Evaluating Macroalgal Hyperspectral Reflectance Separability in Support of Kelp Mapping
Abstract
Highlights
- Kelps and macroalgae show moderate to good spectral separability using only their hyperspectral reflectance profiles.
- Specimens from different regions differed enough in their spectral reflectance to allow them to be accurately labelled by region.
- Kelp and the other macroalgae examined here are suitable candidates for genus-level mapping using high-spatial-resolution, hyperspectral Earth Observation imagery.
- Of the methods examined here, random forest classification is best suited for large-extent kelp mapping as it can handle intra-genus variability caused by geography, environmental conditions, and/or seasonality.
Abstract
1. Introduction
2. Data and Methods
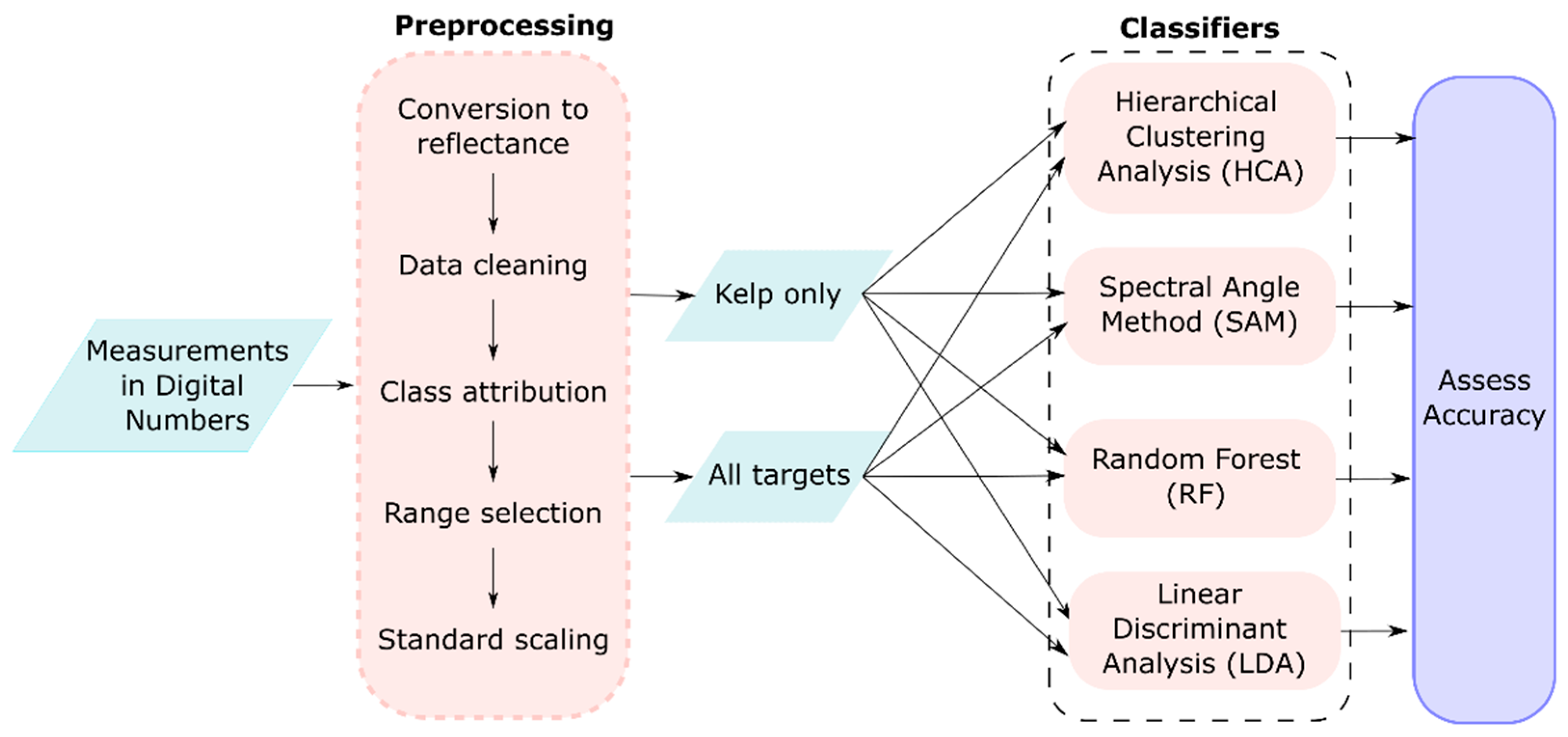
2.1. Methods Overview
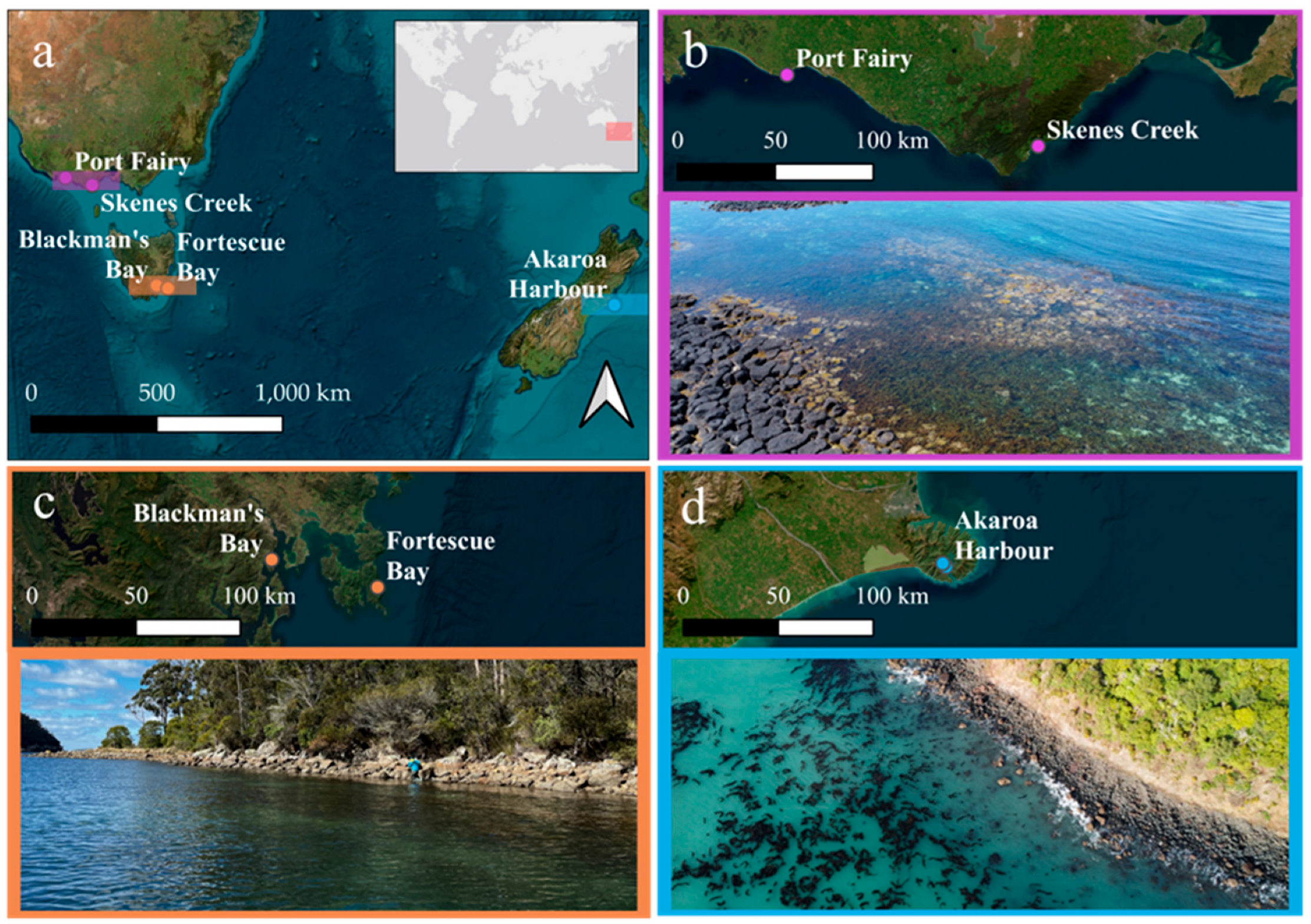
2.2. Field Data Collection Sites
2.3. Spectral Reflectance Collection
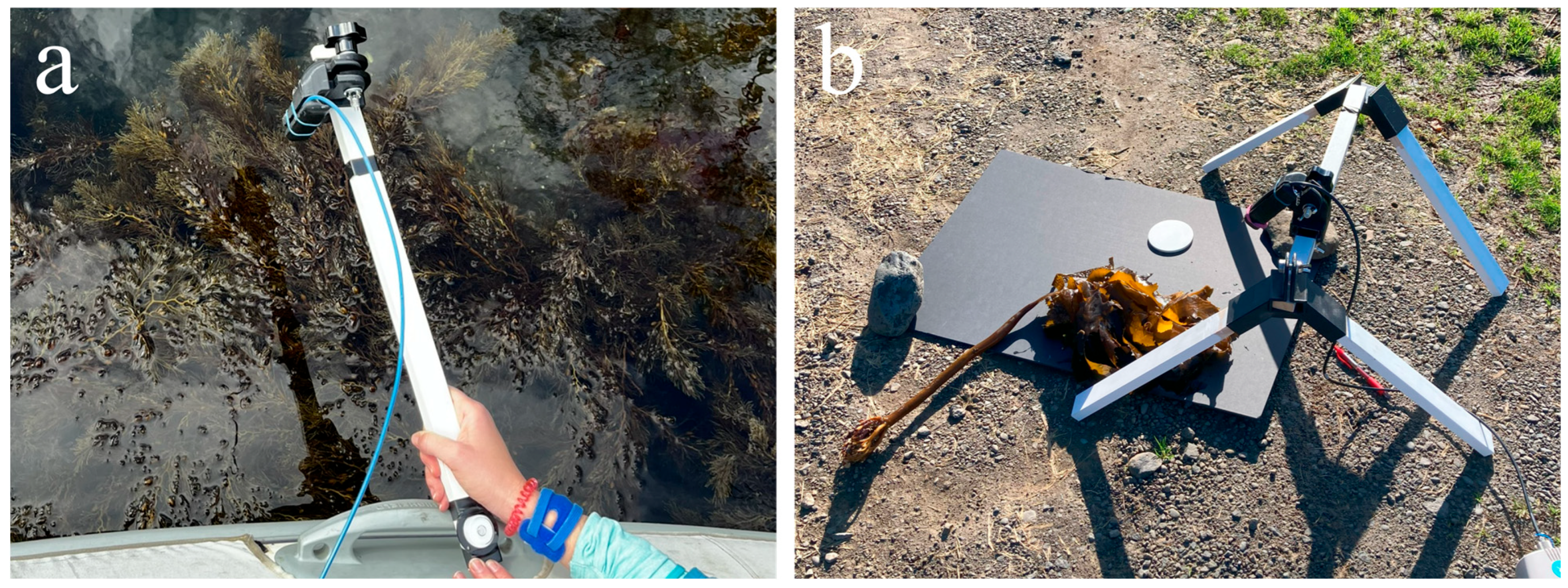
2.3.1. Outdoor In Situ
2.3.2. Outdoor Ex Situ
2.4. Spectral Reflectance Analysis Workflow
2.4.1. Pre-Processing of Field Reflectance Data
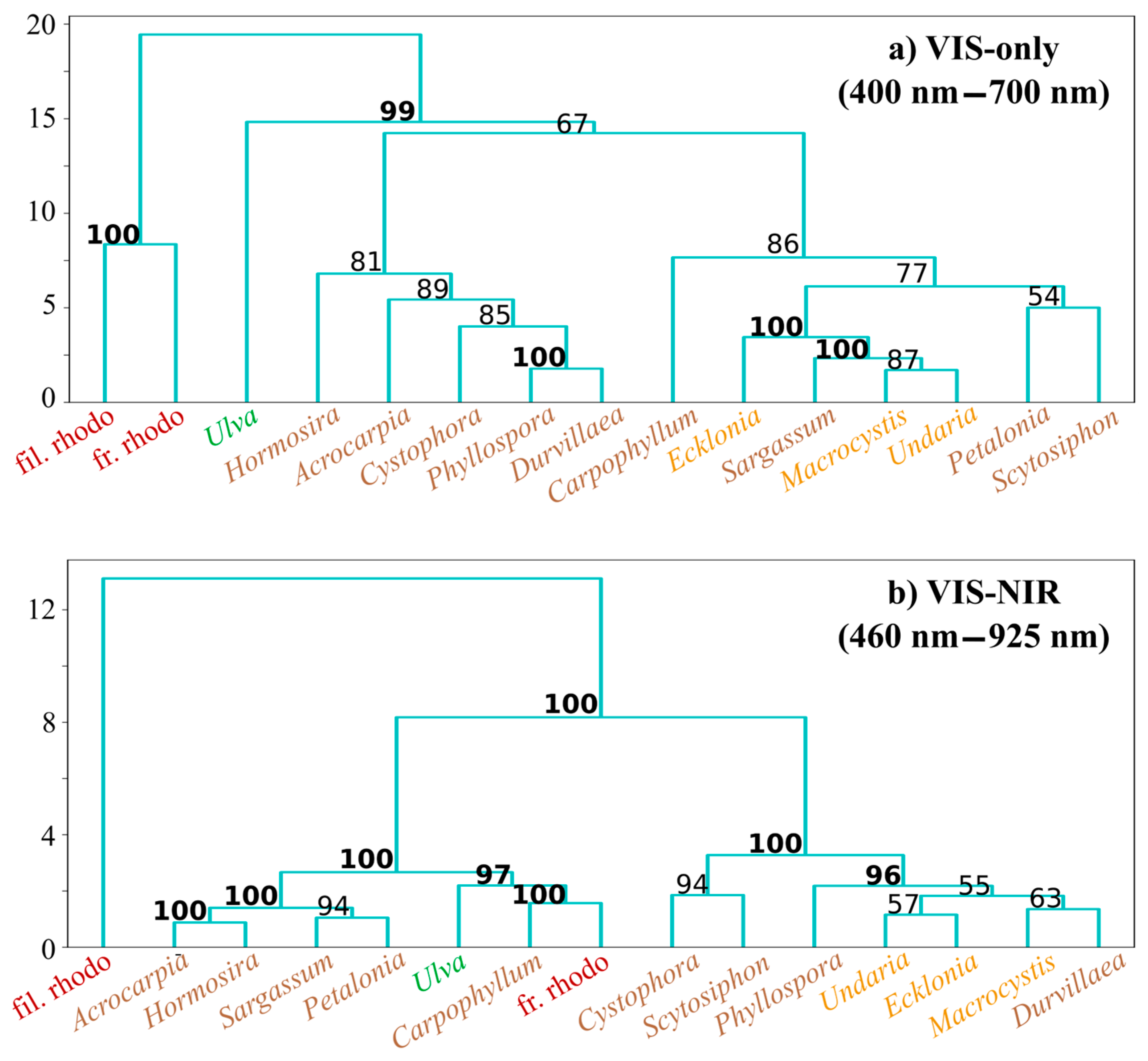
