Spatiotemporal Dynamics and Potential Distribution Prediction of Spartina alterniflora Invasion in Bohai Bay Based on Sentinel Time-Series Data and MaxEnt Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data and Processing
2.2.1. Satellite Data
2.2.2. Sample Data
2.2.3. Environmental Data
2.3. Generation of Feature Set
2.4. S. alterniflora Detection and Prediction
2.4.1. Object-Based Random Forest Algorithm
2.4.2. Accuracy Assessment
2.4.3. Detecting Potential Areas of S. alterniflora Using MaxEnt
- : The probability of class given input ;
- : The partition function, ensuring the probabilities sum to 1;
- : Model parameters or weights for each feature function, learned during training;
- : Feature functions that capture relationships between input x and output y;
- : Number of feature functions.
- (1)
- Model Operation
- (2)
- Suitability Zone Classification
3. Results
3.1. Accuracy Assessment of S. alterniflora Wetland Maps
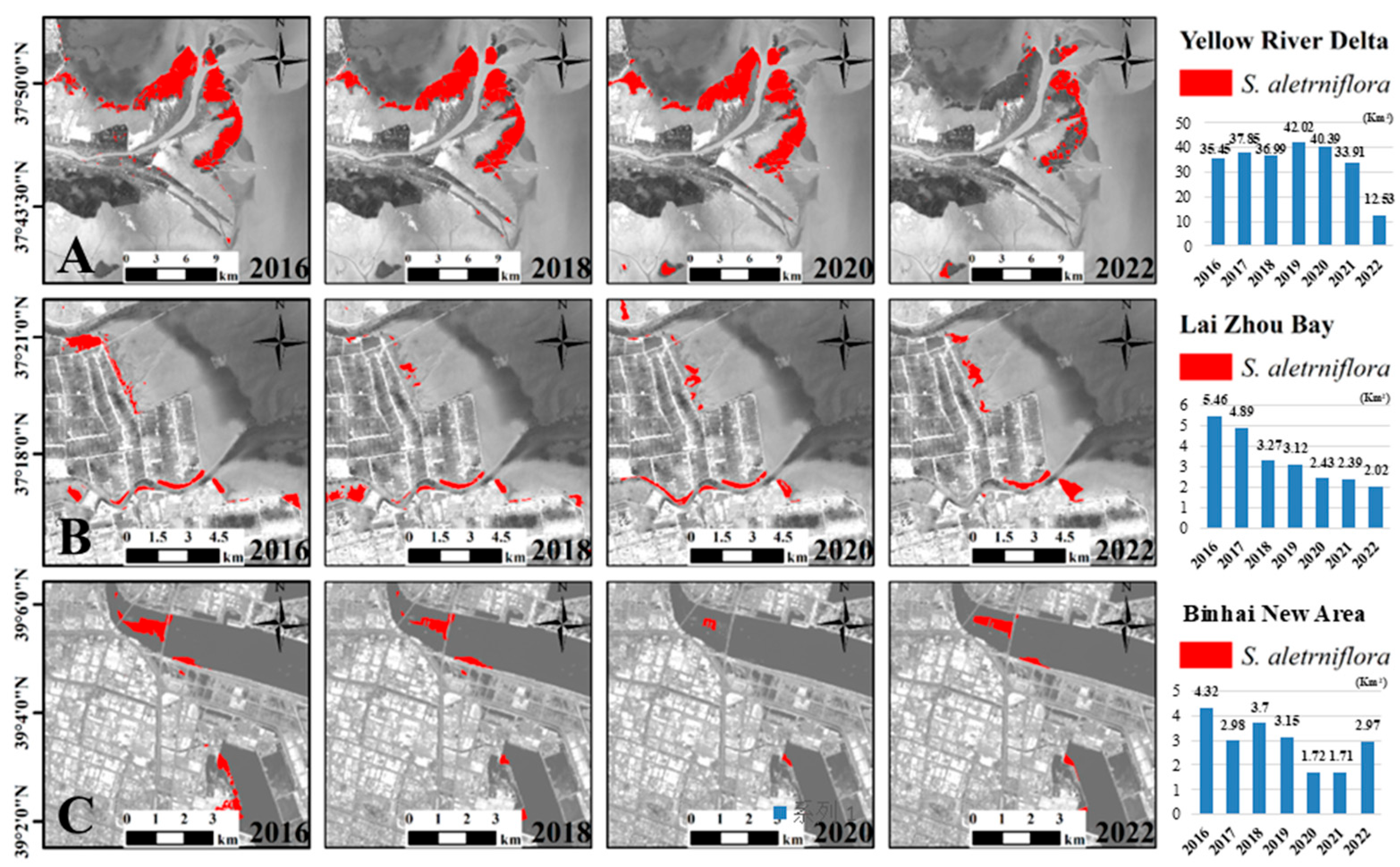
3.2. The Temporal and Spatial Dynamics of S. alterniflora Wetlands from 2016 to 2022
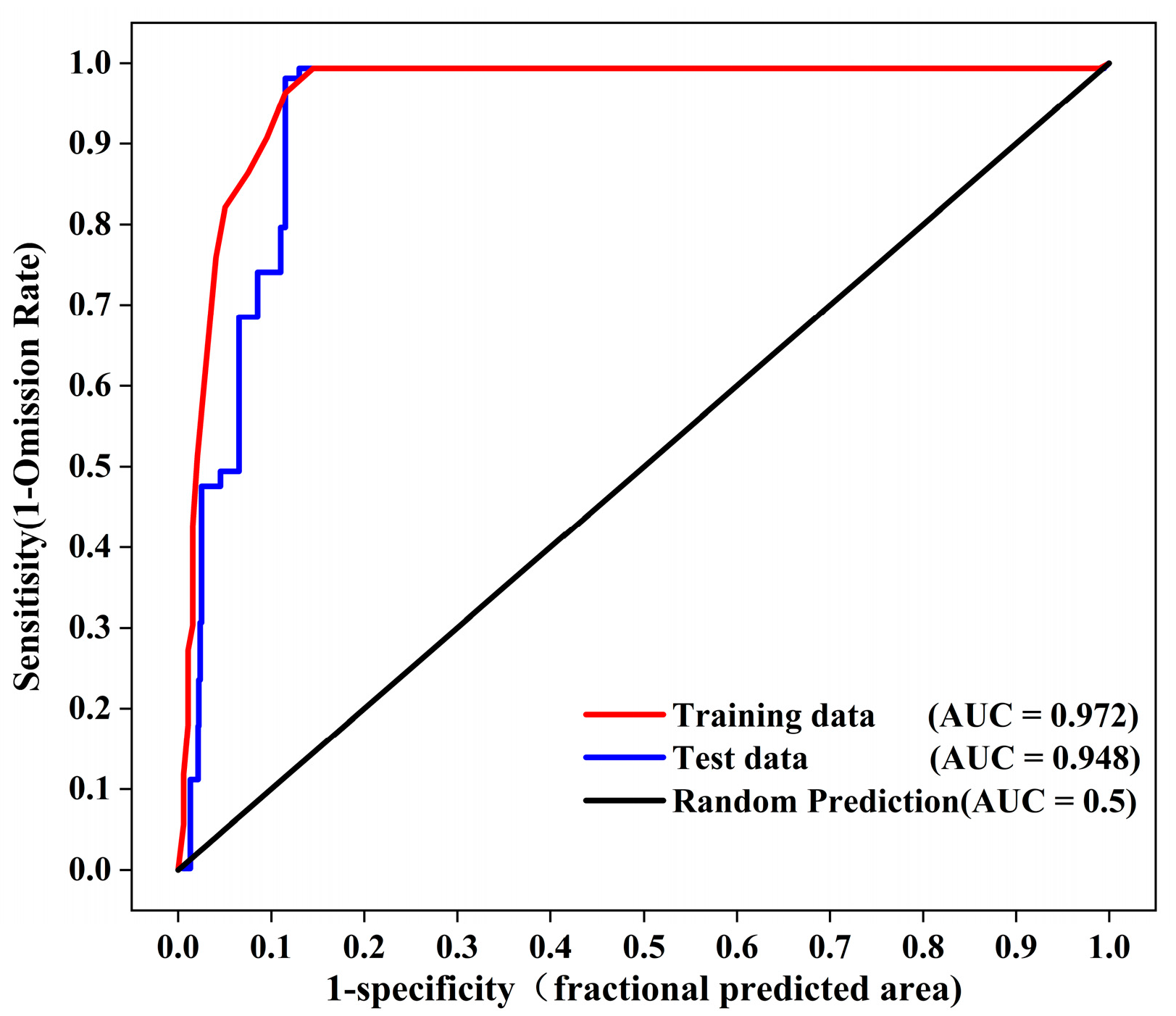
3.3. The Potential Distribution Prediction of S. alterniflora
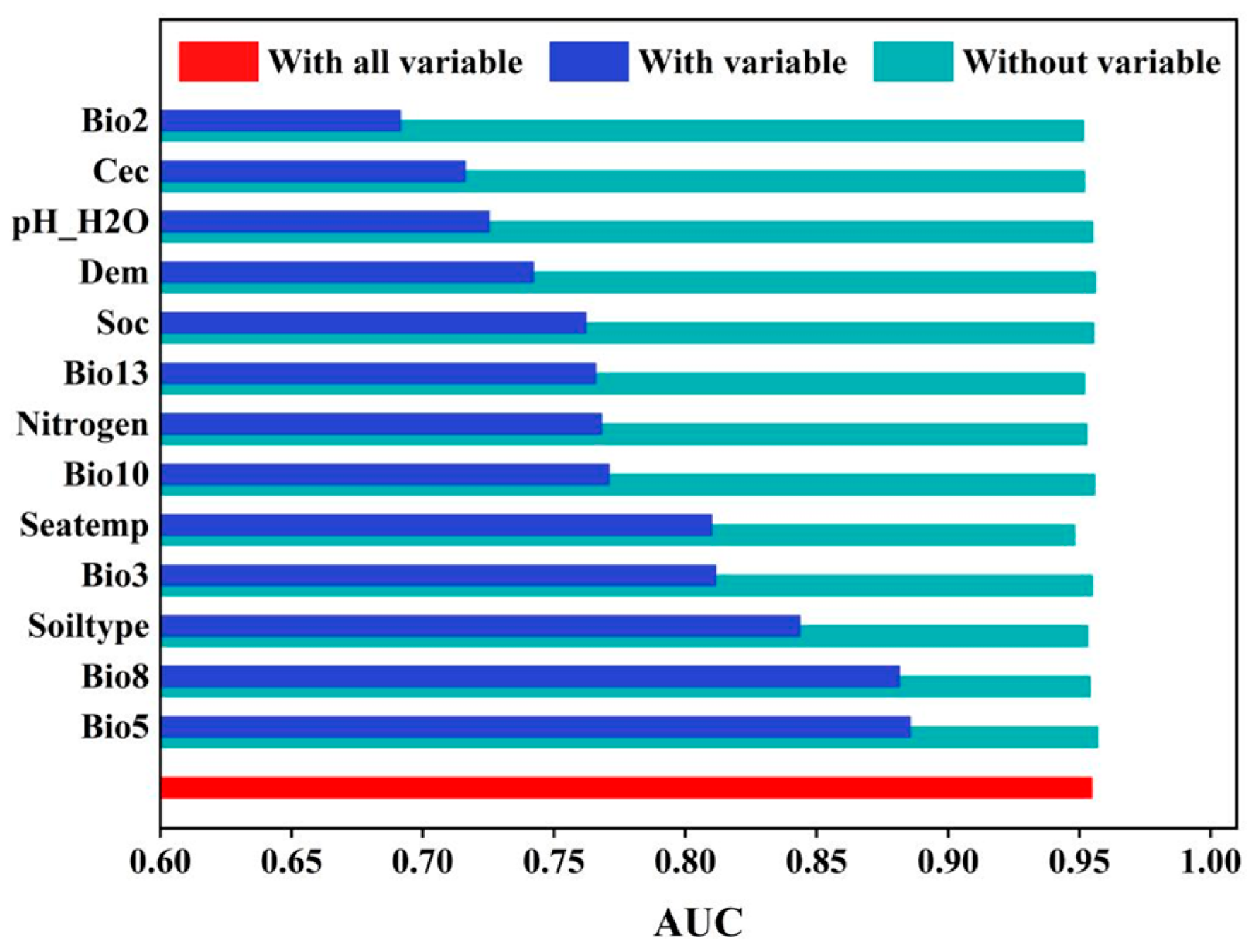
3.3.1. Dominant Factors for the Spatial–Temporal Dynamics of S. alterniflora Wetlands
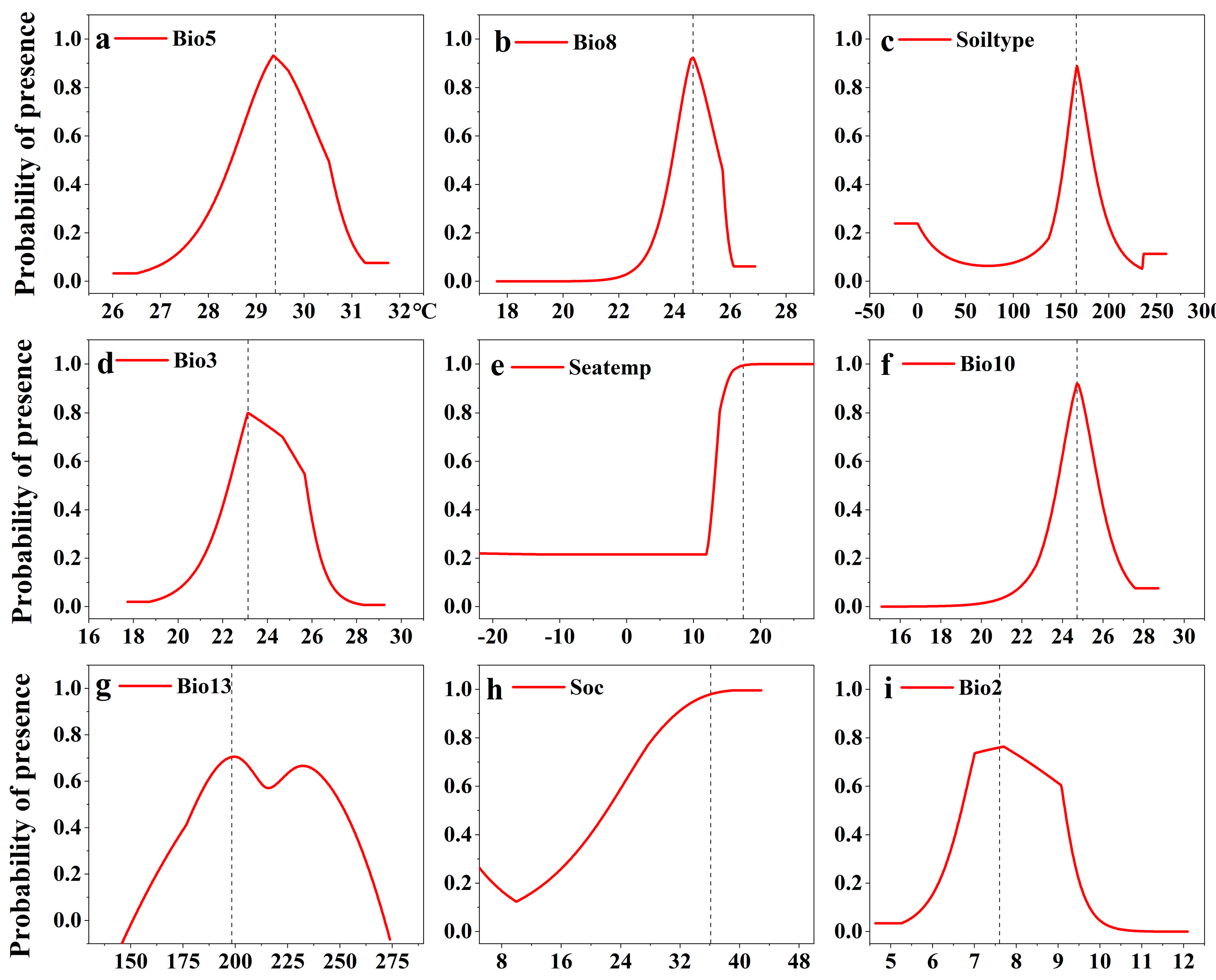
3.3.2. The Response of Potential S. alterniflora Distribution to Environmental Variables
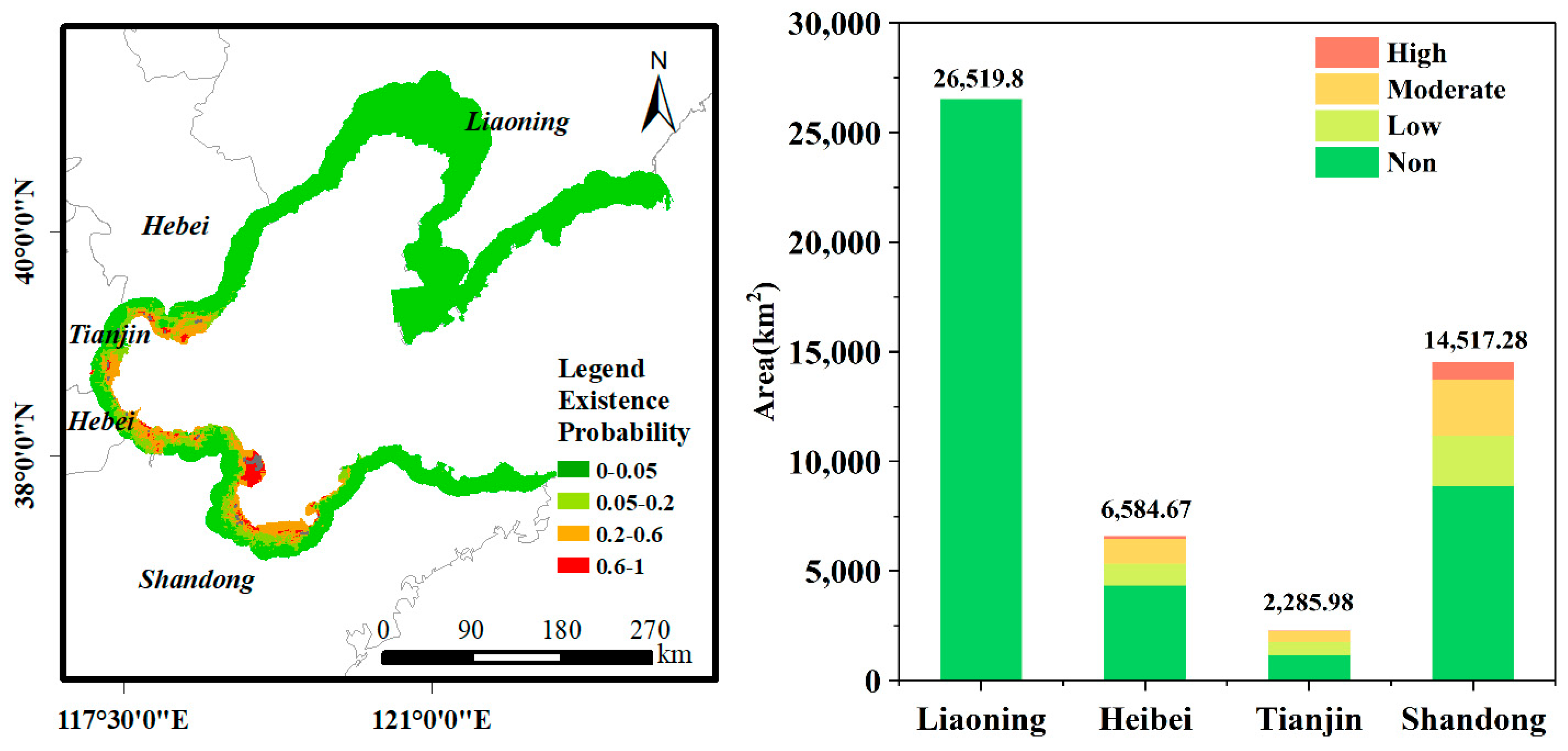
3.3.3. Potential Suitable Areas and Suitability Assessment of S. alterniflora in the Study Area
4. Discussion
4.1. Uncertainty Analysis
4.1.1. Characterization and Monitoring of S. alterniflora Wetlands from Satellite Data Analyses
4.1.2. Prediction of S. alterniflora Wetland Distribution from MaxEnt
4.1.3. Comparison with Previous Studies and Classification Accuracy
4.2. Current Status and Management Recommendations for S. alterniflora Control
4.3. Potential Suitable Areas and Key Regions of Concern for S. alterniflora
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. alterniflora | Spartina alterniflora |
| YRD | Yellow River Delta |
| CGC | Changli Gold Coast |
| GIS | Geographic information systems |
| SAR | Synthetic aperture radar |
| GEE | Google Earth Engine |
| GARP | Genetic algorithm for rule-set production |
| EnFA | Ecological niche factor analysis |
| BIOCLIM | Bioclimatic model |
| MaxEnt | Maximum entropy modeling |
| GRD | Ground range-detected |
| VV | Vertical transmit and vertical receive |
| VH | Vertical transmit and horizontal receive |
| UAVs | Unmanned aerial vehicles |
| GVG | Ground vegetation grids |
| NDVI | Normalized difference vegetation index |
