl. Soc. Bull. 2020, 44, 434–443. [Google Scholar] [CrossRef]
- Hodgson, A.J.; Kelly, N.; Peel, D. Drone images afford more detections of marine wildlife than real-time observers during simultaneous large-scale surveys. PeerJ 2023, 11, e16186. [Google Scholar] [CrossRef]
- Vallecillo, D.; Gauthier-Clerc, M.; Guillemain, M.; Vittecoq, M.; Vandewalle, P.; Roche, B.; Champagnon, J. Reliability of animal counts and implications for the interpretation of trends. Ecol. Evol. 2021, 11, 2249–2260. [Google Scholar] [CrossRef]
- Bird, C.N.; Dawn, A.H.; Dale, J.; Johnston, D.W. A semi-automated method for estimating Adélie penguin colony abundance from a fusion of multispectral and thermal imagery collected with unoccupied aircraft systems. Remote Sens. 2020, 12, 3692. [Google Scholar] [CrossRef]
- LaRue, M.A.; Rotella, J.J.; Garrott, R.A.; Siniff, D.B.; Ainley, D.G.; Stauffer, G.E.; Porter, C.C.; Morin, P.J. Satellite imagery can be used to detect variation in abundance of Weddell seals (Leptonychotes weddellii) in Erebus Bay, Antarctica. Polar Biol. 2011, 34, 1727. [Google Scholar] [CrossRef]
- ESRI. ArcGIS Pro, version 3.2; ESRI: Redlands, CA, USA, 2023. [Google Scholar]
- Scott, L.M.; Janikas, M. V Spatial Statistics in ArcGIS. In Handbook of Applied Spatial Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 27–41. [Google Scholar] [CrossRef]
- QGIS Geographic Information System. QGIS, version 3.34; Open Source Geospatial Foundation Project: Beaverton, OR, USA, 2023; Available online: http://qgis.org/ (accessed on 3 January 2024).
- GRASS Development Team. Geographic Resources Analysis Support System (GRASS) Software, version 8.2; Open Source Geospatial Foundation: Beaverton, OR, USA, 2022; Available online: https://grass.osgeo.org (accessed on 3 January 2024).
- Corrêa, A.A.; Quoos, J.H.; Barreto, A.S.; Groch, K.R.; Eichler, P.P.B. Use of satellite imagery to identify Southern right whales (Eubalaena australis) on a Southwest Atlantic Ocean breeding ground. Mar. Mammal Sci. 2022, 38, 87–101. [Google Scholar] [CrossRef]
- Wada, K. Image Polygonal Annotation with Python. Available online: https://web.archive.org/web/20240103083537/http://labelme.csail.mit.edu/Release3.0 (accessed on 3 January 2024).
- Dutta, A.; Zisserman, A. The VIA Annotation Software for Images, Audio and Video. In Proceedings of the 27th ACM International Conference on Multimedia, Nice, France, 21–25 October 2019; Association for Computing Machinery: New York, NY, USA, 2019; pp. 2276–2279. [Google Scholar]
- Nathans, J.; Thomas, D.; Hogness, D.S. Molecular genetics of human color vision: The genes encoding blue, green, and red pigments. Science 1986, 232, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.G. Physical Principles of Remote Sensing, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012; ISBN 9780511812903. [Google Scholar]
- Ramos, E.A.; Santoya, L.; Verde, J.; Walker, Z.; Castelblanco-Martínez, N.; Kiszka, J.J.; Rieucau, G. Lords of the Rings: Mud ring feeding by Bottlenose dolphins in a Caribbean estuary revealed from sea, air, and space. Mar. Mammal Sci. 2022, 38, 364–373. [Google Scholar] [CrossRef]
- LaRue, M.A.; Lynch, H.J.; Lyver, P.O.B.; Barton, K.; Ainley, D.G.; Pollard, A.; Fraser, W.R.; Ballard, G. A method for estimating colony sizes of Adélie penguins using remote sensing imagery. Polar Biol. 2014, 37, 507–517. [Google Scholar] [CrossRef]
- Raoult, V.; Colefax, A.P.; Allan, B.M.; Cagnazzi, D.; Castelblanco-Martínez, N.; Ierodiaconou, D.; Johnston, D.W.; Landeo-Yauri, S.; Lyons, M.; Pirotta, V.; et al. Operational protocols for the use of drones in marine animal research. Drones 2020, 4, 64. [Google Scholar] [CrossRef]
- Barnas, A.F.; Chabot, D.; Hodgson, A.J.; Johnston, D.W.; Bird, D.M.; Ellis-Felege, S.N. A standardized protocol for reporting methods when using drones for wildlife research. J. Unmanned Veh. Syst. 2020, 8, 89–98. [Google Scholar] [CrossRef]
- Wasowski, R.J. Some ethical aspects of international satellite remote sensing. Photogramm. Eng. Remote Sens. 1991, 57, 41–48. [Google Scholar]
- Ersts, P.J. DotDotGoose, version 1.5.3; Center for Biodiversity and Conservation; American Museum of Natural History: New York, NY, USA, 2022; Available online: https://biodiversityinformatics.amnh.org/open_source/dotdotgoose (accessed on 3 January 2024).
