Inter-Annual Variability of Peatland Vegetation Captured Using Phenocam- and UAV Imagery
Abstract
1. Introduction
1.1. Vegetation Drives Seasonal Carbon Dynamics
1.2. Carbon Fluxes in Northern Peatlands and Responses to Climate Change
1.3. Phenological Monitoring
1.3.1. Manual Surveys and Remote Sensing
1.3.2. Limitations of Current Remote Sensing Approaches
1.3.3. State of the Art: UAVs for Phenological Monitoring
1.4. Research Outline and Aims
2. Materials and Methods
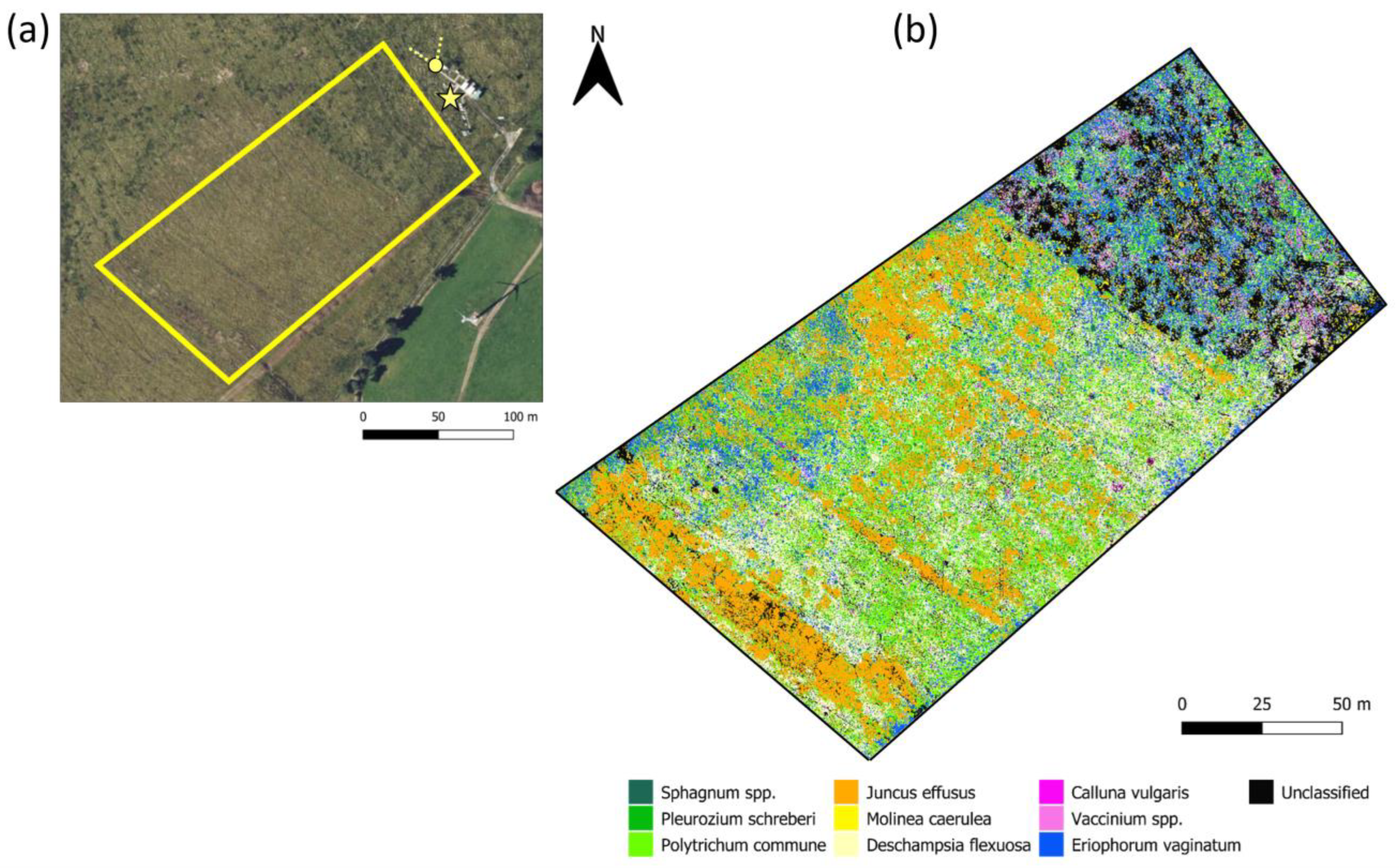
2.1. Study Area
2.2. UAV Data
2.2.1. Data Collection
2.2.2. Data Processing
2.2.3. Classification Map
2.2.4. Extraction of Species-Level VIs
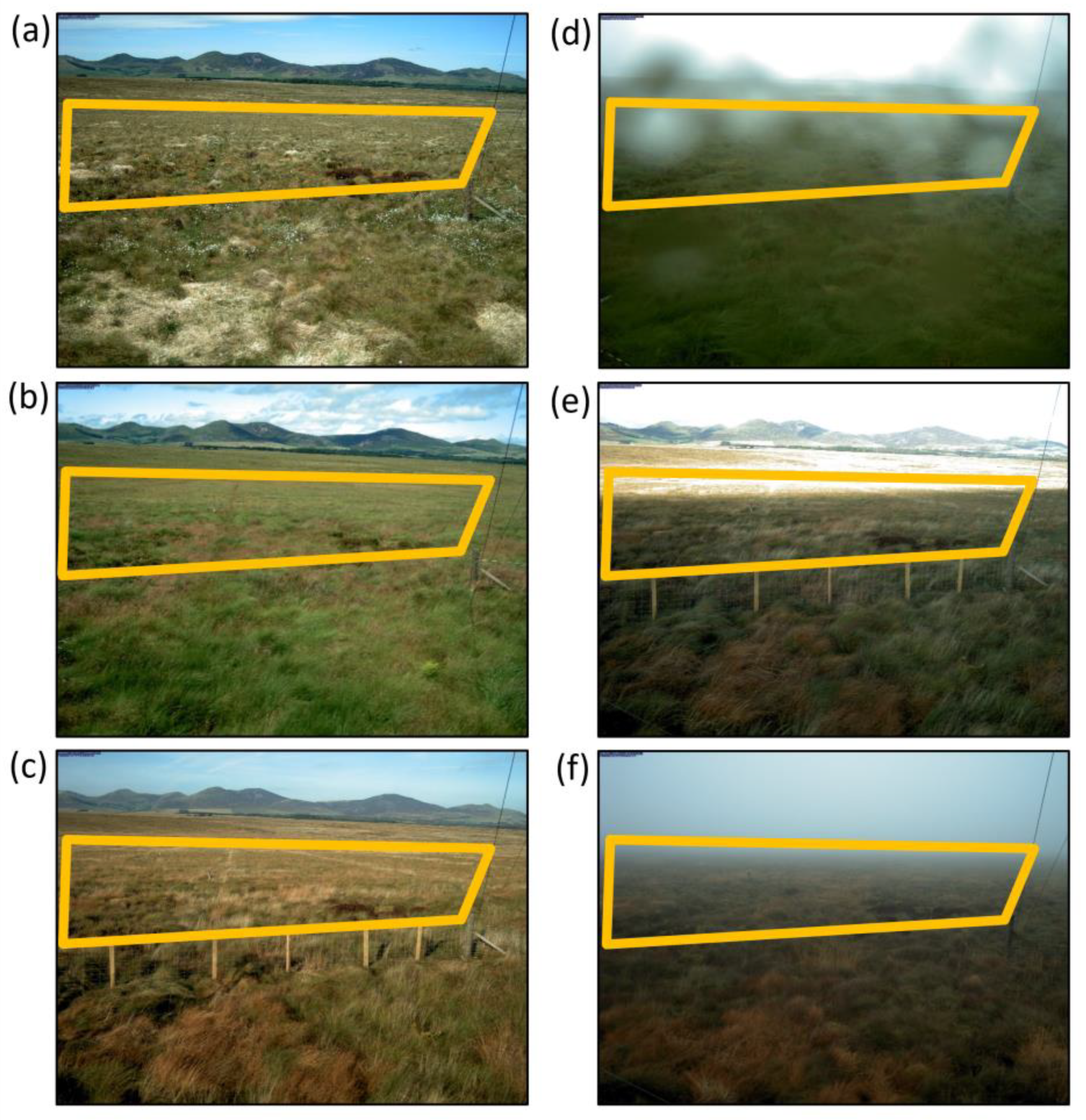
2.3. Phenocam Imagery
2.4. CO2 Flux Measurements and Supporting Meteorological Data
2.4.1. Instrumentation
2.4.2. Flux Calculation and Quality Screening
2.4.3. Flux Partitioning and Gapfilling
2.5. Statistical Analysis
3. Results
3.1. Overview of the Study Period (Hydrometeorological Conditions and CO2 Balance)
3.2. Monitoring Vegetation Phenology
3.2.1. Phenocam Data
3.2.2. UAV Data
3.2.3. Capturing Spatial and Temporal Variability Within the Growing Season
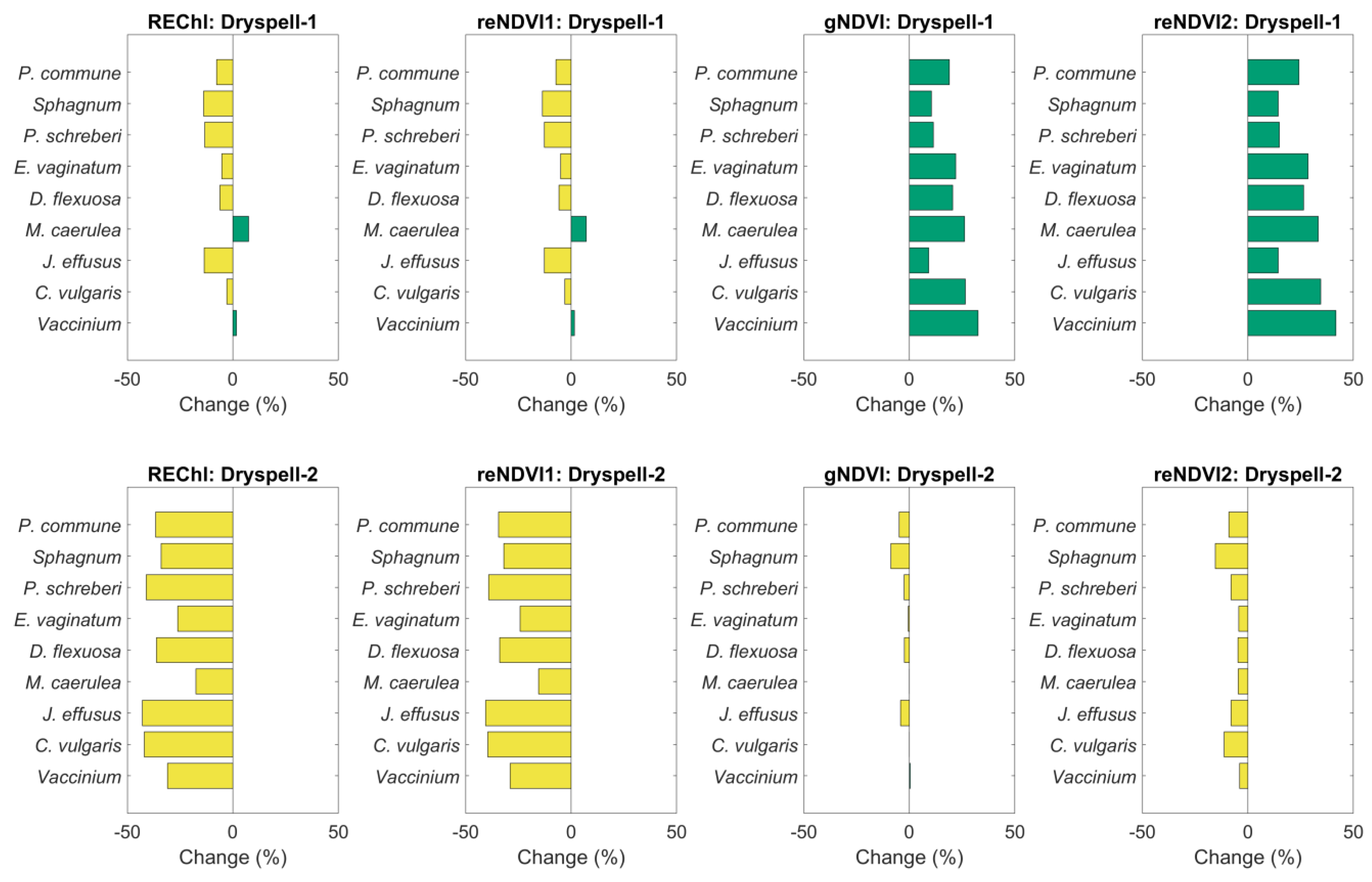
3.2.4. Sensitivity of the Phenological Datasets to Drought
3.3. Seasonal and Inter-Annual Variability in the Relationship Between GPP and Phenology
4. Discussion
4.1. Monitoring Vegetation Phenology
4.1.1. Phenocam Data
4.1.2. UAV Data
4.1.3. Problems Comparing Phenological Datasets
4.2. Links Between Vegetation Greenness and Ecosystem Functioning
4.3. Impact of Dry Spells
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keenan, T.F.; Williams, C.A. The Terrestrial Carbon Sink. Annu. Rev. Environ. Resour. 2018, 43, 219–243. [Google Scholar] [CrossRef]
- D’Arrigo, R.; Jacoby, G.C.; Fung, I.Z. Boreal Forests and Atmosphere–Biosphere Exchange of Carbon Dioxide. Nature 1987, 329, 321–323. [Google Scholar] [CrossRef]
- Hall, C.A.S.; Ekdahl, C.A.; Wartenberg, D.E. A Fifteen-Year Record of Biotic Metabolism in the Northern Hemisphere. Nature 1975, 255, 136–138. [Google Scholar] [CrossRef]
- Lieth, H. Phenology in Productivity Studies. In Analysis of Temperate Forest Ecosystems; Reichle, D.E., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1970; pp. 29–46. [Google Scholar]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate Change, Phenology, and Phenological Control of Vegetation Feedbacks to the Climate System. Agric. For. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Buitenwerf, R.; Rose, L.; Higgins, S.I. Three Decades of Multi-Dimensional Change in Global Leaf Phenology. Nat. Clim. Chang. 2015, 5, 364–368. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting Plant Phenology in Response to Global Change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef]
- Menzel, A.; Yuan, Y.; Matiu, M.; Sparks, T.; Scheifinger, H.; Gehrig, R.; Estrella, N. Climate Change Fingerprints in Recent European Plant Phenology. Glob. Change Biol. 2020, 26, 2599–2612. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Badeck, F.W.; Bondeau, A.; Böttcher, K.; Doktor, D.; Lucht, W.; Schaber, J.; Sitch, S. Responses of Spring Phenology to Climate Change. New Phytol. 2004, 162, 295–309. [Google Scholar] [CrossRef]
