Integrating Dendrochronological and LiDAR Data to Improve Management of Pinus canariensis Forests under Different Thinning and Climatic Scenarios
Abstract
:1. Introduction
2. Materials and Methods
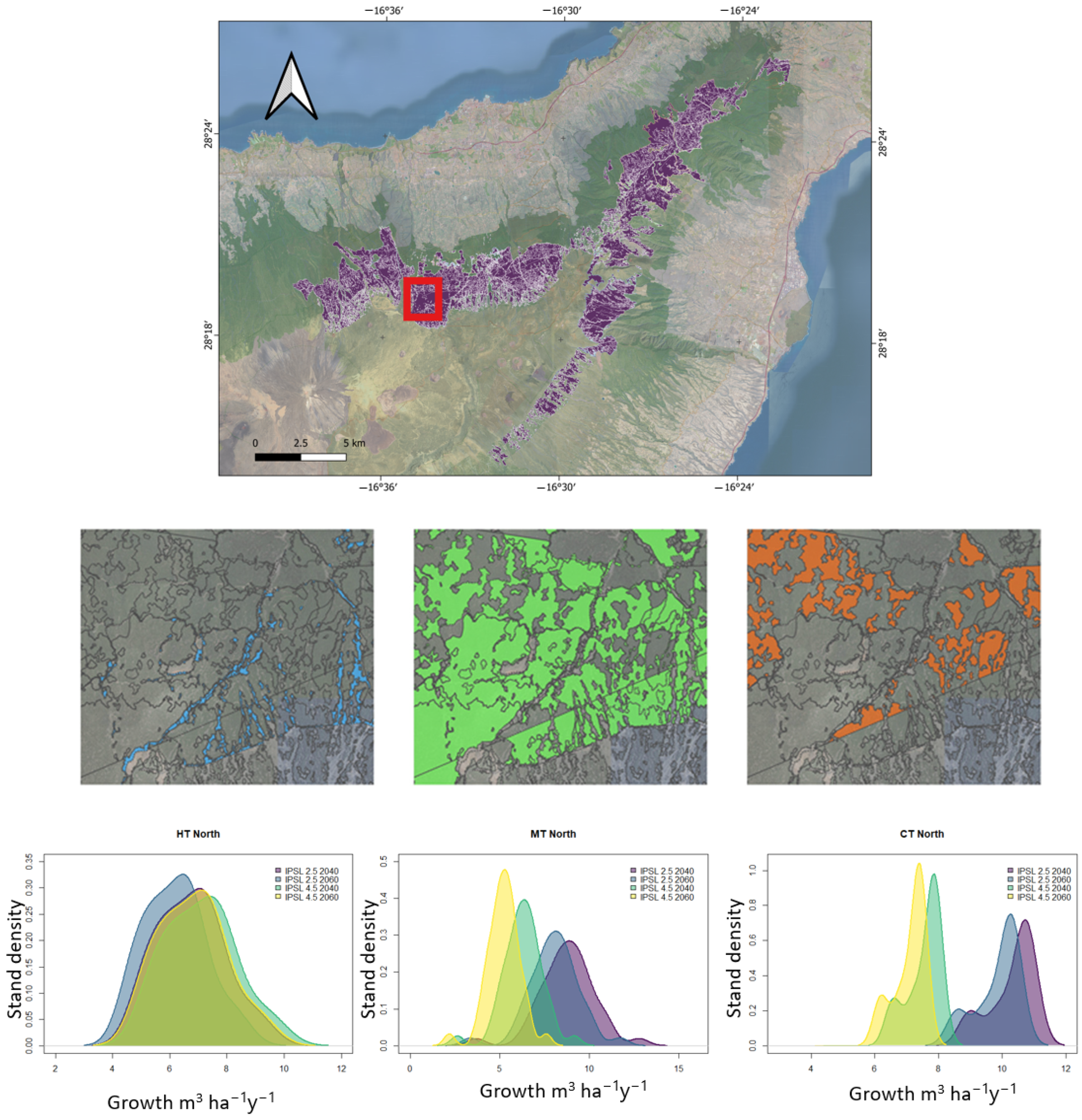
2.1. Study Area
2.2. Experimental Design and Measures
2.3. Dendrochronological Data
2.4. Climate-Driven Growth Models
2.5. LiDAR Stand Segmentation
2.6. Silvicultural Schemes
2.7. Cartography of Silviculture Planning
2.8. Statistical Analysis
3. Results
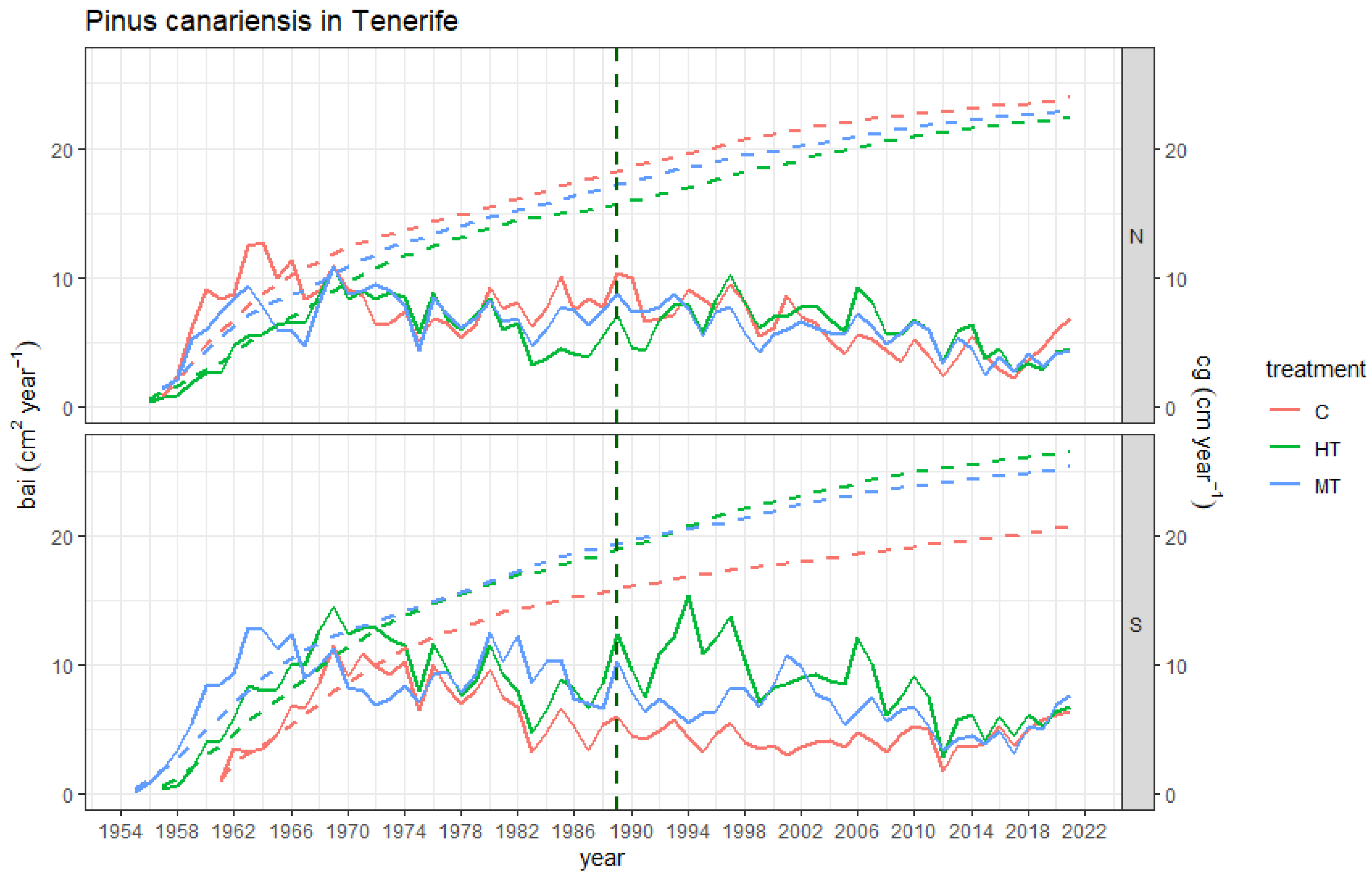
3.1. Stand-Level Growth Responses
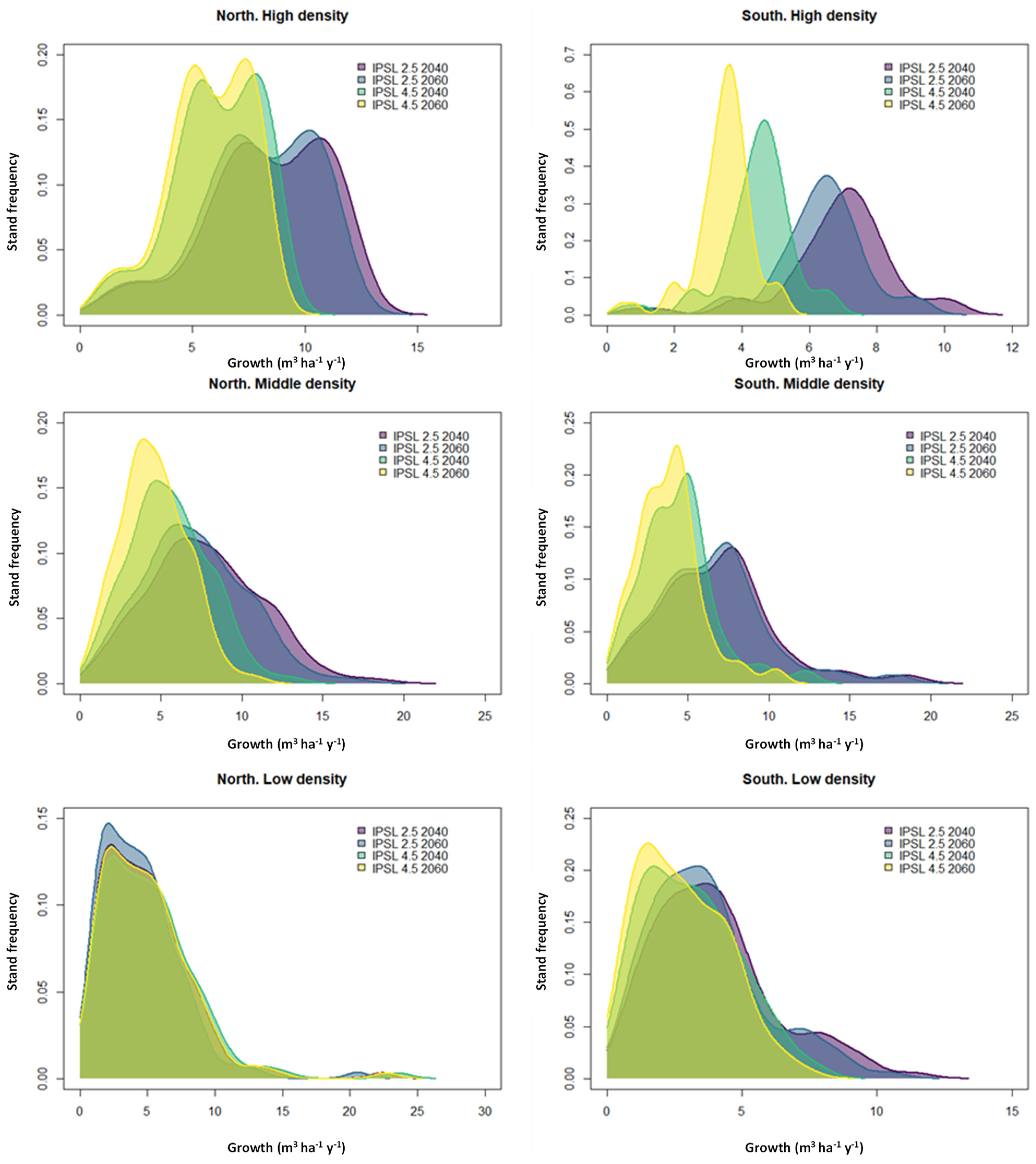
3.2. Growth Projections
3.3. LiDAR Stand Attributes Used to Forecast Growth Trajectories
| Species—Site | Treatment | Timespan | BAI (cm2) | BAI20 (cm2) | BAIpreTH (cm2) | BAIpos10 (cm2) | BAIpostTH (cm2) | AC1 | MS | Rbar |
|---|---|---|---|---|---|---|---|---|---|---|
| P. canariensis—leeward | C | 1957–2021 | 6.94 (0.003) | 4.65 (0.003) | 7.86 (0.005) a | 8.37 (0.003) a | 5.97 (0.003) a | 0.932 | 0.295 | 0.355 |
| MT | 1957–2021 | 6.33 (0.002) | 4.97 (0.003) a | 6.95 (0.003) | 7.42 (0.003) a | 5.74 (0.002) a | 0.887 | 0.344 | 0.286 | |
| HT | 1956–2021 | 5.93 (0.002) a | 5.59 (0.004) | 5.71 (0.004) b | 7.13 (0.005) a | 6.16 (0.003) a | 0.901 | 0.377 | 0.331 | |
| P. canariensis—windward | C | 1961–2021 | 5.54 (0.002) a | 4.42 (0.002) a | 6.84 (0.005) | 4.76 (0.002) b | 4.44 (0.001) b | 0.906 | 0.367 | 0.321 |
| MT | 1955–2021 | 7.48 (0.003) | 5.87 (0.003) | 8.38 (0.005) a | 7.29 (0.004) a | 6.56 (0.003) a | 0.938 | 0.266 | 0.392 | |
| HT | 1957–2021 | 8.46 (0.003) b | 7.11 (0.004) b | 8.40 (0.006) a | 11.49 (0.006) c | 8.51 (0.004) c | 0.884 | 0.353 | 0.255 |
| BAI | BAI20 | BAIpreTH | BAIPost10 | BAIPostTH | CG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| Aspect | 1.586 | 0.209 | 1.311 | 0.254 | 6.349 | 0.012 * | 1.731 | 0.194 | 2.255 | 0.135 | 1.420 | 0.234 |