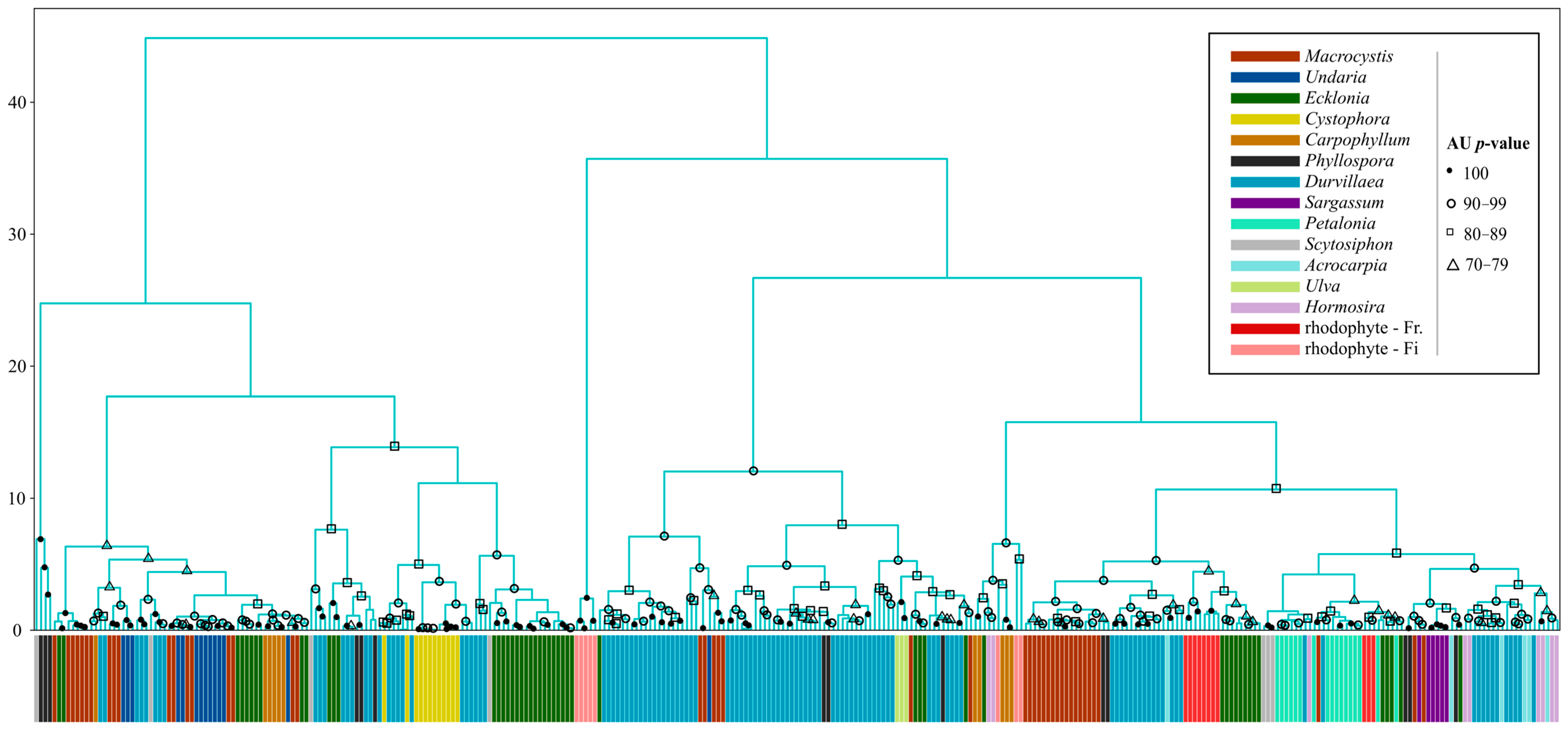
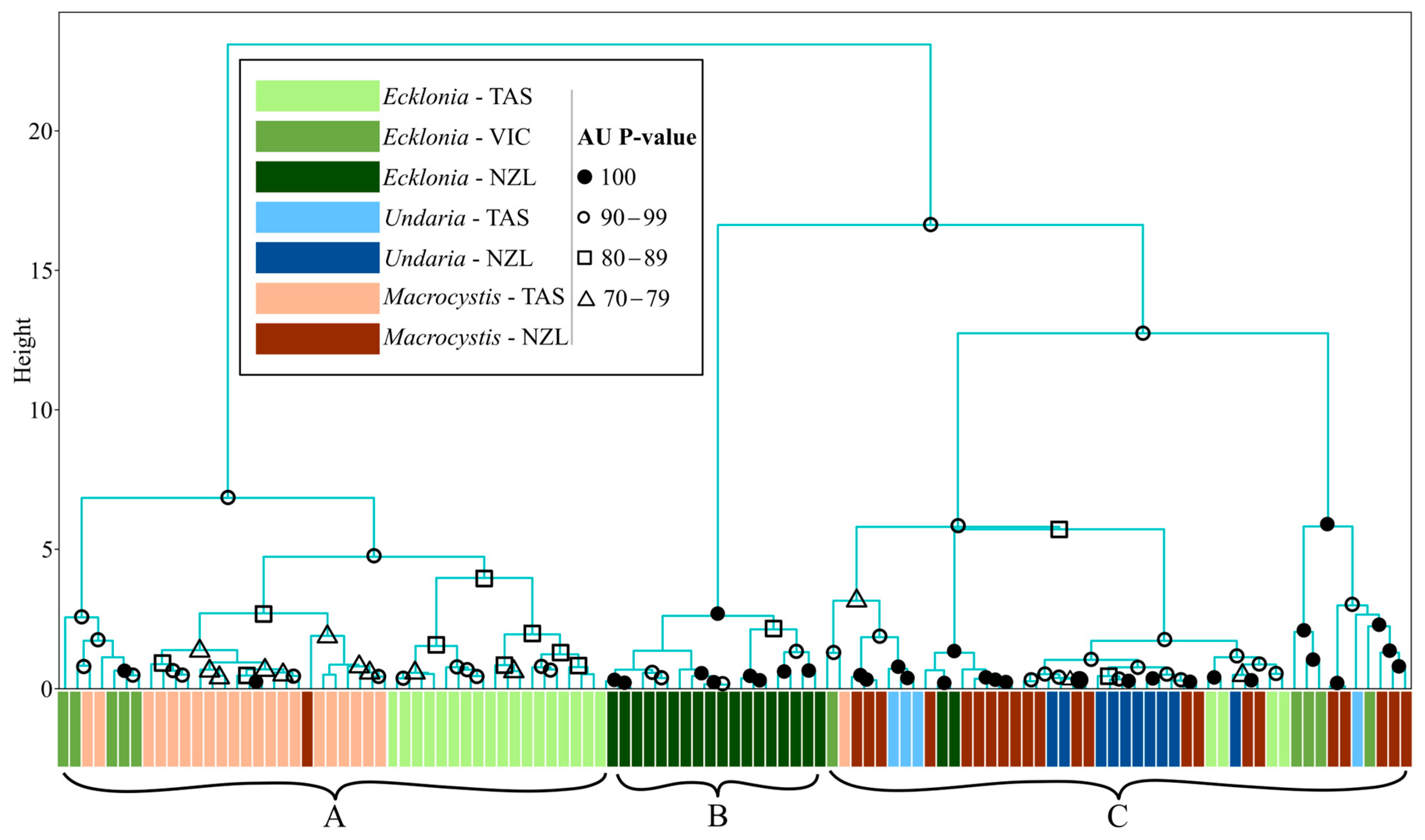
2.4.2. Hierarchical Clustering Analysis (HCA)
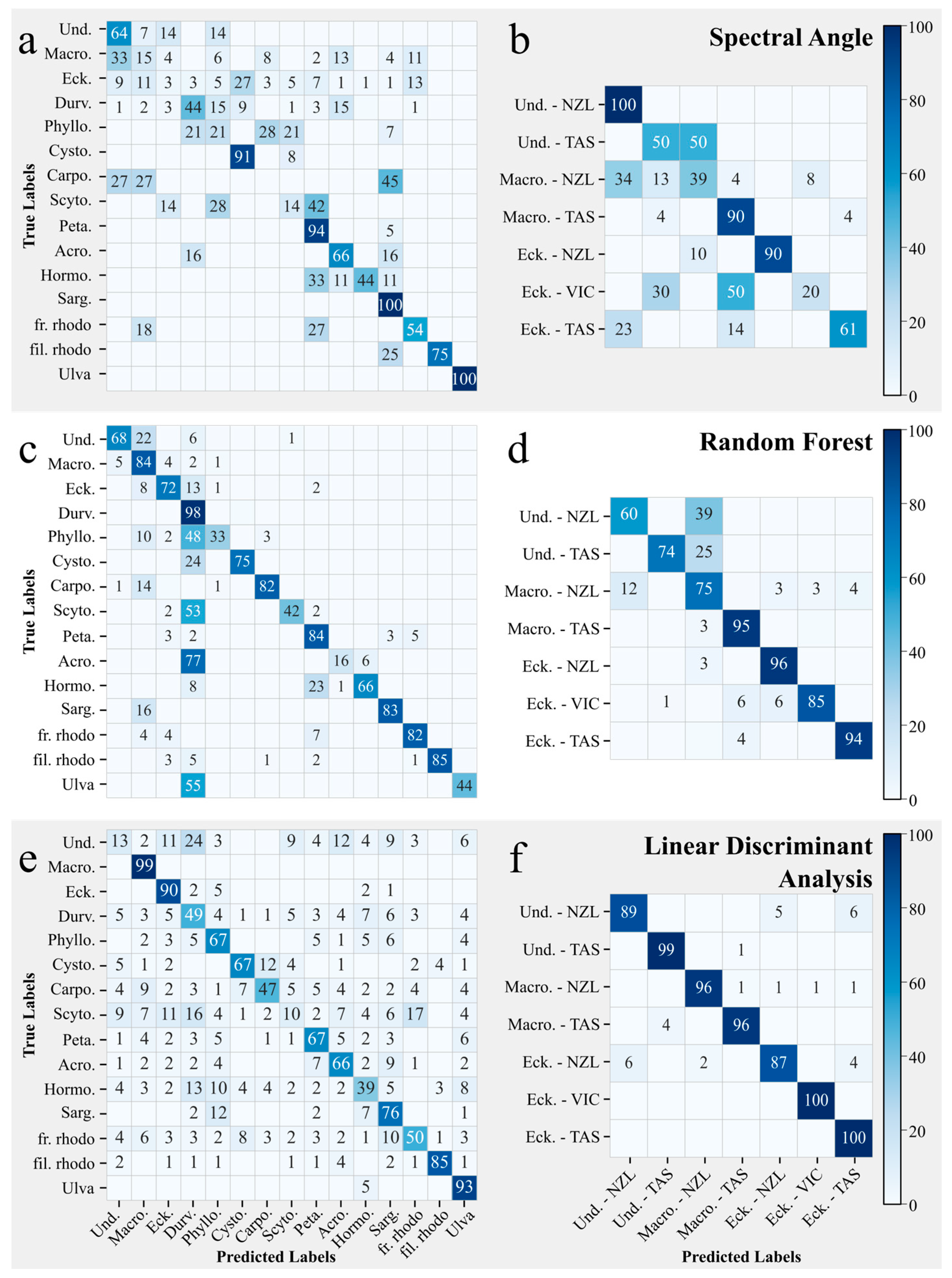
2.4.3. Spectral Angle Classification (SAM)
2.4.4. Random Forest Classification (RF)
2.4.5. Linear Discriminant Analysis (LDA)
3. Results
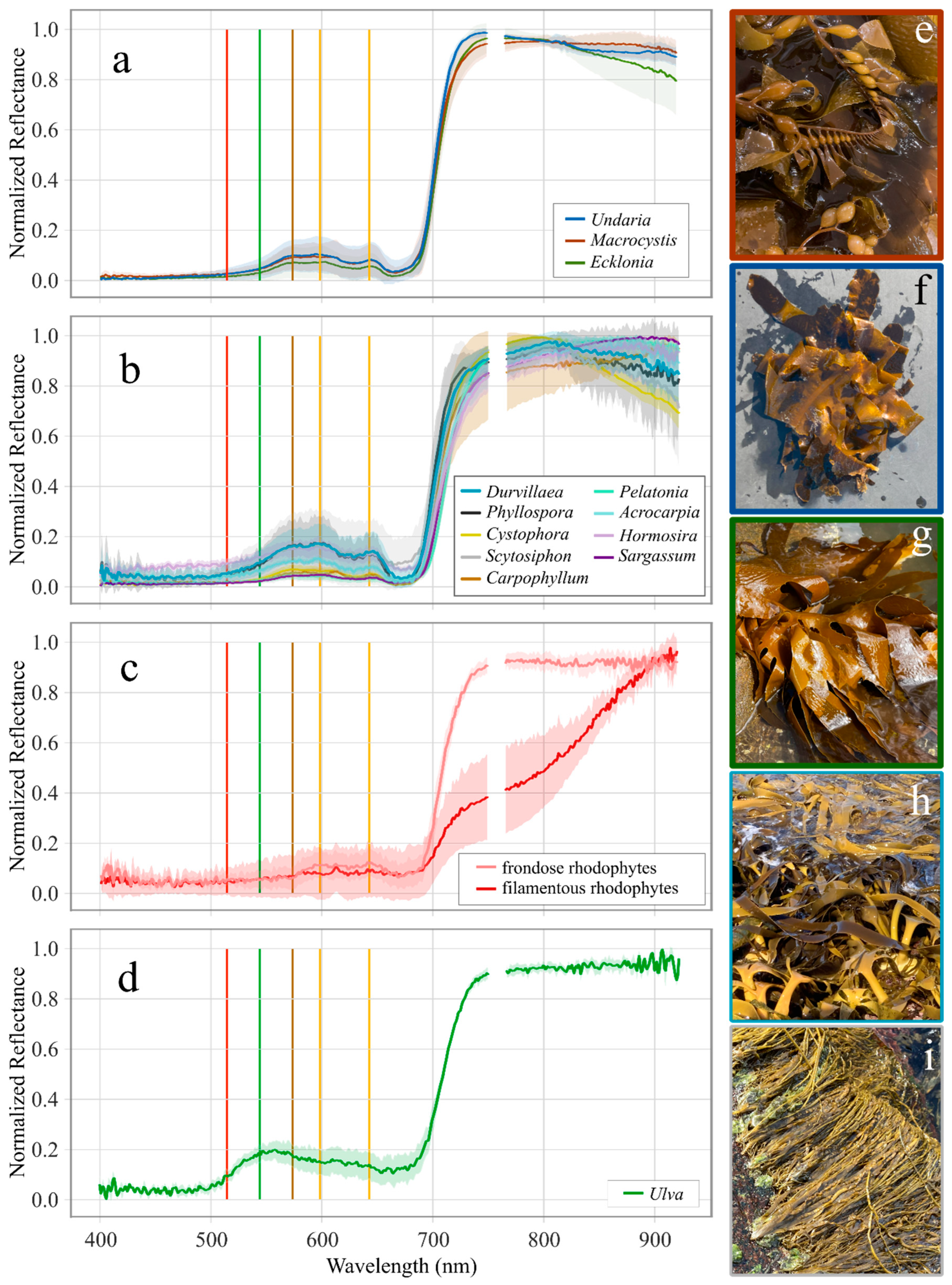
3.1. Reflectance Profiles
3.2. Hierarchical Clustering Analyses
3.2.1. Macroalgal Class Means
3.2.2. Macroalgae by Genus
3.2.3. Kelp by Genus and Site
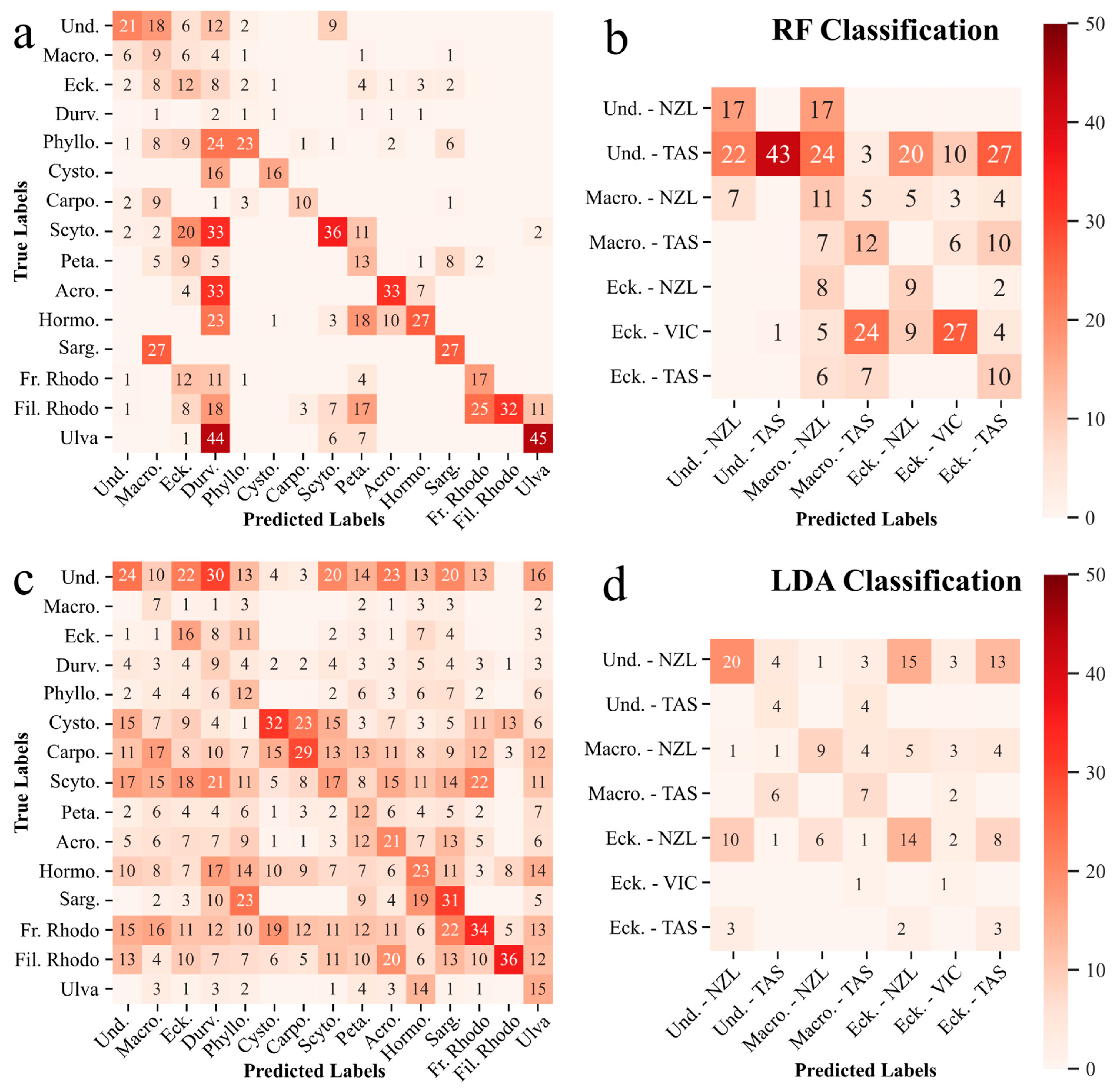
3.3. Spectral Angle, Random Forest, and Linear Discriminant—Image-Applicable Classifications
3.3.1. Macroalgae by Genus
3.3.2. Kelp by Genus and Site
4. Discussion
4.1. Reflectance Profiles
4.2. Hierarchical Clustering Analyses
4.3. Spectral Angle, Random Forest, and Linear Discriminant–Image-Applicable Classifications
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Earth Observation |
| HCA | Hierarchical clustering analysis |
| ANOVA | Analysis of variance |
| ANOSIM | Analysis of similarity |
| VIS | Visible |
| NIR | Near infra-red |
| AI | Artificial Intelligence |
| AUS | Australia |