- Jeong, S. Autumn Greening in a Warming Climate. Nat. Clim. Chang. 2020, 10, 712–713. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology Shifts at Start vs. End of Growing Season in Temperate Vegetation over the Northern Hemisphere for the Period 1982–2008. Glob. Change Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rutishauser, T.; Filella, I. Phenology Feedbacks on Climate Change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Di Luca, A.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and Climate Extreme Events in a Changing Climate. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Huang, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 1513–1766. [Google Scholar]
- Alm, J.; Schulman, L.; Walden, J.; Nykänen, H.; Martikainen, P.J.; Silvola, J. Carbon Balance of a Boreal Bog during a Year with an Exceptionally Dry Summer. Ecology 1999, 80, 161–174. [Google Scholar] [CrossRef]
- Lund, M.; Christensen, T.R.; Lindroth, A.; Schubert, P. Effects of Drought Conditions on the Carbon Dioxide Dynamics in a Temperate Peatland. Environ. Res. Lett. 2012, 7, 045704. [Google Scholar] [CrossRef]
- Keeling, C.D.; Chin, J.F.S.; Whorf, T.P. Increased Activity of Northern Vegetation Inferred from Atmospheric CO2 Measurements. Nature 1996, 382, 146–149. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant Phenology and Global Climate Change: Current Progresses and Challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Gorham, E. Northern Peatlands: Role in the Carbon Cycle and Probable Responses to Climatic Warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Yu, Z.C. Northern Peatland Carbon Stocks and Dynamics: A Review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef]
- Limpens, J.; Berendse, F.; Blodau, C.; Canadell, J.G.; Freeman, C.; Holden, J.; Roulet, N.; Rydin, H.; Schaepman-Strub, G. Peatlands and the Carbon Cycle: From Local Processes to Global Implications—A Synthesis. Biogeosciences 2008, 5, 1475–1491. [Google Scholar] [CrossRef]
- Tarnocai, C.; Stolbovoy, V. Northern Peatlands: Their Characteristics, Development and Sensitivity to Climate Change. Dev. Earth Surf. Process. 2006, 9, 17–51. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Bergholm, F.; Christensen, T.R.; Jonasson, C.; Kokfelt, U.; Johansson, M. A New Climate Era in the Sub-Arctic: Accelerating Climate Changes and Multiple Impacts. Geophys. Res. Lett. 2010, 37, L14705. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M.; Bindi, M.; Brown, S.; Camilloni, I.; Diedhiou, A.; Djalante, R.; Ebi, K.L.; Engelbrecht, F.; et al. Impacts of 1.5 °C Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; IPCC; Masson-Delmotte, V., Zhai, P., Pörtner, H.-O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 175–312. [Google Scholar] [CrossRef]
- Dise, N.B. Peatland Response to Global Change. Science 2009, 326, 810–811. [Google Scholar] [CrossRef]
- Gonsamo, A.; Chen, J.M.; Ooi, Y.W. Peak Season Plant Activity Shift towards Spring Is Reflected by Increasing Carbon Uptake by Extratropical Ecosystems. Glob. Change Biol. 2018, 24, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Post, W.M.; Baldocchi, D.; Andy Black, T.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Phenology of Vegetation Photosynthesis. In Phenology: An Integrative Environmental Science. Tasks for Vegetation Science; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2003; Volume 39, pp. 467–485. [Google Scholar]