| DVI | Difference vegetation index |
| EVI | Difference vegetation index |
| GCI | Green chlorophyll index |
| RVI | Ratio vegetation index |
| CIre | Chlorophyll index using red edge |
| NDre1 | Normalized difference red edge 1 |
| NDre2 | Normalized difference red edge 2 |
| JM | Jeffries–Matusita |
| SNIC | Simple non-iterative clustering |
| PA | Producer’s accuracy |
| UA | User’s accuracy |
| OA | Overall accuracy |
Appendix A
| Code | Environmental Variables | Unit | Dataset | Source |
|---|---|---|---|---|
| Bio1 | Annual mean temperature | °C | Climate factor | BIOCLIM WorldClim 2.1 dataset within the WorldClim database (https://worldclim.org/, accessed on 15 April 2024) |
| Bio2 | Mean diurnal range (mean of monthly (max temp–min temp)) | °C | ||
| Bio3 | Isothermality (Bio2/Bio7) (×100) | |||
| Bio4 | Temperature seasonality (standard deviation ×100) | |||
| Bio5 | Max temperature of warmest month | °C | ||
| Bio6 | Min temperature of coldest month | °C | ||
| Bio7 | Temperature annual range (Bio5–Bio6) | °C | ||
| fBio8 | Mean temperature of wettest quarter | °C | ||
| Bio9 | Mean temperature of driest quarter | °C | ||
| Bio10 | Mean temperature of warmest quarter | °C | ||
| Bio11 | Mean temperature of coldest quarter | °C | ||
| Bio12 | Annual precipitation | mm | ||
| Bio13 | Precipitation of wettest month | mm | ||
| Bio14 | Precipitation of driest month | mm | ||
| Bio15 | Precipitation seasonality (coefficient of variation) | |||
| Bio16 | Precipitation of wettest quarter | mm | ||
| Bio17 | Precipitation of driest quarter | mm | ||
| Bio18 | Precipitation of warmest quarter | |||
| Bio19 | Precipitation of coldest quarter | mm | ||
| Dem | Elevation | m | Terrain factor | ASTER GDEM digital elevation data (https://www.gscloud.cn/, accessed on 18 April 2024) |
| Slope | Slope | ° | ||
| Cec | Cation exchange capacity of the soil | cmol©/kg | Soil factor | SoilGrids global soil dataset (https://soilgrids.org/, accessed on 26 April 2024) |
| pH_H2O | Soil pH | pH | ||
| Nitrogen | Total nitrogen (N) | g/kg | ||
| Ocd | Organic carbon density | kg/dm3 | ||
| Ocs | Organic carbon stocks | kg/m2 | ||
| Clay | Proportion of clay particles (<0.002 mm) in the fine earth fraction | g/100 g (%) | ||
| Sand | Proportion of sand particles (>0.05 mm) in the fine earth fraction | g/100 g (%) | ||
| Soc | Soil organic carbon content in the fine earth fraction | g/kg | ||
| Silt | Proportion of silt particles (≥0.002 mm and ≤0.05 mm) in the fine earth fraction | g/100 g (%) | ||
| Soiltype | Soil type | |||