| NZL | New Zealand |
| AU | Approximately unbiased |
| SAM | Spectral angle method |
| RF | Random forest |
| LDA | Linear discriminant analysis |
| PCA | Principle component analysis |
| VIC | Victoria |
| TAS | Tasmania |
References
- National Ocean Account, Experimental Estimates. Available online: https://www.abs.gov.au/statistics/environment/environmental-accounts/national-ocean-account-experimental-estimates/nov-2022 (accessed on 27 March 2025).
- McHenry, J.; Okamoto, D.K.; Filbee-Dexter, K.; Krumhansl, K.A.; MacGregor, K.A.; Hessing-Lewis, M.; Timmer, B.; Archambault, P.; Attridge, C.M.; Cottier, D.; et al. A Blueprint for National Assessments of the Blue Carbon Capacity of Kelp Forests Applied to Canada’s Coastline. NPJ Ocean Sustain. 2025, 4, 30. [Google Scholar] [CrossRef]
- Franco, J.N.; Sainz Meyer, H.; Babe, Ó.; Martins, M.; Reis, B.; Sanchez-Gallego, Á.; Lemos, M.F.L.; Dolbeth, M.; Sousa-Pinto, I.; Arenas, F. Potential Blue Carbon in the Fringe of Southern European Kelp Forests. Sci. Rep. 2025, 15, 29573. [Google Scholar] [CrossRef]