- Peñuelas, J.; Filella, I. Responses to a Warming World. Science 2001, 294, 793–795. [Google Scholar] [CrossRef]
- Antala, M.; Juszczak, R.; van der Tol, C.; Rastogi, A. Impact of Climate Change-Induced Alterations in Peatland Vegetation Phenology and Composition on Carbon Balance. Sci. Total Environ. 2022, 827, 154294. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing Season Extension and Its Impact on Terrestrial Carbon Cycle in the Northern Hemisphere over the Past 2 Decades. Glob. Biogeochem. Cycles 2007, 21, GB3018. [Google Scholar] [CrossRef]
- Barel, J.M.; Moulia, V.; Hamard, S.; Sytiuk, A.; Jassey, V.E.J. Come Rain, Come Shine: Peatland Carbon Dynamics Shift Under Extreme Precipitation. Front. Environ. Sci. 2021, 9, 233. [Google Scholar] [CrossRef]
- Lafleur, P.M.; Roulet, N.T.; Bubier, J.L.; Frolking, S.; Moore, T.R. Interannual Variability in the Peatland-Atmosphere Carbon Dioxide Exchange at an Ombrotrophic Bog. Glob. Biogeochem. Cycles 2003, 17, 1036. [Google Scholar] [CrossRef]
- Brown, T.B.; Hultine, K.R.; Steltzer, H.; Denny, E.G.; Denslow, M.W.; Granados, J.; Henderson, S.; Moore, D.; Nagai, S.; Sanclements, M.; et al. Using Phenocams to Monitor Our Changing Earth: Toward a Global Phenocam Network. Front. Ecol. Environ. 2016, 14, 84–93. [Google Scholar] [CrossRef]
- Richardson, A.D. Tracking Seasonal Rhythms of Plants in Diverse Ecosystems with Digital Camera Imagery. New Phytol. 2019, 222, 1742–1750. [Google Scholar] [CrossRef]
- Richardson, A.D. PhenoCam: An Evolving, Open-Source Tool to Study the Temporal and Spatial Variability of Ecosystem-Scale Phenology. Agric. For. Meteorol. 2023, 342, 109751. [Google Scholar] [CrossRef]
- Arndt, K.A.; Santos, M.J.; Ustin, S.; Davidson, S.J.; Stow, D.; Oechel, W.C.; Tran, T.T.P.; Graybill, B.; Zona, D. Arctic Greening Associated with Lengthening Growing Seasons in Northern Alaska. Environ. Res. Lett. 2019, 14, 125018. [Google Scholar] [CrossRef]
- Arroyo-Mora, J.P.; Kalacska, M.; Soffer, R.; Ifimov, G.; Leblanc, G.; Schaaf, E.S.; Lucanus, O. Evaluation of Phenospectral Dynamics with Sentinel-2A Using a Bottom-up Approach in a Northern Ombrotrophic Peatland. Remote Sens. Environ. 2018, 216, 544–560. [Google Scholar] [CrossRef]
- Kross, A.S.E.; Roulet, N.T.; Moore, T.R.; Lafleur, P.M.; Humphreys, E.R.; Seaquist, J.W.; Flanagan, L.B.; Aurela, M. Phenology and Its Role in Carbon Dioxide Exchange Processes in Northern Peatlands. J. Geophys. Res. Biogeosci. 2014, 119, 1370–1384. [Google Scholar] [CrossRef]
- Bannari, A.; Morin, D.; Bonn, F.; Huete, A.R. A Review of Vegetation Indices. Remote Sens. Rev. 1995, 13, 95–120. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring Vegetation Phenology Using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Cremonese, E.; Filippa, G.; Galvagno, M.; Siniscalco, C.; Oddi, L.; Morra di Cella, U.; Migliavacca, M. Heat Wave Hinders Green Wave: The Impact of Climate Extreme on the Phenology of a Mountain Grassland. Agric. For. Meteorol. 2017, 247, 320–330. [Google Scholar] [CrossRef]
- Hufkens, K.; Filippa, G.; Cremonese, E.; Migliavacca, M.; D’Odorico, P.; Peichl, M.; Gielen, B.; Hörtnagl, L.; Soudani, K.; Papale, D.; et al. Assimilating Phenology Datasets Automatically across ICOS Ecosystem Stations. Int. Agrophysics 2018, 32, 677–687. [Google Scholar] [CrossRef]