| Seatemp | Sea surface temperatures | °C | Marine factor | Sentinel-3 SLSTR (https://earthengine.google.com/, accessed on 26 April 2024) ImageCollection (“COPERNICUS/S3/OLCI”) |
| Seasalt | Seawater salinity | % | the Global Ocean Data Assimilation Experiment |
References
- Ke, N.; Lu, X.; Fei, W. Spatiotemporal evolution of a tidal channel under Spartina alterniflora invasion and expansion in a coastal Wetland, Jiangsu, China. Remote Sens. Lett. 2024, 15, 389–400. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Tian, J.; Wang, L.; Yin, D.; Li, X.; Diao, C.; Gong, H.; Shi, C.; Menenti, M.; Ge, Y.; Nie, S.; et al. Development of spectral-phenological features for deep learning to understand Spartina alterniflora invasion. Remote Sens. Environ. 2020, 242, 111745. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Liu, M.; Liu, X.; Tao, J. Species Distribution Models of the Spartina alterniflora Loisel in Its Origin and Invasive Country Reveal an Ecological Niche Shift. Front. Plant Sci. 2021, 12, 738769. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhao, S.; Engel, B.A.; Zhang, M.; Zhang, Z. Can We Protect China’s Northern Coast Wetlands from Tidal Dynamics by Restoring Native Species After Spartina alterniflora Is Eradicated? A Model-Based Analysis. Land Degrad. Dev. 2024, 35, 5439–5451. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L. An experimental study on physical controls of an exotic plant Spartina alterniflora in Shanghai, China. Ecol. Eng. 2008, 32, 11–21. [Google Scholar] [CrossRef]
- Xu, R.; Zhao, S.; Ke, Y. A Simple Phenology-Based Vegetation Index for Mapping Invasive Spartina Alterniflora Using Google Earth Engine. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 190–201. [Google Scholar] [CrossRef]
- Liu, M.; Mao, D.; Wang, Z.; Li, L.; Man, W.; Jia, M.; Ren, C.; Zhang, Y. Rapid Invasion of Spartina alterniflora in the Coastal Zone of Mainland China: New Observations from Landsat OLI Images. Remote Sens. 2018, 10, 1933. [Google Scholar] [CrossRef]
- Qiu, Z.; Mao, D.; Feng, K.; Wang, M.; Xiang, H.; Wang, Z. High-Resolution Mapping Changes in the Invasion of Spartina Alterniflora in the Yellow River Delta. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 6445–6455. [Google Scholar] [CrossRef]
- Li, B.; Liao, C.-h.; Zhang, X.-d.; Chen, H.-l.; Wang, Q.; Chen, Z.-y.; Gan, X.-j.; Wu, J.-h.; Zhao, B.; Ma, Z.-j.; et al. Spartina alterniflora invasions in the Yangtze River estuary, China: An overview of current status and ecosystem effects. Ecol. Eng. 2009, 35, 511–520. [Google Scholar] [CrossRef]