| Treatment | 0.813 | 0.463 | 5.953 | 0.003 ** | 0.826 | 0.439 | 23.416 | <0.001 *** | 17.089 | <0.001 *** | 0.463 | 0.630 |
| Exposition x Aspect | 11.100 | <0.001 *** | 8.096 | <0.001 *** | 7.145 | 0.001 ** | 29.190 | <0.001 *** | 21.632 | <0.001 *** | 5.657 | 0.003 ** |
3.4. Cartographying Silvicultural Management Plans
4. Discussion
4.1. Thinning Intensity and Growth Responses
4.2. Projecting Tree Growth Based on Climate Scenarios
4.3. LiDAR Metrics Used to Describe Thinning Growth Trajectories
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef]
- Seidl, R.; Albrich, K.; Erb, K.; Formayer, H.; Leidinger, D.; Leitinger, G.; Rammer, W. What drives the future supply of regulating ecosystem services in a mountain forest landscape? For. Ecol. Manag. 2019, 445, 37–47. [Google Scholar] [CrossRef]
- Legg, S. Climate change 2021-the physical science basis. Interaction 2021, 49, 44–45. [Google Scholar]
- Turner, M.G.; Calder, W.J.; Cumming, G.S.; Hughes, T.P.; Jentsch, A.; LaDeau, S.L.; Carpenter, S.R. Climate change, ecosystems and abrupt change: Science priorities. Philos. Trans. R. Soc. B 2020, 375, 20190105. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.R.; Lenton, T.M.; Bascompte, J.; Brock, W.; Dakos, V.; Vandermeer, J. Anticipating critical transitions. Science 2012, 338, 344–348. [Google Scholar] [CrossRef]
- Seidl, R.; Vigl, F.; Rössler, G.; Neumann, M.; Rammer, W. Assessing the resilience of Norway spruce forests through a model-based reanalysis of thinning trials. Forest Ecol. Manag. 2017, 388, 3–12. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.W.; Palik, B.J. Building on the last “new” thing: Exploring the compatibility of ecological and adaptation silviculture. Can. J. For. Res. 2021, 51, 172–180. [Google Scholar] [CrossRef]