- Kross, A.; Seaquist, J.W.; Roulet, N.T.; Fernandes, R.; Sonnentag, O. Estimating Carbon Dioxide Exchange Rates at Contrasting Northern Peatlands Using MODIS Satellite Data. Remote Sens. Environ. 2013, 137, 234–243. [Google Scholar] [CrossRef]
- Dixon Hamil, K.A.; Iannone, B.V.; Huang, W.K.; Fei, S.; Zhang, H. Cross-Scale Contradictions in Ecological Relationships. Landsc. Ecol. 2016, 31, 7–18. [Google Scholar] [CrossRef]
- Newman, E.A.; Breckheimer, I.K.; Park, D.S. Disentangling the Effects of Climate Change, Landscape Heterogeneity, and Scale on Phenological Metrics. bioRxiv 2021. [Google Scholar] [CrossRef]
- Park, D.S.; Newman, E.A.; Breckheimer, I.K. Scale Gaps in Landscape Phenology: Challenges and Opportunities. Trends Ecol. Evol. 2021, 36, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Gao, F.; Liu, Y.; Schaaf, C.; Friedl, M.; Yu, Y.; Jayavelu, S.; Gray, J.; Liu, L.; et al. Exploration of Scaling Effects on Coarse Resolution Land Surface Phenology. Remote Sens. Environ. 2017, 190, 318–330. [Google Scholar] [CrossRef]
- Jelinski, D.E.; Wu, J. The Modifiable Areal Unit Problem and Implications for Landscape Ecology. Landsc. Ecol. 1996, 11, 129–140. [Google Scholar] [CrossRef]
- Woodcock, C.E.; Strahler, A.H. The Factor of Scale in Remote Sensing. Remote Sens. Environ. 1987, 21, 311–332. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F.; Vadeboncoeur, M.A. Green Leaf Phenology at Landsat Resolution: Scaling from the Field to the Satellite. Remote Sens. Environ. 2006, 100, 265–279. [Google Scholar] [CrossRef]
- Xie, Y.; Wilson, A.M. Change Point Estimation of Deciduous Forest Land Surface Phenology. Remote Sens. Environ. 2020, 240, 111698. [Google Scholar] [CrossRef]
- Linkosalmi, M.; Tuovinen, J.-P.; Nevalainen, O.; Peltoniemi, M.; Tanis, C.M.; Arslan, A.N.; Rainne, J.; Lohila, A.; Laurila, T.; Aurela, M. Tracking Vegetation Phenology of Pristine Northern Boreal Peatlands by Combining Digital Photography with CO2 Flux and Remote Sensing Data. Biogeosciences 2022, 19, 4747–4765. [Google Scholar] [CrossRef]
- Davidson, S.J.; Goud, E.M.; Malhotra, A.; Estey, C.O.; Korsah, P.; Strack, M. Linear Disturbances Shift Boreal Peatland Plant Communities Toward Earlier Peak Greenness. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006403. [Google Scholar] [CrossRef]
- Anderson, K.; Gaston, K.J. Lightweight Unmanned Aerial Vehicles Will Revolutionize Spatial Ecology. Front. Ecol. Environ. 2013, 11, 138–146. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R.; Barr, S. Assessing Spring Phenology of a Temperate Woodland: A Multiscale Comparison of Ground, Unmanned Aerial Vehicle and Landsat Satellite Observations. Remote Sens. Environ. 2019, 223, 229–242. [Google Scholar] [CrossRef]
- Klosterman, S.; Melaas, E.; Wang, J.; Martinez, A.; Frederick, S.; O’Keefe, J.; Orwig, D.A.; Wang, Z.; Sun, Q.; Schaaf, C.; et al. Fine-Scale Perspectives on Landscape Phenology from Unmanned Aerial Vehicle (UAV) Photography. Agric. For. Meteorol. 2018, 248, 397–407. [Google Scholar] [CrossRef]
- Berra, E.F.; Gaulton, R.; Barr, S. Use of a Digital Camera Onboard a UAV to Monitor Spring Phenology at Individual Tree Level. In Proceedings of the 2016 IEEE International Geoscience and Remote Sensing Symposium (IGARSS), Beijing, China, 10–15 July 2016; pp. 3496–3499. [Google Scholar] [CrossRef]