- Liu, W.; Strong, D.R.; Pennings, S.C.; Zhang, Y. Provenance-by-environment interaction of reproductive traits in the invasion of Spartina alterniflora in China. Ecology 2017, 98, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mao, D.; Wang, Z.; Huang, X.; Li, L.; Jia, M. Invasion of Spartina alterniflora in the coastal zone of mainland China: Control achievements from 2015 to 2020 towards the Sustainable Development Goals. J. Environ. Manag. 2022, 323, 116242. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.; Jialin, L.; Yongchao, L. Tracking annual changes in the distribution and composition of saltmarsh vegetation on the Jiangsu coast of China using Landsat time series–based phenological parameters. Remote Sens. Environ. 2023, 284, 113370. [Google Scholar] [CrossRef]
- Zhou, X.; Zuo, Y.; Zheng, K. Monitoring the Invasion of S. alterniflora on the Yangtze River Delta, China, Using Time Series Landsat Images during 1990–2022. Remote Sens. 2024, 16, 1377. [Google Scholar] [CrossRef]
- Marpu, P.R.; Gamba, P.; Niemeyer, I. Hyperspectral data classification using an ensemble of class-dependent neural networks. In Proceedings of the 2009 First Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing, Grenoble, France, 26–28 August 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Tian, Y.; Jia, M.; Wang, Z.; Mao, D.; Du, B.; Wang, C. Monitoring Invasion Process of Spartina alterniflora by Seasonal Sentinel-2 Imagery and an Object-Based Random Forest Classification. Remote Sens. 2020, 12, 1383. [Google Scholar] [CrossRef]
- Ma, Y.; Zhuo, L.; Cao, J. Mapping Invasive Spartina alterniflora Using Phenological Information and Red-Edge Bands of Sentinel-2 Time-Series Data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2025, 18, 13–24. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, M.; Wang, J.; Song, X.; Gui, Y.; Zhang, Y.; Li, W. Multiple attention network for spartina alterniflora segmentation using multitemporal remote sensing images. IEEE Trans. Geosci. Remote Sens. 2023, 61, 1–15. [Google Scholar] [CrossRef]
- Chen, W.; Shi, C. Fine-scale mapping of Spartina alterniflora-invaded mangrove forests with multi-temporal WorldView-Sentinel-2 data fusion. Remote Sens. Environ. 2023, 295, 113690. [Google Scholar] [CrossRef]
- Sun, C.; Li, J.; Liu, Y.; Liu, Y.; Liu, R. Plant species classification in salt marshes using phenological parameters derived from Sentinel-2 pixel-differential time-series. Remote Sens. Environ. 2021, 256, 112320. [Google Scholar] [CrossRef]
- Drusch, M.; Bello, U.D.; Carlier, S.; Colin, O.; Fernandez, V.; Gascon, F.; Hoersch, B.; Isola, C.; Laberinti, P.; Martimort, P.; et al. Sentinel-2: ESA’s Optical High-Resolution Mission for GMES Operational Services. Remote Sens. Environ. 2012, 120, 25–36. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Wang, X.; Xu, X.; Qiu, S.; Pan, L.; Ma, J.; Ju, R.; Wu, J.; Li, B. Continual expansion of Spartina alterniflora in the temperate and subtropical coastal zones of China during 1985–2020. Int. J. Appl. Earth Obs. Geoinf. 2023, 117, 103192. [Google Scholar] [CrossRef]