- Nyland, R.D. Silviculture: Concepts and Applications; Waveland Press: Long Grove, IL, USA, 2016. [Google Scholar]
- Gleason, K.E.; Bradford, J.B.; Bottero, A.; D’Amato, A.W.; Fraver, S.; Palik, B.J.; Kern, C.C. Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere 2017, 8, e01849. [Google Scholar] [CrossRef]
- Bradford, J.B.; Shriver, R.K.; Robles, M.D.; McCauley, L.A.; Woolley, T.J.; Andrews, C.A.; Bell, D.M. Tree mortality response to drought-density interactions suggests opportunities to enhance drought resistance. J. Appl. Ecol. 2022, 59, 549–559. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Bradford, J.B.; Fraver, S.; Battaglia, M.A.; Asherin, L.A. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Powers, J.; Cochard, H.; Choat, B. Hanging by a thread? Forests and drought. Science 2020, 368, 261–266. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.W.; Bradford, J.B.; Fraver, S.; Palik, B.J. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol Appl. 2013, 23, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cerrillo, R.M.; Cachinero-Vivar, A.M.; Pérez-Priego, Ó.; Cantón, R.A.; Begueria, S.; Camarero, J.J. Developing alternatives to adaptive silviculture: Thinning and tree growth resistance to drought in a Pinus species on an elevated gradient in Southern Spain. Forest Ecol. Manag. 2023, 537, 120936. [Google Scholar] [CrossRef]
- Manrique-Alba, À.; Beguería, S.; Molina, A.J.; González-Sanchis, M.; Tomàs-Burguera, M.; Del Campo, A.D.; Camarero, J.J. Long-term thinning effects on tree growth, drought response and water use efficiency at two Aleppo pine plantations in Spain. Sci. Total Environ. 2020, 728, 138536. [Google Scholar] [CrossRef]