- Borowicz, A.; Le, H.; Humphries, G.; Nehls, G.; Höschle, C.; Kosarev, V.; Lynch, H.J. Aerial-trained deep learning networks for surveying cetaceans from satellite imagery. PLoS ONE 2019, 14, e0212532. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Weinstein, B.G. A computer vision for animal ecology. J. Anim. Ecol. 2018, 87, 533–545. [Google Scholar] [CrossRef]
- Gray, P.C.; Fleishman, A.B.; Klein, D.J.; McKown, M.W.; Bézy, V.S.; Lohmann, K.J.; Johnston, D.W. A convolutional neural network for detecting sea turtles in drone imagery. Methods Ecol. Evol. 2019, 10, 345–355. [Google Scholar] [CrossRef]
- Ren, S.; He, K.; Girshick, R.; Sun, J. Faster r-cnn: Towards real-time object detection with region proposal networks. Adv. Neural Inf. Process. Syst. 2015, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, B.C.; Spitzbart, B.; Lynch, H.J. SealNet: A fully-automated pack-ice seal detection pipeline for sub-meter satellite imagery. Remote Sens. Environ. 2020, 239, 111617. [Google Scholar] [CrossRef]
- Hoekendijk, J.P.A.; Kellenberger, B.; Aarts, G.; Brasseur, S.; Poiesz, S.S.H.; Tuia, D. Counting using deep learning regression gives value to ecological surveys. Sci. Rep. 2021, 11, 23209. [Google Scholar] [CrossRef]
- Forsyth, D.; Ponce, J. Computer Vision: A Modern Approach, 2nd ed.; Prentice Hall Professional Technical Reference: Hoboken, NJ, USA, 2011; ISBN 013608592X. [Google Scholar]
- Shrader-Frechette, K. Ethics of Scientific Research, 1st ed.; Rowan & Littlefield Publishers: Lanham, MD, USA, 1994. [Google Scholar]
- Planet Labs. Introducing the Pelican Constellation: Rapid, Responsive, Resilient. Available online: https://www.planet.com/products/pelican/ (accessed on 3 January 2024).
- Hilker, T.; Lyapustin, A.I.; Tucker, C.J.; Sellers, P.J.; Hall, F.G.; Wang, Y. Remote sensing of tropical ecosystems: Atmospheric correction and cloud masking matter. Remote Sens. Environ. 2012, 127, 370–384. [Google Scholar] [CrossRef]
- The European Space Agency (ESA). Umbra SAR Commercial Microsatellite Constellation. Available online: https://www.eoportal.org/other-space-activities/umbra#launches-of-umbra-satellites-in-reverse-order (accessed on 3 January 2024).
- European Space Agency. EoPortal. Available online: https://www.eoportal.org/satellite-missions (accessed on 15 November 2023).
- Commission of Earth Observation Satellites. CEOS Database. Available online: https://database.eohandbook.com/ (accessed on 15 November 2023).
- World Meterological Organisation Observing Systems Capability Analysis and Review Tool (OSCAR). Available online: https://database.eohandbook.com/ (accessed on 15 November 2023).
- Satellite Imaging Corporation. Satellite Sensors. Available online: https://www.satimagingcorp.com/satellite-sensors/ (accessed on 3 January 2024).
- Kelso, T.S. CelesTrak. Available online: https://celestrak.org/satcat/search.php (accessed on 17 November 2023).
- Krebs, G.D. Gunter’s Space Page. Available online: https://space.skyrocket.de/ (accessed on 3 January 2024).
- World Meterological Organisation. UMBRA-SAR. Available online: https://space.oscar.wmo.int/instruments/view/umbra_sar (accessed on 3 January 2024).
- e-geos. Price List 2021. Available online: https://www.e-geos.it/assets/images/test-img/cosmo-document/gd-com-20-001-e-geos-official-pricelist-june-22nd-2020.pdf (accessed on 3 January 2024).
- Virelli, M.; Coletta, A.; Tapete, D.; Cigna, F. COSMO-SkyMed: A satellite tool for monitoring cultural heritage. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2023, XLVIII-M-2, 1621–1627. [Google Scholar] [CrossRef]
- Tapete, D.; Cigna, F. COSMO-SkyMed SAR for Detection and Monitoring of Archaeological and Cultural Heritage Sites. Remote Sens. 2019, 11, 1326. [Google Scholar] [CrossRef]
- The European Space Agency (ESA). COSMO-SkyMed SG Overview. Available online: https://earth.esa.int/eogateway/missions/cosmo-skymed-second-generation/overview (accessed on 3 January 2024).
- Cloud Earth Observation Services (CLEOS). Radar. Available online: https://www.cleos.earth/SATELLITEIMAGERY/Radar?cclcl=en_US (accessed on 3 January 2024).