- Kuwae, T.; Yoshida, G.; Hori, M.; Watanabe, K.; Tanaya, T.; Okada, T.; Umezawa, Y.; Sasaki, J. Nationwide estimate of the annual uptake of atmospheric carbon dioxide by shallow coastal ecosystems in Japan. J. JSCE 2023, 11, 23-00139. [Google Scholar] [CrossRef]
- Eger, A.M.; Marzinelli, E.M.; Beas-Luna, R.; Blain, C.O.; Blamey, L.K.; Byrnes, J.E.K.; Carnell, P.E.; Choi, C.G.; Hessing-Lewis, M.; Kim, K.Y.; et al. The Value of Ecosystem Services in Global Marine Kelp Forests. Nat. Commun. 2023, 14, 1894. [Google Scholar] [CrossRef]
- Dayton, P.K. Ecology of Kelp Communities. Annu. Rev. Ecol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Cavanaugh, K.C.; Bell, T.; Costa, M.; Eddy, N.E.; Gendall, L.; Gleason, M.G.; Hessing-Lewis, M.; Martone, R.; McPherson, M.; Pontier, O.; et al. A Review of the Opportunities and Challenges for Using Remote Sensing for Management of Surface-Canopy Forming Kelps. Front. Mar. Sci. 2021, 8, 753531. [Google Scholar] [CrossRef]
- Schroeder, S.B.; Dupont, C.; Boyer, L.; Juanes, F.; Costa, M. Passive Remote Sensing Technology for Mapping Bull Kelp (Nereocystis luetkeana): A Review of Techniques and Regional Case Study. Glob. Ecol. Conserv. 2019, 19, e00683. [Google Scholar] [CrossRef]