- Tague, C.L.; Moritz, M.; Hanan, E. The changing water cycle: The eco-hydrologic impacts of forest density reduction in Mediterranean (seasonally dry) regions. Water 2019, 6, e1350. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Naranjo-Cigala, A.; Fernández-Palacios, J.M.; Fernández Lugo, S. Ecology and management of natural and reforested Canary Island pine stands. In Ecology and Management of Natural and Reforested Canary Island Pine Stands; Wallace, E.B., Ed.; Woodlands; Ecology, Management and Conservation Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Fernández-Palacios, J.M.; de Nicolás, J.P. Altitudinal pattern of vegetation variation on Tenerife. J. Veg. Sci. 1995, 6, 183–190. [Google Scholar] [CrossRef]
- Gieger, T.; Leuschner, C. Altitudinal change in needle water relations of Pinus canariensis and possible evidence of a drought-induced alpine timberline on Mt. Teide, Tenerife. Flora 2004, 199, 100–109. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Palacios, J.M. From pine plantations to natural stands. Ecological restoration of a Pinus canariensis Sweet, ex Spreng forest. Plant Ecol. 2005, 181, 217–226. [Google Scholar] [CrossRef]
- Molina, A.J.; Navarro-Cerrillo, R.M.; Pérez-Romero, J.; Alejano, R.; Bellot, J.F.; Blanco, J.A.; del Campo, A.D. SilvAdapt. Net: A Site-Based Network of Adaptive Forest Management Related to Climate Change in Spain. Forests 2021, 12, 1807. [Google Scholar] [CrossRef]
- Madrigal, A.; Domínguez, M.L.; Peraza, M.; Barber, J.R.; Herrero, J.; Rodríguez-Solano, R. Estudio de la Selvicultura de las Masas Artificiales de Pinus Canariensis Sweet ex Spreng; Gobierno de Canarias, Dirección General del Medio Ambiente; Fundación General de la Universidad Politécnica de Madrid, Departamento de Silvopascicultura: Santa Cruz de Tenerife, Madrid, Spain, 1989. [Google Scholar]
- Aboal, J.R.; Jiménez, M.S.; Morales, D. Gil P Effects of thinning on throughfall in Canary Islands pine forest—The role of fog. J Hydrol. 2000, 238, 218–230. [Google Scholar] [CrossRef]