- Fawcett, D.; Bennie, J.; Anderson, K. Monitoring Spring Phenology of Individual Tree Crowns Using Drone-Acquired NDVI Data. Remote Sens. Ecol. Conserv. 2021, 7, 227–244. [Google Scholar] [CrossRef]
- Burkart, A.; Hecht, V.L.; Kraska, T.; Rascher, U. Phenological Analysis of Unmanned Aerial Vehicle Based Time Series of Barley Imagery with High Temporal Resolution. Precis. Agric. 2018, 19, 134–146. [Google Scholar] [CrossRef]
- Räsänen, A.; Aurela, M.; Juutinen, S.; Kumpula, T.; Lohila, A.; Penttilä, T.; Virtanen, T. Detecting Northern Peatland Vegetation Patterns at Ultra-High Spatial Resolution. Remote Sens. Ecol. Conserv. 2020, 6, 457–471. [Google Scholar] [CrossRef]
- Assmann, J.J.; Myers-Smith, I.H.; Kerby, J.T.; Cunliffe, A.M.; Daskalova, G.N. Drone Data Reveal Heterogeneity in Tundra Greenness and Phenology Not Captured by Satellites. Environ. Res. Lett. 2020, 15, 125002. [Google Scholar] [CrossRef]
- Lees, K.J.; Khomik, M.; Quaife, T.; Clark, J.M.; Hill, T.; Klein, D.; Ritson, J.; Artz, R.R.E. Assessing the Reliability of Peatland GPP Measurements by Remote Sensing: From Plot to Landscape Scale. Sci. Total Environ. 2021, 766, 142613. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, C. Understanding the Role of Phenology and Summer Physiology in Controlling Net Ecosystem Production: A Multiscale Comparison of Satellite, PhenoCam and Eddy Covariance Data. Environ. Res. Lett. 2020, 15, 104086. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hufkens, K.; Milliman, T.; Frolking, S. Intercomparison of Phenological Transition Dates Derived from the PhenoCam Dataset V1.0 and MODIS Satellite Remote Sensing. Sci. Rep. 2018, 8, 5679. [Google Scholar] [CrossRef] [PubMed]
- Billett, M.F.; Palmer, S.M.; Hope, D.; Deacon, C.; Storeton-West, R.; Hargreaves, K.J.; Flechard, C.; Fowler, D. Linking Land-Atmosphere-Stream Carbon Fluxes in a Lowland Peatland System. Glob. Biogeochem. Cycles 2004, 18, GB1024. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and Future Köppen-Geiger Climate Classification Maps at 1-Km Resolution. Sci. Data 2018, 5, 1–12. [Google Scholar] [CrossRef]
- Assmann, J.J.; Kerby, J.T.; Cunliffe, A.M.; Myers-Smith, I.H. Vegetation Monitoring Using Multispectral Sensors—Best Practices and Lessons Learned from High Latitudes. J. Unmanned Veh. Syst. 2019, 7, 54–75. [Google Scholar] [CrossRef]
- Stow, D.; Nichol, C.J.; Wade, T.; Assmann, J.J.; Simpson, G.; Helfter, C. Illumination Geometry and Flying Height Influence Surface Reflectance and NDVI Derived from Multispectral UAS Imagery. Drones 2019, 3, 55. [Google Scholar] [CrossRef]
- Simpson, G.; Nichol, C.J.; Wade, T.; Helfter, C.; Hamilton, A.; Gibson-Poole, S. Species-Level Classification of Peatland Vegetation Using Ultra-High-Resolution UAV Imagery. Drones 2024, 8, 97. [Google Scholar] [CrossRef]
- Wingate, L.; Cremonese, E.; Migliavacca, M.; Brown, T.; D’Odorico, R.; Peichl, M.; Gielen, B.; Hortnagl, L. ICOS Protocol—Phenocamera: Automated Phenology Monitoring (Version: 3); ICOS Ecosystem Thematic Centre: Viterbo, Italy, 2015; Available online: https://european-webcam-network.net/data/ICOS_Phenocamera_WG_Protocol_final.pdf (accessed on 5 April 2020).