- Li, X.; Tian, J.; Li, X.; Yu, Y.; Ou, Y.; Zhu, L.; Zhu, X.; Zhou, B.; Gong, H. Annual mapping of Spartina alterniflora with deep learning and spectral-phenological features from 2017 to 2021 in the mainland of China. Int. J. Remote Sens. 2024, 45, 3172–3199. [Google Scholar] [CrossRef]
- Ayres, D.R.; Smith, D.L.; Zaremba, K.; Klohr, S.; Strong, D.R. Spread of Exotic Cordgrasses and Hybrids (Spartina sp.) in the Tidal Marshes of San Francisco Bay, California, USA. Biol. Invasions 2004, 6, 221–231. [Google Scholar] [CrossRef]
- Cui, L.; Berger, U.; Cao, M.; Zhang, Y.; He, J.; Pan, L.; Jiang, J. Conservation and Restoration of Mangroves in Response to Invasion of Spartina alterniflora Based on the MaxEnt Model: A Case Study in China. Forests 2023, 14, 1220. [Google Scholar] [CrossRef]
- Drake, J.M.; Lodge, D.M. Forecasting Potential Distributions of Nonindigenous Species with a Genetic Algorithm. Fisheries 2006, 31, 9–16. [Google Scholar] [CrossRef]
- Thameur, B.H.; Soufiène, S.; Ammar, H.H.; Hammami, S. Spatial distribution and habitat selection of culicoides imicola: The potential vector of bluetongue virus in Tunisia. Onderstepoort J. Vet. Res. 2021, 88, e1–e9. [Google Scholar] [CrossRef]
- Hu, A.L.; Yang, J.; Liu, B.L.; Zou, Y. Prediction on the changes in potential suitable areas for mangroves along the coast of Guangxi and the threat from Spartina alterniflora invasion. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2024, 35, 669–677. [Google Scholar]
- Carpenter, G.; Gillison, A.N.; Winter, J. DOMAIN: A flexible modelling procedure for mapping potential distributions of plants and animals. Biodivers. Conserv. 1993, 2, 667–680. [Google Scholar] [CrossRef]
- Zhang, H.; Song, J.; Zhao, H.; Li, M.; Han, W. Predicting the Distribution of the Invasive Species Leptocybe invasa: Combining MaxEnt and Geodetector Models. Insects 2021, 12, 92. [Google Scholar] [CrossRef]
- Amindin, A.; Pourghasemi, H.R.; Safaeian, R.; Rahmanian, S.; Tiefenbacher, J.P.; Naimi, B. Predicting Current and Future Habitat Suitability of an Endemic Species Using Data-Fusion Approach: Responses to Climate Change. Rangel. Ecol. Manag. 2024, 94, 149–162. [Google Scholar] [CrossRef]
- Blanco-Sacristán, J.; Johansen, K.; Duarte, C.M.; Daffonchio, D.; Hoteit, I.; McCabe, M.F. Mangrove distribution and afforestation potential in the Red Sea. Sci. Total Environ. 2022, 843, 157098. [Google Scholar] [CrossRef] [PubMed]