- Pérez-de-Lis, G.; García-González, I.; Rozas, V.; Arévalo, J.R. Effects of thinning intensity on radial growth patterns and temperature sensitivity in Pinus canariensis afforestations on Tenerife Island, Spain. Ann. For. Sci. 2011, 68, 1093–1104. [Google Scholar] [CrossRef]
- Rozas, V.; García-González, I.; Pérez-de-Lis, G.; Arévalo, J.R. Local and large-scale climatic factors controlling tree-ring growth of Pinus canariensis on an oceanic island. Clim. Res. 2013, 56, 197–207. [Google Scholar] [CrossRef]
- Rozas, V.; Pérez-de-Lis, G.; García-González, I.; Arévalo, J.R. Contrasting effects of wildfire and climate on radial growth of Pinus canariensis on windward and leeward slopes on Tenerife, Canary Islands. Trees 2011, 25, 895–905. [Google Scholar] [CrossRef]
- Otto, R.; García-del-Rey, E.; Méndez, J.; Fernández-Palacios, J.M. Effects of thinning on seed rain, regeneration and understory vegetation in a Pinus canariensis plantation (Tenerife, Canary Islands). For. Ecol. Manag. 2012, 280, 71–81. [Google Scholar] [CrossRef]
- Correa-Díaz, A.; Silva, L.C.R.; Horwath, W.R.; Gómez-Guerrero, A.; Vargas-Hernández, J.; Villanueva-Díaz, J.; Velázquez-Martínez, A. From trees to ecosystems: Spatiotemporal scaling of climatic impacts on montane landscapes using dendrochronological, isotopic, and remotely sensed data. Glob. Biogeochem. Cycles 2020, 34, e2019GB006325. [Google Scholar] [CrossRef]
- Zhou, Y.; Yi, Y.; Jia, W.; Cai, Y.; Yang, W.; Li, Z. Applying dendrochronology and remote sensing to explore climate-drive in montane forests over space and time. Quat. Sci. Rev. 2020, 237, 106292. [Google Scholar] [CrossRef]
- Kangas, A.; Gobakken, T.; Puliti, S.; Hauglin, M.; Naesset, E. Value of airborne laser scanning and digital aerial photogrammetry data in forest decision making. Silva Fenn. 2018, 52, 9923. [Google Scholar] [CrossRef]