- Mora-Soto, A.; Palacios, M.; Macaya, E.; Gómez, I.; Huovinen, P.; Pérez-Matus, A.; Young, M.; Golding, N.; Toro, M.; Yaqub, M.; et al. A High-Resolution Global Map of Giant Kelp (Macrocystis Pyrifera) Forests and Intertidal Green Algae (Ulvophyceae) with Sentinel-2 Imagery. Remote Sens. 2020, 12, 694. [Google Scholar] [CrossRef]
- Olmedo-Masat, O.M.; Raffo, M.P.; Rodríguez-Pérez, D.; Arijón, M.; Sánchez-Carnero, N. How Far Can We Classify Macroalgae Remotely? An Example Using a New Spectral Library of Species from the South West Atlantic (Argentine Patagonia). Remote Sens. 2020, 12, 3870. [Google Scholar] [CrossRef]
- Douay, F.; Verpoorter, C.; Duong, G.; Spilmont, N.; Gevaert, F. New Hyperspectral Procedure to Discriminate Intertidal Macroalgae. Remote Sens. 2022, 14, 346. [Google Scholar] [CrossRef]
- Rowan, G.S.L.; Kalacska, M. A Review of Remote Sensing of Submerged Aquatic Vegetation for Non-Specialists. Remote Sens. 2021, 13, 623. [Google Scholar] [CrossRef]
- Smart, J.N.; Schmid, M.; Paine, E.R.; Britton, D.; Revill, A.; Hurd, C.L. Seasonal Ammonium Uptake Kinetics of Four Brown Macroalgae: Implications for Use in Integrated Multi-Trophic Aquaculture. J. Appl. Phycol. 2022, 34, 1693–1708. [Google Scholar] [CrossRef]