- Charrua, A.B.; Bandeira, S.O.; Catarino, S.; Cabral, P.; Romeiras, M.M. Assessment of the vulnerability of coastal mangrove ecosystems in Mozambique. Ocean Coast. Manag. 2020, 189, 105145. [Google Scholar] [CrossRef]
- Min, Y.; Ke, Y.; Han, Y.; Yin, X.; Zhou, D. Dynamic monitoring of invasive Spartina alterniflora clearance via fusion of Sentinel-2 and GF-1 time series images. Natl. Remote Sens. Bull. 2023, 27, 1467–1479. [Google Scholar] [CrossRef]
- Chen, P.; Xia, J.; Ma, H.; Gao, F.; Dong, M.; Xing, X.; Li, C. Analysis of spatial distribution pattern and its influencing factors of the Tamarix chinensis population on the beach of the muddy coastal zone of Bohai Bay. Ecol. Indic. 2022, 140, 109016. [Google Scholar] [CrossRef]
- Wang, M.; Mao, D.; Wang, Y.; Xiao, X.; Xiang, H.; Feng, K.; Luo, L.; Jia, M.; Song, K.; Wang, Z. Wetland mapping in East Asia by two-stage object-based Random Forest and hierarchical decision tree algorithms on Sentinel-1/2 images. Remote Sens. Environ. 2023, 297, 113793. [Google Scholar] [CrossRef]
- Pouyan, S.; Rahmanian, S.; Amindin, A.; Pourghasemi, H.R. Chapter 15—Spatial and seasonal modeling of the land surface temperature using random forest. In Computers in Earth and Environmental Sciences; Pourghasemi, H.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 221–234. [Google Scholar] [CrossRef]
- Yan, D.; Li, J.; Yao, X.; Luan, Z. Quantifying the Long-Term Expansion and Dieback of Spartina Alterniflora Using Google Earth Engine and Object-Based Hierarchical Random Forest Classification. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 9781–9793. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, J.-S.; Kim, J.H.; Kwon, Y.S.; Lee, D.-H. Modeling the distribution of invasive species (Ambrosia spp.) using regression kriging and Maxent. Front. Ecol. Evol. 2022, 10, 1036816. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, J.; Ren, G.; Zhao, K.; Wang, X. Global potential distribution prediction of Xanthium italicum based on Maxent model. Sci. Rep. 2021, 11, 16545. [Google Scholar] [CrossRef]
- Fan, Y.; Yu, S.; Wang, J.; Li, P.; Chen, S.; Ji, H.; Li, P.; Dou, S. Changes of Inundation Frequency in the Yellow River Delta and Its Response to Wetland Vegetation. Land 2022, 11, 1647. [Google Scholar] [CrossRef]
- Dai, G.; Yang, B.; Huang, Y. Current situation investigation and invasion process analysis of Spartina alterniflora in marine reserve. Mar. Environ. Sci. 2022, 41, 697–704. [Google Scholar]
- Tesfamariam, B.G.; Gessesse, B.; Melgani, F. MaxEnt-based modeling of suitable habitat for rehabilitation of Podocarpus forest at landscape-scale. Environ. Syst. Res. 2022, 11, 4. [Google Scholar] [CrossRef]