- Coops, N.C.; Tompalski, P.; Goodbody, T.R.; Queinnec, M.; Luther, J.E.; Bolton, D.K.; Hermosilla, T. Modelling lidar-derived estimates of forest attributes over space and time: A review of approaches and future trends. Remote Sens. Environ. 2021, 260, 112477. [Google Scholar] [CrossRef]
- Wulder, M.A.; White, J.C.; Nelson, R.F.; Næsset, E.; Ørka, H.O.; Coops, N.C.; Hilker, T.; Bater, C.W.; Gobakken, T. Lidar sampling for large-area forest characterization: A review. Remote Sens. Environ. 2012, 121, 196–209. [Google Scholar] [CrossRef]
- Pretzsch, H.; del Río, M.; Grote, R.; Klemmt, H.J.; Ordóñez, C.; Bravo Oviedo, F. Tracing drought effects from the tree to the stand growth in temperate and Mediterranean forests: Insights and consequences for forest ecology and management. Eur. J. For. Res. 2022, 141, 727–751. [Google Scholar] [CrossRef]
- MITECO Cuarto Inventario Forestal Español Canarias. Ministerio para la Transición Ecológica y el Reto Demográfico (MITECO) Madrid 2020. Available online: www.miteco.gob.es (accessed on 12 January 2023).
- Fernández-Caldas, E.; Tejedor, M.; Quantin, P. Los Suelos Volcánicos de Canarias; Servicio de Publicaciones de la Universidad de La Laguna: La Laguna, Spain, 1985. [Google Scholar]
- Fritts, H. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Holmes, R.L. Computer-assisted quality control in tree-ring dating and measurement. Tree Ring Res. 1983, 43, 51–67. [Google Scholar]
- Bunn, A.; Korpela, M.; Biondi, F.; Campelo, F.; Mérian, P.; Qeadan, F.; Klesse, S. Package ‘dplR’. Dendrochronology Program Library in R, Version, 1, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2022. Available online: https://www.R-project.org/ (accessed on 9 January 2023).