- Richardson, A.D.; Braswell, B.H.; Hollinger, D.Y.; Jenkins, J.P.; Ollinger, S.V. Near-Surface Remote Sensing of Spatial and Temporal Variation in Canopy Phenology. Ecol. Appl. 2009, 19, 1417–1428. [Google Scholar] [CrossRef]
- Filippa, G.; Cremonese, E.; Migliavacca, M.; Galvagno, M.; Forkel, M.; Wingate, L.; Tomelleri, E.; Morra di Cella, U.; Richardson, A.D. Phenopix: A R Package for Image-Based Vegetation Phenology. Agric. For. Meteorol. 2016, 220, 141–150. [Google Scholar] [CrossRef]
- Sonnentag, O.; Hufkens, K.; Teshera-Sterne, C.; Young, A.M.; Friedl, M.; Braswell, B.H.; Milliman, T.; O’Keefe, J.; Richardson, A.D. Digital Repeat Photography for Phenological Research in Forest Ecosystems. Agric. For. Meteorol. 2012, 152, 159–177. [Google Scholar] [CrossRef]
- Gu, L.; Post, W.M.; Baldocchi, D.D.; Black, T.A.; Suyker, A.E.; Verma, S.B.; Vesala, T.; Wofsy, S.C. Characterizing the Seasonal Dynamics of Plant Community Photosynthesis across a Range of Vegetation Types. In Phenology of Ecosystem Processes: Applications in Global Change Research; Noormets, A., Ed.; Springer: New York, NY, USA, 2009; ISBN 9781441900258. [Google Scholar]
- Aubinet, M.; Vesala, T.; Papale, D. Eddy Covariance: A Practical Guide to Measurement and Data Analysis, 1st ed.; Aubinet, M., Vesala, T., Papale, D., Eds.; Springer Atmospheric Sciences: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-007-2350-4. [Google Scholar]
- Baldocchi, D.; Valentini, R.; Running, S.; Oechel, W.; Dahlman, R. Strategies for Measuring and Modelling Carbon Dioxide and Water Vapour Fluxes over Terrestrial Ecosystems. Glob. Change Biol. 1996, 2, 159–168. [Google Scholar] [CrossRef]
- Baldocchi, D.D. Assessing the Eddy Covariance Technique for Evaluating Carbon Dioxide Exchange Rates of Ecosystems: Past, Present and Future. Glob. Change Biol. 2003, 9, 479–492. [Google Scholar] [CrossRef]
- Helfter, C.; Campbell, C.; Dinsmore, K.J.; Drewer, J.; Coyle, M.; Anderson, M.; Skiba, U.; Nemitz, E.; Billett, M.F.; Sutton, M.A. Drivers of Long-Term Variability in CO2 Net Ecosystem Exchange in a Temperate Peatland. Biogeosciences 2015, 12, 1799–1811. [Google Scholar] [CrossRef]
- UKCEH; Coyle, M.; Roberts, E.; Jones, M.; Leeson, S.; Mullinger, N.; Simmons, I.; Van Dijk, N.; Kentisbeer, J.; Leith, I.; et al. Auchencorth Moss Atmospheric Observatory (AU): Annual Half-Hourly Meteorology since 1995, Near Edinburgh, UK. Available online: https://catalogue.ceda.ac.uk/uuid/8e6cbb111cfd41a19c92aadcb2d040fd/ (accessed on 14 March 2022).
- Vickers, D.; Mahrt, L. Quality Control and Flux Sampling Problems for Tower and Aircraft Data. J. Atmos. Ocean. Technol. 1997, 14, 512–526. [Google Scholar] [CrossRef]
- Mauder, M.; Foken, T. Documentation and Instruction Manual of the Eddy-Covariance Software Package TK2. Universität Bayreuth, Abt. Mikrometeorologie: Bayreuth, Germany, 2004. [Google Scholar]
- Papale, D.; Reichstein, M.; Aubinet, M.; Canfora, E.; Bernhofer, C.; Kutsch, W.; Longdoz, B.; Rambal, S.; Valentini, R.; Vesala, T.; et al. Towards a Standardized Processing of Net Ecosystem Exchange Measured with Eddy Covariance Technique: Algorithms and Uncertainty Estimation. Biogeosciences 2006, 3, 571–583. [Google Scholar] [CrossRef]
- Foken, T.; Wichura, B. Tools for Quality Assessment of Surface-Based Flux Measurements. Agric. For. Meteorol. 1996, 78, 83–105. [Google Scholar] [CrossRef]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the Separation of Net Ecosystem Exchange into Assimilation and Ecosystem Respiration: Review and Improved Algorithm. Glob. Change Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Wutzler, T.; Lucas-Moffat, A.; Migliavacca, M.; Knauer, J.; Sickel, K.; Šigut, L.; Menzer, O.; Reichstein, M. Basic and Extensible Post-Processing of Eddy Covariance Flux Data with REddyProc. Biogeosciences 2018, 15, 5015–5030. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y. A Method to Estimate the Additional Uncertainty in Gap-Filled NEE Resulting from Long Gaps in the CO2 Flux Record. Agric. For. Meteorol. 2007, 147, 199–208. [Google Scholar] [CrossRef]
- Lloyd, J.; Taylor, J.A. On the Temperature Dependence of Soil Respiration. Funct. Ecol. 1994, 8, 315. [Google Scholar] [CrossRef]
- McCarthy, M.; Christidis, N.; Dunstone, N.; Fereday, D.; Kay, G.; Klein-Tank, A.; Lowe, J.; Petch, J.; Scaife, A.; Stott, P. Drivers of the UK Summer Heatwave of 2018. Weather 2019, 74, 390–396. [Google Scholar] [CrossRef]
- Turner, S.; Barker, L.J.; Hannaford, J.; Muchan, K.; Parry, S.; Sefton, C. The 2018/2019 Drought in the UK: A Hydrological Appraisal. Weather 2021, 76, 248–253. [Google Scholar] [CrossRef]
- Evans, C.D.; Peacock, M.; Baird, A.J.; Artz, R.R.E.; Burden, A.; Callaghan, N.; Chapman, P.J.; Cooper, H.M.; Coyle, M.; Craig, E.; et al. Overriding Water Table Control on Managed Peatland Greenhouse Gas Emissions. Nature 2021, 593, 548–552. [Google Scholar] [CrossRef]
- Ahrends, H.E.; Brügger, R.; Stöckli, R.; Schenk, J.; Michna, P.; Jeanneret, F.; Wanner, H.; Eugster, W.; Ahrends, C.; Brügger, R.; et al. Quantitative Phenological Observations of a Mixed Beech Forest in Northern Switzerland with Digital Photography. J. Geophys. Res. Biogeosci. 2008, 113, 4004. [Google Scholar] [CrossRef]