- Alexandre, M.J.C.W.; Mercedes Román, D.; Wartini, N.; Alex, B.M. Spatial evaluation of the soils capacity and condition to store carbon across Australia. Geoderma 2024, 442, 116805. [Google Scholar] [CrossRef]
- Esfanjani, J.; Ghorbani, A.; Zare Chahouki, M.A. MaxEnt Modeling for Predicting Impacts of Environmental Factors on the Potential Distribution of Artemisia aucheri and Bromus tomentellus-Festuca ovina in Iran. Pol. J. Environ. Stud. 2018, 27, 1041–1047. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, B.; Niu, Y.; Chen, C.; Zhou, Y. Classification of coastal salt marsh based on Sentinel-1 time series backscattering characteristics:The case of the Yangtze River delta. Natl. Remote Sens. Bull. 2022, 26, 672–682. [Google Scholar] [CrossRef]
- Dai, J.; Luo, F.; Chen, Z.; Zhou, G.; Zeng, J. Pixel-based unique phenological feature composite method: Tackle the challenges of Spartina alterniflora identification and salt marsh classification in mixed vegetations area. J. Appl. Remote Sens. 2023, 17, 024510. [Google Scholar] [CrossRef]
- Dong, D.; Huang, H.; Gao, Q. Tracking the Dynamics of Spartina alterniflora with WorldView-2/3 and Sentinel-1/2 Imagery in Zhangjiang Estuary, China. Water 2024, 16, 1780. [Google Scholar] [CrossRef]
- Vitharana, U.W.A.; Nora, J.C.; Darshani, K.; Umakant, M.; Karl, F.-H. Factors controlling the spatial heterogeneity of soil organic carbon concentrations and stocks in a boreal forest. Geoderma Reg. 2024, 36, e00749. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.; Ren, G.; Wang, J. Aboveground biomass of salt-marsh vegetation in coastal wetlands: Sample expansion of in situ hyperspectral and Sentinel-2 data using a generative adversarial network. Remote Sens. Environ. 2022, 270, 112885. [Google Scholar] [CrossRef]
- Peng, H.-B.; Shi, J.; Gan, X.; Zhang, J.; Ma, C.; Piersma, T.; Melville, D.S. Efficient removal of Spartina alterniflora with low negative environmental impacts using imazapyr. Front. Mar. Sci. 2022, 9, 1054402. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Liu, X.; Song, H.; Liu, S.; Qi, J.; Jing, F.; Gao, Z. Ecological analysis and multi-scenario simulation of Yellow River Delta wetland under clearing of Spartina alterniflora. Glob. Ecol. Conserv. 2024, 56, e03287. [Google Scholar] [CrossRef]
- Sha, C.; Wang, Z.; Cao, J.; Chen, J.; Shen, C.; Zhang, J.; Wang, Q.; Wang, M. Management of Spartina alterniflora: Assessing the Efficacy of Plant Growth Regulators on Ecological and Microbial Dynamics. Sustainability 2024, 16, 7848. [Google Scholar] [CrossRef]