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Lebourgeois, F.; Pierrat, J.C.; Perez, V.; Piedallu, C.; Cecchini, S.; Ulrich, E. Simulating phenological shifts in French temperate forests under two climatic change scenarios and four driving global circulation models. Int. J. Biometeorol. 2010, 54, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Bert, D.; Lebourgeois, F.; Ouayjan, A.; Ducousso, A.; Ogée, J.; Hampe, A. Past and future radial growth and water-use efficiency of Fagus sylvatica and Quercus robur in a long-term climate refugium. Dendrochronologia 2022, 72, 125939. [Google Scholar] [CrossRef]
- Cabildo de Tenerife. Proyecto Para la Ejecución de Tratamientos Selvícolas de Mejora en Pino Canario Procedente de Repoblación; Agresta: Cabildo de Tenerife: Santa Cruz de Tenerife, Spain, 2014. [Google Scholar]
- Arias-Rodil, M.; Diéguez-Aranda, U.; Álvarez-González, J.G.; Pérez-Cruzado, C.; Castedo-Dorado, F.; González-Ferreiro, E. Modeling diameter distributions in radiata pine plantations in Spain with existing countrywide LiDAR data. Ann. For. Sci. 2018, 75, 36. [Google Scholar] [CrossRef]
- Gorgoso-Varela, J.J.; Rojo-Alboreca, A. Use of Gumbel and Weibull functions to model extreme values of diameter distributions in forest stands. Ann. For. Sci. 2014, 71, 741–750. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sakar, D. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1 2020, 1–149. [Google Scholar]
- McGaughey, B. FUSION Version 3.30; USDA Forest Service: Seattle, WA, USA, 2018. [Google Scholar]
- Varo-Martínez, M.Á.; Navarro-Cerrillo, R.M. Stand delineation of Pinus sylvestris L. plantations suffering decline processes based on biophysical tree crown variables: A necessary tool for adaptive silviculture. Rem. Sens. 2021, 13, 436. [Google Scholar] [CrossRef]
- Næsset, E. Predicting forest stand characteristics with airborne scanning laser using a practical two-stage procedure and field data. Remote Sens. Environ. 2002, 80, 88–99. [Google Scholar] [CrossRef]
- López-Senespleda, E.; Climent, J.M.; Montero, G. Selvicultura de Pinus canariensis Sweet. In Compendio de Selvicultura Aplicada en España; INIA: Madrid, Spain, 2008. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Green, P. Package ‘lme4’. 2009. Available online: http://lme4.r-forge.r-project (accessed on 21 January 2023).
- Anderegg, W.R.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D. Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 2012, 3, 292–297. [Google Scholar] [CrossRef]
- Yi, C.; Hendrey, G.; Niu, S.; McDowell, N.; Allen, C.D. Tree mortality in a warming world: Causes, patterns, and implications. Environ. Res. Lett. 2022, 17, 030201. [Google Scholar] [CrossRef]
- Sankey, T.; Tatum, J. Thinning increases forest resiliency during unprecedented drought. Sci. Rep. 2022, 12, 9041. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.; Cubera, E. Impact of stand density on water status and leaf gas exchange in Quercus ilex. For. Ecol. Manag. 2008, 254, 74–84. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Carreira, J.A. Plastic responses of Abies pinsapo xylogenesis to drought and competition. Tree Physiol. 2009, 29, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Martín-Benito, D.; Del Río, M.; Heinrich, I.; Helle, G.; Cañellas, I. Response of climate-growth relationships and water use efficiency to thinning in a Pinus nigra afforestation. For. Ecol. Manag. 2010, 259, 967–975. [Google Scholar] [CrossRef]
- Lechuga, V.; Carraro, V.; Viñegla, B.; Carreira, J.A.; Linares, J.C. Managing drought-sensitive forests under global change. Low competition enhances long-term growth and water uptake in Abies pinsapo. For. Ecol. Manag. 2017, 406, 72–82. [Google Scholar] [CrossRef]
- Climent, J.; Chambel, M.R.; Pérez, E.; Gil, L.; Pardos, J. Relationship between heartwood radius and early radial growth, tree age, and climate in Pinus canariensis. Can. J. For. Res. 2002, 32, 103–111. [Google Scholar] [CrossRef]
- Blanco, J.A.; Imbert, J.B.; Castillo, F.J. Nutrient return via litterfall in two contrasting Pinus sylvestris forests in the Pyrenees under different thinning intensities. For. Ecol. Manag. 2008, 256, 1840–1852. [Google Scholar] [CrossRef]