- Ide, R.; Oguma, H. Use of Digital Cameras for Phenological Observations. Ecol. Inform. 2010, 5, 339–347. [Google Scholar] [CrossRef]
- Koebsch, F.; Sonnentag, O.; Järveoja, J.; Peltoniemi, M.; Alekseychik, P.; Aurela, M.; Arslan, A.N.; Dinsmore, K.; Gianelle, D.; Helfter, C.; et al. Refining the Role of Phenology in Regulating Gross Ecosystem Productivity across European Peatlands. Glob. Change Biol. 2020, 26, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Mora, J.P.; Kalacska, M.; Lucanus, O.; Soffer, R.; Leblanc, G. Spectro-Spatial Relationship between UAV Derived High Resolution DEM and SWIR Hyperspectral Data: Application to an Ombrotrophic Peatland. In Proceedings of the SPIE 10421, Remote Sensing for Agriculture, Ecosystems, and Hydrology XIX, SPIE, Warsaw, Poland, 2 November 2017; p. 104210P. [Google Scholar]
- Harris, A.; Bryant, R.G.; Baird, A.J. Mapping the Effects of Water Stress on Sphagnum: Preliminary Observations Using Airborne Remote Sensing. Remote Sens. Environ. 2006, 100, 363–378. [Google Scholar] [CrossRef]
- Hufkens, K.; Friedl, M.; Sonnentag, O.; Braswell, B.H.; Milliman, T.; Richardson, A.D. Linking Near-Surface and Satellite Remote Sensing Measurements of Deciduous Broadleaf Forest Phenology. Remote Sens. Environ. 2012, 117, 307–321. [Google Scholar] [CrossRef]
- Liu, Y.; Hill, M.J.; Zhang, X.; Wang, Z.; Richardson, A.D.; Hufkens, K.; Filippa, G.; Baldocchi, D.D.; Ma, S.; Verfaillie, J.; et al. Using Data from Landsat, MODIS, VIIRS and PhenoCams to Monitor the Phenology of California Oak/Grass Savanna and Open Grassland across Spatial Scales. Agric. For. Meteorol. 2017, 237–238, 311–325. [Google Scholar] [CrossRef]
- Papale, D.; Nicolini, G. ICOS Ecosystem Instructions for Setting Up the Spatial Sampling Scheme (Version 20170802); ICOS Ecosystem Thematic Centre: Viterbo, Italy, 2016. [Google Scholar] [CrossRef]
- Deering, D.W.; Middleton, E.M.; Irons, J.R.; Blad, B.L.; Walter-Shea, E.A.; Hays, C.L.; Walthall, C.; Eck, T.F.; Ahmad, S.P.; Banerjee, B.P. Prairie Grassland Bidirectional Reflectances Measured by Different Instruments at the FIFE Site. J. Geophys. Res. Atmos. 1992, 97, 18887–18903. [Google Scholar] [CrossRef]
- Epiphanio, J.C.N.; Huete, A.R. Dependence of NDVI and SAVI on Sun/Sensor Geometry and Its Effect on FAPAR Relationships in Alfalfa. Remote Sens. Environ. 1995, 51, 351–360. [Google Scholar] [CrossRef]
- Woolley, J.T. Reflectance and Transmittance of Light by Leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Darby, B.; Felts, E.; Sonnentag, O.; Friedl, M.A.; Hufkens, K.; O’Keefe, J.; Klosterman, S.; Munger, J.W.; Toomey, M.; et al. Tracking Forest Phenology and Seasonal Physiology Using Digital Repeat Photography: A Critical Assessment. Ecol. Appl. 2014, 24, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation Phenology from Sentinel-2 and Field Cameras for a Dutch Barrier Island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Migliavacca, M.; Galvagno, M.; Cremonese, E.; Rossini, M.; Meroni, M.; Sonnentag, O.; Cogliati, S.; Manca, G.; Diotri, F.; Busetto, L.; et al. Using Digital Repeat Photography and Eddy Covariance Data to Model Grassland Phenology and Photosynthetic CO2 Uptake. Agric. For. Meteorol. 2011, 151, 1325–1337. [Google Scholar] [CrossRef]
- Järveoja, J.; Nilsson, M.B.; Gažovič, M.; Crill, P.M.; Peichl, M. Partitioning of the Net CO2 Exchange Using an Automated Chamber System Reveals Plant Phenology as Key Control of Production and Respiration Fluxes in a Boreal Peatland. Glob. Change Biol. 2018, 24, 3436–3451. [Google Scholar] [CrossRef]
- Knox, S.H.; Dronova, I.; Sturtevant, C.; Oikawa, P.Y.; Matthes, J.H.; Verfaillie, J.; Baldocchi, D. Using Digital Camera and Landsat Imagery with Eddy Covariance Data to Model Gross Primary Production in Restored Wetlands. Agric. For. Meteorol. 2017, 237–238, 233–245. [Google Scholar] [CrossRef]
- Linkosalmi, M.; Aurela, M.; Tuovinen, J.P.; Peltoniemi, M.; Tanis, C.M.; Arslan, A.N.; Kolari, P.; Böttcher, K.; Aalto, T.; Rainne, J.; et al. Digital Photography for Assessing the Link between Vegetation Phenology and CO2 Exchange in Two Contrasting Northern Ecosystems. Geosci. Instrum. Methods Data Syst. 2016, 5, 417–426. [Google Scholar] [CrossRef]
- Peichl, M.; Gažovič, M.; Vermeij, I.; De Goede, E.; Sonnentag, O.; Limpens, J.; Nilsson, M.B. Peatland Vegetation Composition and Phenology Drive the Seasonal Trajectory of Maximum Gross Primary Production. Sci. Rep. 2018, 8, 8012. [Google Scholar] [CrossRef]
- Richardson, A.D.; Anderson, R.S.; Arain, M.A.; Barr, A.G.; Bohrer, G.; Chen, G.; Chen, J.M.; Ciais, P.; Davis, K.J.; Desai, A.R.; et al. Terrestrial Biosphere Models Need Better Representation of Vegetation Phenology: Results from the North American Carbon Program Site Synthesis. Glob. Change Biol. 2012, 18, 566–584. [Google Scholar] [CrossRef]
- Strachan, I.B.; Pelletier, L.; Bonneville, M.C. Inter-Annual Variability in Water Table Depth Controls Net Ecosystem Carbon Dioxide Exchange in a Boreal Bog. Biogeochemistry 2016, 127, 99–111. [Google Scholar] [CrossRef]