- McIlwaine, B.; Casado, M.R.; Leinster, P. Using 1st Derivative Reflectance Signatures within a Remote Sensing Framework to Identify Macroalgae in Marine Environments. Remote Sens. 2019, 11, 704. [Google Scholar] [CrossRef]
- Chao Rodríguez, Y.; Domínguez Gómez, J.A.; Sánchez-Carnero, N.; Rodríguez-Pérez, D. A Comparison of Spectral Macroalgae Taxa Separability Methods Using an Extensive Spectral Library. Algal Res. 2017, 26, 463–473. [Google Scholar] [CrossRef]
- Kotta, J.; Remm, K.; Vahtmäe, E.; Kutser, T.; Orav-Kotta, H. In-Air Spectral Signatures of the Baltic Sea Macrophytes and Their Statistical Separability. J. Appl. Remote Sens. 2014, 8, 083634. [Google Scholar] [CrossRef]
- Integrated Marine and Coastal Regionalisation of Australia (IMCRA) v4.0 Mesoscale Bioregions. Available online: https://fed.dcceew.gov.au/datasets/erin::integrated-marine-and-coastal-regionalisation-of-australia-imcra-v4-0-meso-scale-bioregions/explore (accessed on 12 July 2025).
- Environment Canterbury; New Zealand Department of Conservation; Te Rūnanga o Ōnuku; Te Rūnanga o Wairewa; Te Rūnanga o Rāpaki; University of Otago Iongairo. Available online: https://storymaps.arcgis.com/collections/4c59cf39bcee4875b117cfe02f4f0a47 (accessed on 30 June 2025).
- North, W.J. The Biology of Giant Kelp Beds (Macrocystis) in California; Beihefte zur Nova Hedwigia; Verlag Von J. Cramer: Stuttgart, Germany, 1971; Volume 32, ISBN 3768254321. [Google Scholar]
- Braune, W.; Guiry, M. Seaweeds: A Colour Guide to Common Benthic Green, Brown and Red Algae of the World’s Oceans; Koeltz Scientific Books: Königstein, Germany, 2011; ISBN 978-3-906166-90-2. [Google Scholar]
- Jupp, D.; Daniel, P. Notes on the Use of Radiometers for Spectral Reflectances; CSIRO: Acton, Australia, 1993; p. 4. [Google Scholar]
- ASD, Inc. ViewSpec Pro User Manual; Analytical Spectral Devices, Inc.: Boulder, CO, USA, 2008; p. 28. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Suzuki, R.; Shimodaira, H. Pvclust: An R Package for Assessing the Uncertainty in Hierarchical Clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef]
- Turanjanin, A. Stability of Hierarchical Clustering. 2020. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?lang=eng&id=121038 (accessed on 24 March 2025).