- Kagawa, A.; Battipaglia, G. Post-photosynthetic carbon, oxygen and hydrogen isotope signal transfer to tree rings—How timing of cell formations and turnover of stored carbohydrates affect intra-annual isotope variations. In Stable Isotopes in Tree Rings: Inferring Physiological, Climatic and Environmental Responses; Springer: Cham, Switzerland, 2022; pp. 429–462. [Google Scholar]
- Zhirnova, D.F.; Belokopytova, L.V.; Barabantsova, A.E.; Babushkina, E.A.; Vaganov, E.A. What prevails in climatic response of Pinus sylvestris in-between its range limits in mountains: Slope aspect or elevation? Int. J. Biometeorol. 2020, 64, 333–344. [Google Scholar] [CrossRef]
- Brito, P.; Wieser, G.; Oberhuber, W.; Gruber, A.; Lorenzo, J.R.; González-Rodríguez, Á.M.; Jiménez, M.S. Water availability drives stem growth and stem water deficit of Pinus canariensis in a drought-induced treeline in Tenerife. Plant Ecol. 2017, 218, 277–290. [Google Scholar] [CrossRef]
- Bauwe, A.; Jurasinski, G.; Scharnweber, T.; Schroeder, C.; Lennartz, B. Impact of climate change on tree-ring growth of Scots pine, common beech and pedunculate oak in northeastern Germany. iForest 2015, 9, 1. [Google Scholar] [CrossRef]
- Vieira, J.; Carvalho, A.; Campelo, F. Tree growth under climate change: Evidence from xylogenesis timings and kinetics. Front. Plant Sci. 2020, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Cerrillo, R.M.; Sánchez-Salguero, R.; Rodriguez, C.; Lazo, J.D.; Moreno-Rojas, J.M.; Palacios-Rodriguez, G.; Camarero, J.J. Is thinning an alternative when trees could die in response to drought? The case of planted Pinus nigra and P. sylvestris stands in southern Spain. Forest Ecol. Manag. 2019, 433, 313–324. [Google Scholar] [CrossRef]
- Blanco, A.; Castroviejo, M.; Fraile, J.L.; Gandullo, J.M.; Muñoz, L.A.; Sánchez Palomares, O. Estudio Ecológico del Pino Canario; ICONA-MAPA: Madrid, Spain, 1989. [Google Scholar]
- Hill, A.; Buddenbaum, H.; Mandallaz, D. Combining canopy height and tree species map information for large-scale timber volume estimations under strong heterogeneity of auxiliary data and variable sample plot sizes. Eur. J. For. Res. 2018, 137, 489–505. [Google Scholar] [CrossRef]
- Hashida, Y.; Lewis, D.J. The intersection between climate adaptation, mitigation, and natural resources: An empirical analysis of forest management. J. Assoc. Environ. Resour. Econ. 2019, 6, 893–926. [Google Scholar] [CrossRef]
- Velasco Pereira, E.A.; Varo Martínez, M.A.; Ruiz Gómez, F.J.; Navarro-Cerrillo, R.M. Temporal Changes in Mediterranean Pine Forest Biomass Using Synergy Models of ALOS PALSAR-Sentinel 1-Landsat 8 Sensors. Remote Sens. 2023, 15, 3430. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Cerrillo, R.M.; Padrón Cedrés, E.; Cachinero-Vivar, A.M.; Valeriano, C.; Camarero, J.J. Integrating Dendrochronological and LiDAR Data to Improve Management of Pinus canariensis Forests under Different Thinning and Climatic Scenarios. Remote Sens. 2024, 16, 850. https://doi.org/10.3390/rs16050850
Navarro-Cerrillo RM, Padrón Cedrés E, Cachinero-Vivar AM, Valeriano C, Camarero JJ. Integrating Dendrochronological and LiDAR Data to Improve Management of Pinus canariensis Forests under Different Thinning and Climatic Scenarios. Remote Sensing. 2024; 16(5):850. https://doi.org/10.3390/rs16050850
Chicago/Turabian StyleNavarro-Cerrillo, Rafael M., Eva Padrón Cedrés, Antonio M. Cachinero-Vivar, Cristina Valeriano, and Jesús Julio Camarero. 2024. "Integrating Dendrochronological and LiDAR Data to Improve Management of Pinus canariensis Forests under Different Thinning and Climatic Scenarios" Remote Sensing 16, no. 5: 850. https://doi.org/10.3390/rs16050850
APA StyleNavarro-Cerrillo, R. M., Padrón Cedrés, E., Cachinero-Vivar, A. M., Valeriano, C., & Camarero, J. J. (2024). Integrating Dendrochronological and LiDAR Data to Improve Management of Pinus canariensis Forests under Different Thinning and Climatic Scenarios. Remote Sensing, 16(5), 850. https://doi.org/10.3390/rs16050850