- Johnson, D.; Vachon, J.; Britton, A.J.; Helliwell, R.C. Drought Alters Carbon Fluxes in Alpine Snowbed Ecosystems through Contrasting Impacts on Graminoids and Forbs. New Phytol. 2011, 190, 740–749. [Google Scholar] [CrossRef]
- Kuiper, J.J.; Mooij, W.M.; Bragazza, L.; Robroek, B.J.M. Plant Functional Types Define Magnitude of Drought Response in Peatland CO2 Exchange. Ecology 2014, 95, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, N.; Laine, A.M.; Korrensalo, A.; Nijp, J.; Limpens, J.; Mehtätalo, L.; Männistö, E.; Tuittila, E.S. A Deepened Water Table Increases the Vulnerability of Peat Mosses to Periodic Drought. J. Ecol. 2024, 112, 1210–1224. [Google Scholar] [CrossRef]
- Taylor, K.; Rowland, A.P.; Jones, H.E. Molinia caerulea (L.) Moench. J. Ecol. 2001, 89, 126–144. [Google Scholar] [CrossRef]
- Wein, R.W. Eriophorum Vaginatum L. J. Ecol. 1973, 61, 601–615. [Google Scholar] [CrossRef]
- Richards, P.W.; Clapham, A.R. Juncus Effusus L. (Juncus Communis β Effusus E. Mey). J. Ecol. 1941, 29, 375–380. [Google Scholar] [CrossRef]
- Peichl, M.; Sonnentag, O.; Nilsson, M.B. Bringing Color into the Picture: Using Digital Repeat Photography to Investigate Phenology Controls of the Carbon Dioxide Exchange in a Boreal Mire. Ecosystems 2015, 18, 115–131. [Google Scholar] [CrossRef]






| Band Name | Central Band Wavelength ± Width (nm) |
|---|---|
| Green | 550 ± 40 |
| Red | 660 ± 40 |
| Red-Edge | 735 ± 10 |
| Near Infra-Red | 790 ± 40 |
| Plant Functional Type | Dominant Species |
|---|---|
| Shrub | Calluna vulgaris Vaccinium spp. (V. myrtillus, V. oxycoccus) |
| Bryophyte (moss) | Polytrichum commune Sphagnum spp. Pleurozium schreberi |
| Grasses | Deschampsia flexuosa Molinia caerulea |
| Rush | Juncus effusus |
| Sedge | Eriophorum vaginatum |
| Vegetation Index (VI) | Abbreviation | Equation | Description |
|---|---|---|---|
| Red-Edge Chlorophyll | REChl | Indicator of photosynthetic activity of vegetation canopy | |
| Green Chlorophyll | gChl | Used to estimate leaf chlorophyll content | |
| Red Chlorophyll | rChl | Used to identify senescence and areas of yellowing vegetation | |
| Normalised Difference | NDVI * | Sensitive to structural and physiological properties. Used to estimate leaf area index | |
| Normalised Difference Red-Edge | reNDVI1 * | Modification of the NDVI utilising the red-edge band | |
| Normalised Difference Red-Edge/red | reNDVI2 * | Modification of the NDVI utilising the red-edge and red bands | |
| Green Normalised Difference | gNDVI * | Sensitive to chlorophyll content and has a higher point of saturation than the NDVI | |
| Normalised Green/Red Difference | NGRDI * | Modification of the NDVI employing only reflectance in the visible (green and red) bands |
| (1) Data completeness |
| Check the number of raw data points (N) per 30-min flux averaging period ≥90% of the possible total (i.e., N ≥ 1620 for 10 Hz data) |
| (2) Raw data spikes |
| Data excluded if spikes (i.e., values > ± 2 standard deviations from the mean) in vertical wind velocity, CO2 mole fraction, or water-vapour (H2O) mole fraction > 1% total number of data points per 30-min flux averaging period (i.e., ≥18 for 10 Hz data) |
| (3) Sufficient turbulence |
| Discard measurements with insufficient turbulence, defined by a friction velocity (u*) threshold [83] |
| (4) Stationarity |
| Check that the time-series is in steady state [84] |
| (5) Flux within sensible range for site |
| Discard half-hourly CO2 fluxes which fall outside the range ± 30 µ mol CO2 m−2 s−1 |
| Year | Green-Up | Peak Growing Season | Green-Down | Complete Growing Season |
|---|---|---|---|---|
| 2018 | - | - | - | - |
| 2019 | DOY 119–156 (37 days) | DOY 156–269 (113 days) | DOY 269–321 (52 days) | DOY 119–321 (202 days) |
| 2020 | DOY 127–186 (59 days) | DOY 186–253 (67 days) | DOY 253–317 (64 days) | DOY 127–317 (190 days) |
| 2021 | DOY 135–171 (36 days) | DOY 171–254 (83 days) | DOY 254–329 (75 days) | DOY 135–329 (194 days) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simpson, G.; Wade, T.; Helfter, C.; Jones, M.R.; Yeung, K.; Nichol, C.J. Inter-Annual Variability of Peatland Vegetation Captured Using Phenocam- and UAV Imagery. Remote Sens. 2025, 17, 526. https://doi.org/10.3390/rs17030526
Simpson G, Wade T, Helfter C, Jones MR, Yeung K, Nichol CJ. Inter-Annual Variability of Peatland Vegetation Captured Using Phenocam- and UAV Imagery. Remote Sensing. 2025; 17(3):526. https://doi.org/10.3390/rs17030526
Chicago/Turabian StyleSimpson, Gillian, Tom Wade, Carole Helfter, Matthew R. Jones, Karen Yeung, and Caroline J. Nichol. 2025. "Inter-Annual Variability of Peatland Vegetation Captured Using Phenocam- and UAV Imagery" Remote Sensing 17, no. 3: 526. https://doi.org/10.3390/rs17030526
APA StyleSimpson, G., Wade, T., Helfter, C., Jones, M. R., Yeung, K., & Nichol, C. J. (2025). Inter-Annual Variability of Peatland Vegetation Captured Using Phenocam- and UAV Imagery. Remote Sensing, 17(3), 526. https://doi.org/10.3390/rs17030526