- ICEYE. ESA Third Party Mission Data. Available online: https://www.iceye.com/free-data/esa-tpm?utm_campaign=032020%20-%20Research%20Program&utm_medium=email&_hsmi=116544214&_hsenc=p2ANqtz-_mqqLikxPGFXjTtNa7s9d-hUDqO8JSW98ghRbtxk92AKIvv3U9y1BfVV4sMZsZFWKZW6svTF5OpcMOQwvBSUqtGVE21Q&utm_content=116544214&utm_source=hs (accessed on 3 January 2024).
- Rey, N.; Volpi, M.; Joost, S.; Tuia, D. Detecting animals in African Savanna with UAVs and the crowds. Remote Sens. Environ. 2017, 200, 341–351. [Google Scholar] [CrossRef]
- LaRue, M.A.; Ainley, D.G.; Pennycook, J.; Stamatiou, K.; Salas, L.; Nur, N.; Stammerjohn, S.; Barrington, L. Engaging ‘the crowd’ in remote sensing to learn about habitat affinity of the Weddell seal in Antarctica. Remote Sens. Ecol. Conserv. 2020, 6, 70–78. [Google Scholar] [CrossRef]
- Salas, L.; LaRue, M.A.; Nur, N.; Ainley, D.; Stammerjohn, S.; Pennycook, J.; Rotella, J.; Paterson, J.T.; Siniff, D.; Stamatiou, K. Reducing error and increasing reliability of wildlife counts from citizen science surveys: Counting Weddell Seals in the Ross Sea from satellite images. bioRxiv 2020. [Google Scholar] [CrossRef]
- LaRue, M.; Brooks, C.; Wege, M.; Salas, L.; Gardiner, N. High-resolution satellite imagery meets the challenge of monitoring remote marine protected areas in the Antarctic and beyond. Conserv. Lett. 2022, e12884. [Google Scholar] [CrossRef]
- Wege, M.; Salas, L.; LaRue, M.A. Citizen science and habitat modelling facilitates conservation planning for crabeater seals in the Weddell Sea. Divers. Distrib. 2020, 26, 1291–1304. [Google Scholar] [CrossRef]
- Wood, S.A.; Robinson, P.W.; Costa, D.P.; Beltran, R.S. Accuracy and precision of citizen scientist animal counts from drone imagery. PLoS One 2021, 16, e0244040. [Google Scholar] [CrossRef]
- World Wildlife Fund (WWF). Walrus From Space. Available online: https://www.wwf.org.uk/learn/walrus-from-space (accessed on 3 January 2024).
- Wild Southwest. Drones for Ducks. Available online: https://www.zooniverse.org/projects/rowan-aspire/drones-for-ducks (accessed on 15 November 2023).
- Wildlife From Space. Albatrosses From Space. Available online: https://www.bas.ac.uk/project/wildlife-from-space/albatrosses-from-space/ (accessed on 3 January 2024).


| 1. Species/Habitat Specific Traits | 2. Key Metrics | 3. Platform/Sensor Selection | 4. Mission Planning/Execution | 5. Data Management/Processing |
|---|---|---|---|---|
|
|
|
|
|
| Fixed Wing | Multirotor | Transitional | |
|---|---|---|---|
| Launch area | Large | Small | Small |
| Flight duration | >1 h | <1 h | 30–60 min |
| Payload | Light (<1 kg) | Heavy (several kg) | Light (<1 kg) |
| Piolet experience | Substantial training | Minimal, however, larger systems may need more training | Intermediate |
| Launch area | Large | Small | Small |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attard, M.R.G.; Phillips, R.A.; Bowler, E.; Clarke, P.J.; Cubaynes, H.; Johnston, D.W.; Fretwell, P.T. Review of Satellite Remote Sensing and Unoccupied Aircraft Systems for Counting Wildlife on Land. Remote Sens. 2024, 16, 627. https://doi.org/10.3390/rs16040627
Attard MRG, Phillips RA, Bowler E, Clarke PJ, Cubaynes H, Johnston DW, Fretwell PT. Review of Satellite Remote Sensing and Unoccupied Aircraft Systems for Counting Wildlife on Land. Remote Sensing. 2024; 16(4):627. https://doi.org/10.3390/rs16040627
Chicago/Turabian StyleAttard, Marie R. G., Richard A. Phillips, Ellen Bowler, Penny J. Clarke, Hannah Cubaynes, David W. Johnston, and Peter T. Fretwell. 2024. "Review of Satellite Remote Sensing and Unoccupied Aircraft Systems for Counting Wildlife on Land" Remote Sensing 16, no. 4: 627. https://doi.org/10.3390/rs16040627
APA StyleAttard, M. R. G., Phillips, R. A., Bowler, E., Clarke, P. J., Cubaynes, H., Johnston, D. W., & Fretwell, P. T. (2024). Review of Satellite Remote Sensing and Unoccupied Aircraft Systems for Counting Wildlife on Land. Remote Sensing, 16(4), 627. https://doi.org/10.3390/rs16040627