- Shimodaira, H. An Approximately Unbiased Test of Phylogenetic Tree Selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef]
- Kruse, F.A.; Lefkoff, A.B.; Boardman, J.W.; Heidebrecht, K.B.; Shapiro, A.T.; Barloon, P.J.; Goetz, A.F.H. The Spectral Image Processing System (SIPS)—Interactive Visualization and Analysis of Imaging Spectrometer Data. Remote Sens. Environ. 1993, 44, 145–163. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and Physiological Basis for the Reflectance of Visible and Near-Infrared Radiation from Vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Hardy, R.F.; Hochberg, E.J. Spectral and Spatial Requirements of Remote Measurements of Pelagic Sargassum Macroalgae. Remote Sens. Environ. 2015, 167, 229–246. [Google Scholar] [CrossRef]
- Timmer, B.; Reshitnyk, L.Y.; Hessing-Lewis, M.; Juanes, F.; Costa, M. Comparing the Use of Red-Edge and Near-Infrared Wavelength Ranges for Detecting Submerged Kelp Canopy. Remote Sens. 2022, 14, 2241. [Google Scholar] [CrossRef]
- Colombo-Pallotta, M.F.; García-Mendoza, E.; Ladah, L.B. Photosynthetic Performance, Light Absorption, and Pigment Composition of Macrocystis Pyrifera (Laminariales, Phaeophyceae) Blades from Different Depths1. J. Phycol. 2006, 42, 1225–1234. [Google Scholar] [CrossRef]
- Bell, T.W.; Cavanaugh, K.C.; Siegel, D.A. Remote Monitoring of Giant Kelp Biomass and Physiological Condition: An Evaluation of the Potential for the Hyperspectral Infrared Imager (HyspIRI) Mission. Remote Sens. Environ. 2015, 167, 218–228. [Google Scholar] [CrossRef]
- Cavanaugh, K.; Siegel, D.; Reed, D.; Dennison, P. Environmental Controls of Giant-Kelp Biomass in the Santa Barbara Channel, California. Mar. Ecol. Prog. Ser. 2011, 429, 1–17. [Google Scholar] [CrossRef]
- Bell, T.W.; Cavanaugh, K.C.; Saccomanno, V.R.; Cavanaugh, K.C.; Houskeeper, H.F.; Eddy, N.; Schuetzenmeister, F.; Rindlaub, N.; Gleason, M. Kelpwatch: A New Visualization and Analysis Tool to Explore Kelp Canopy Dynamics Reveals Variable Response to and Recovery from Marine Heatwaves. PLoS ONE 2023, 18, e0271477. [Google Scholar] [CrossRef]
- Nijland, W.; Reshitnyk, L.; Rubidge, E. Satellite Remote Sensing of Canopy-Forming Kelp on a Complex Coastline: A Novel Procedure Using the Landsat Image Archive. Remote Sens. Environ. 2019, 220, 41–50. [Google Scholar] [CrossRef]
- Mabin, C.J.T.; Johnson, C.R.; Wright, J.T. Physiological Response to Temperature, Light, and Nitrates in the Giant Kelp Macrocystis Pyrifera from Tasmania, Australia. Mar. Ecol. Prog. Ser. 2019, 614, 1–19. [Google Scholar] [CrossRef]
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-0-521-14595-4. [Google Scholar]
- Hurd, C.L.; Pilditch, C.A. Flow-Induced Morphological Variations Affect Diffusion Boundary-Layer Thickness of Macrocystis Pyrifera (Heterokontophyta, Laminariales). J. Phycol. 2011, 47, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Case, B.S.; White, W.L. Effects of Location and Season on Seaweed Spectral Signatures. Front. Ecol. Evol. 2021, 9, 581852. [Google Scholar] [CrossRef]
- Schmid, M.; Guihéneuf, F.; Stengel, D.B. Ecological and Commercial Implications of Temporal and Spatial Variability in the Composition of Pigments and Fatty Acids in Five Irish Macroalgae. Mar. Biol. 2017, 164, 158. [Google Scholar] [CrossRef]
- Palacios, M.; Osman, D.; Ramírez, J.; Huovinen, P.; Gómez, I. Photobiology of the Giant Kelp Macrocystis Pyrifera in the Land-Terminating Glacier Fjord Yendegaia (Tierra Del Fuego): A Look into the Future? Sci. Total Environ. 2021, 751, 141810. [Google Scholar] [CrossRef] [PubMed]
- Arafeh-Dalmau, N.; Montaño-Moctezuma, G.; Martínez, J.A.; Beas-Luna, R.; Schoeman, D.S.; Torres-Moye, G. Extreme Marine Heatwaves Alter Kelp Forest Community Near Its Equatorward Distribution Limit. Front. Mar. Sci. 2019, 6, 499. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Bell, T.W.; Caselle, J.E.; Campagna, C.; Goodell, W.; Hüne, M.; Muñoz, A.; Salinas-de-León, P.; Sala, E.; et al. Kelp Forests at the End of the Earth: 45 Years Later. PLoS ONE 2020, 15, e0229259. [Google Scholar] [CrossRef] [PubMed]
- Houskeeper, H.F.; Rosenthal, I.S.; Cavanaugh, K.C.; Pawlak, C.; Trouille, L.; Byrnes, J.E.K.; Bell, T.W.; Cavanaugh, K.C. Automated Satellite Remote Sensing of Giant Kelp at the Falkland Islands (Islas Malvinas). PLoS ONE 2022, 17, e0257933. [Google Scholar] [CrossRef]
| Classifier | Description | Input Data | Classifier Data Division | Evaluation Metric |
|---|---|---|---|---|
| Hierarchical Clustering Analysis (HCA) | A sample is compared to every other sample on a one-to-one basis. The two most similar are clustered, with that cluster becoming a point of comparison instead of the two component samples. These comparisons are iteratively performed until one cluster contains all samples. | Reflectance spectra | One-to-one comparisons | Approximately Unbiased (AU) p-Value |
| Spectral Angle Method (SAM) | Class labels are assigned by minimizing the angle between the vector representing a sample in n-dimensional space (where n is the number of spectral bands) and the vector of a class’s average or reference spectrum. | Reflectance spectra | Leave-one-out | F1 score |
| Random Forest (RF) | A series of binary decisions are made based on the spectral features to assign a sample to a class, forming a decision tree. The random forest aggregates the results of many decision trees | Principal Component transformed spectra | 70% training 30% testing | F1 score |
| Linear Discriminant Analysis (LDA) | Labelled data is projected into multidimensional space. A new set of orthogonal axes are defined to maximize the separation between classes on the fewest possible axes. | Reflectance spectra | 70% training 30% testing | F1 score |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowan, G.S.L.; Smart, J.N.; Roelfsema, C.; Phinn, S.R. Evaluating Macroalgal Hyperspectral Reflectance Separability in Support of Kelp Mapping. Remote Sens. 2025, 17, 3491. https://doi.org/10.3390/rs17203491
Rowan GSL, Smart JN, Roelfsema C, Phinn SR. Evaluating Macroalgal Hyperspectral Reflectance Separability in Support of Kelp Mapping. Remote Sensing. 2025; 17(20):3491. https://doi.org/10.3390/rs17203491
Chicago/Turabian StyleRowan, Gillian S. L., Joanna N. Smart, Chris Roelfsema, and Stuart R. Phinn. 2025. "Evaluating Macroalgal Hyperspectral Reflectance Separability in Support of Kelp Mapping" Remote Sensing 17, no. 20: 3491. https://doi.org/10.3390/rs17203491
APA StyleRowan, G. S. L., Smart, J. N., Roelfsema, C., & Phinn, S. R. (2025). Evaluating Macroalgal Hyperspectral Reflectance Separability in Support of Kelp Mapping. Remote Sensing, 17(20), 3491. https://doi.org/10.3390/rs17203491