- Song, Z.; Huang, Y.; Liu, Q.; Hu, X. Discovering the Characteristics of Community Structures and Functional Properties of Epiphytic Bacteria on Spartina alterniflora in the Coastal Salt Marsh Area. J. Mar. Sci. Eng. 2022, 10, 1981. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, Q.; Liao, A.; Xu, C.; Liu, M. Plant Adaptability and Vegetation Differentiation in the Coastal Beaches of Yellow–Bohai Sea in China. Int. J. Environ. Res. Public Health 2022, 19, 2225. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Wu, Z.; Zhang, S.; Wu, X.; Wang, H.; Bi, N. Temporal and Spatial Variation of Wetland Vegetation in the Yellow River(Huanghe)Mouth and Its Influencing Factors. Period. Ocean Univ. China 2022, 52, 81–94. [Google Scholar]
- Duan, H.; Yang, C.; Yu, X. Evaluation of historical and future coastal wetland change in the Yellow and Bohai Seas using satellite images and a land use model. J. Environ. Manag. 2024, 351, 119986. [Google Scholar] [CrossRef]
- Xie, B.; Han, G.; Qiao, P.; Mei, B.; Wang, Q.; Zhou, Y.; Zhang, A.; Song, W.; Guan, B. Effects of mechanical and chemical control on invasive Spartina alterniflora in the Yellow River Delta, China. PeerJ 2019, 7, e7655. [Google Scholar] [CrossRef]
- Yao, H.; Chen, M.; Huang, Z.; Huang, Y.; Wang, M.; Liu, Y. Remote sensing monitoring and potential distribution analysis of Spartina alterniflora in coastal zone of Guangxi. Ecol. Evol. 2024, 14, e11469. [Google Scholar] [CrossRef]
- Dong, D.; Chen, L.; Zou, Z.; Jiang, H.; Huang, H.; Wei, Z.; Xu, Y.; Zeng, J.; Tian, S. Monitoring coastal salt marsh area changes based on high-resolution satellite and unmanned aerial vehicle imagery in Guangxi. J. Appl. Oceanogr. 2024, 43, 84–94. [Google Scholar]
- Huang, Y.; Liu, Z.; Zheng, G.; Zhao, C. Identification of Spartina alterniflora habitat expansion in a Suaeda salsa dominated coastal wetlands. Ecol. Indic. 2022, 145, 109704. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Wang, Y.; Zhao, R.; Huang, M.; Wang, Y.; Yang, G.; Meng, X. Research progress and prospects of hyperspectral remote sensing for global wetland from 2010 to 2022. Natl. Remote Sens. Bull. 2023, 27, 1281–1299. [Google Scholar]
- Nie, M.; Liu, W.; Pennings, S.C.; Li, B. Lessons from the invasion of in coastal China. Ecology 2023, 104, e3874. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, G. Study on the change monitoring of typical estuarine wetland and its effect on ecological factors in Bohai Rim region, China. Front. Ecol. Evol. 2022, 10, 967593. [Google Scholar] [CrossRef]

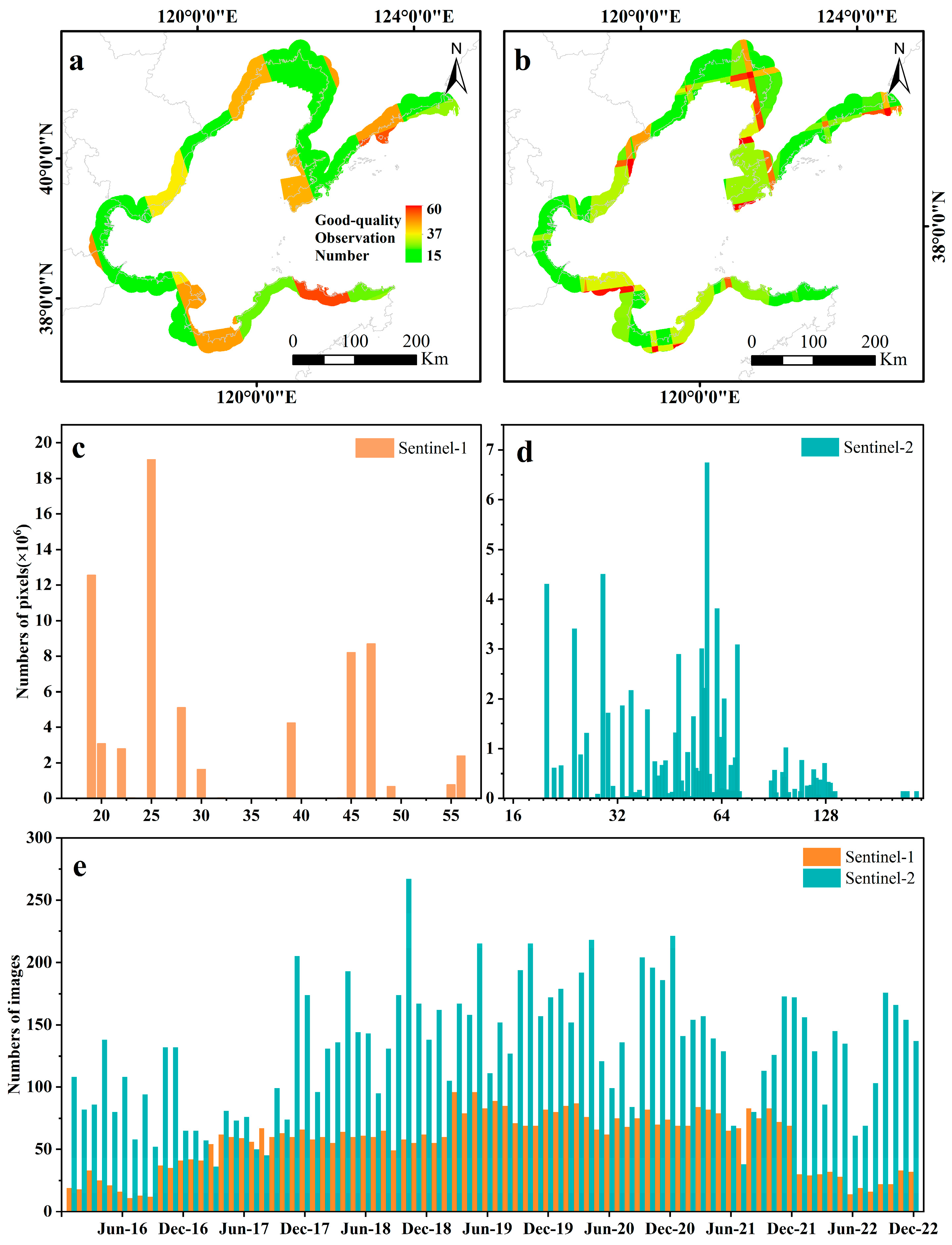
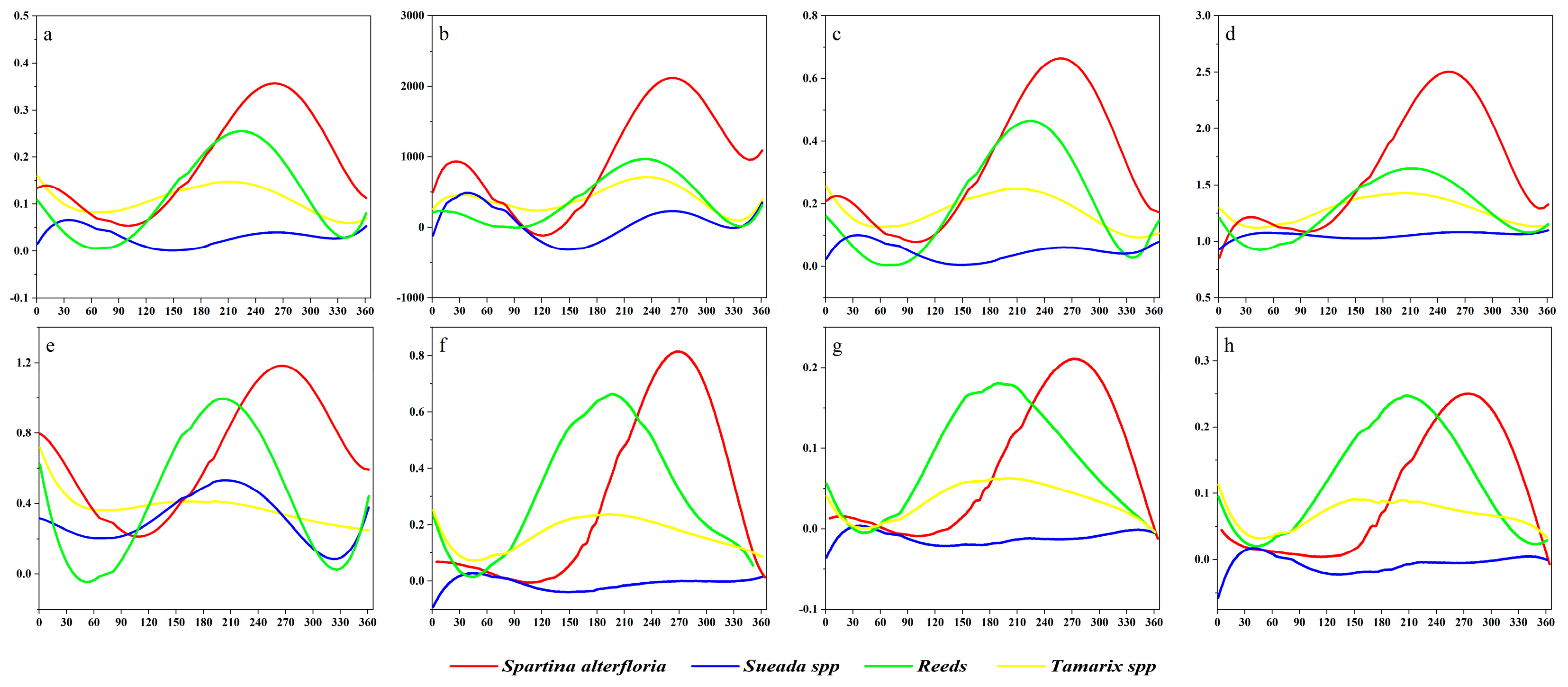
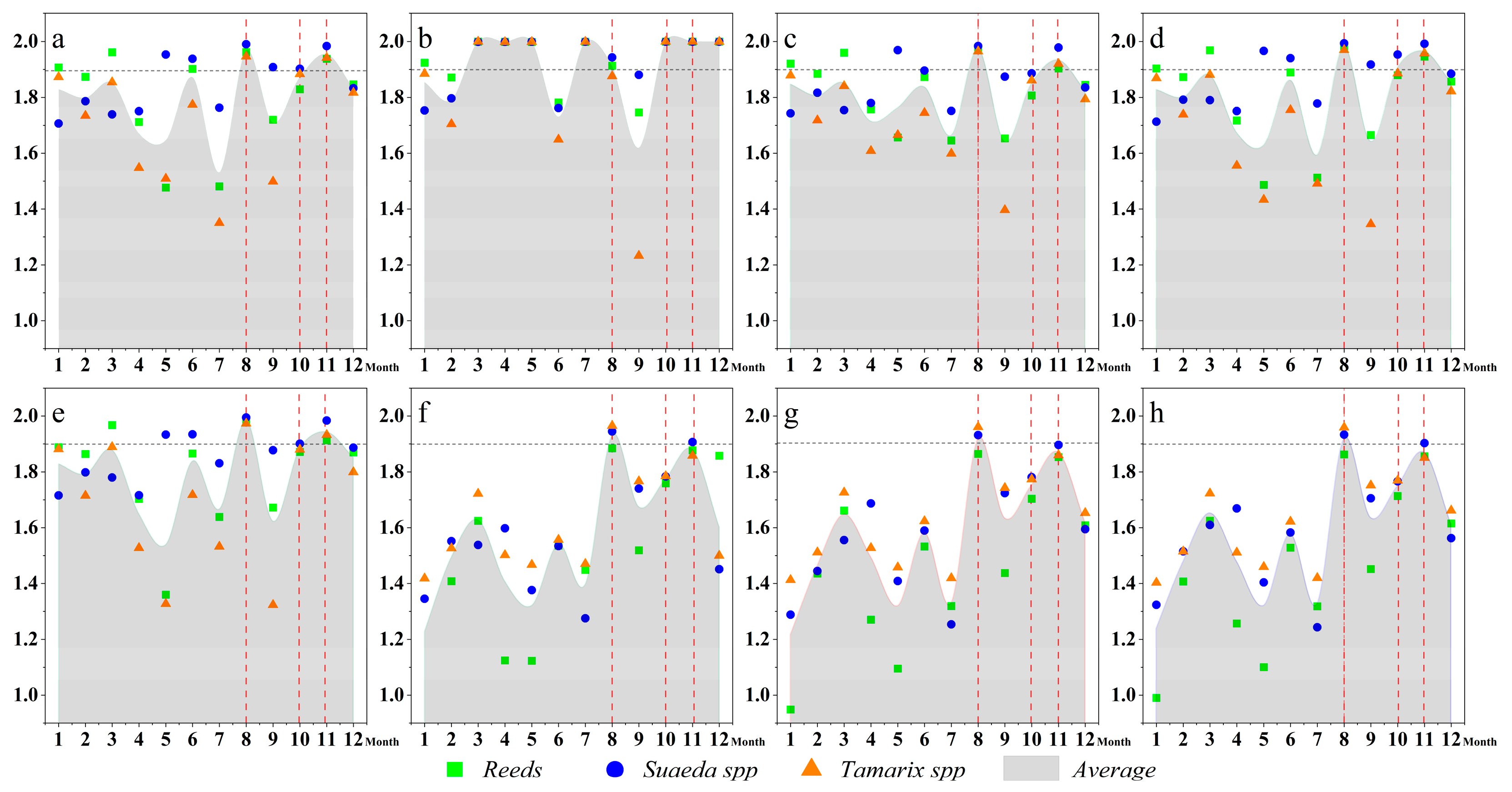
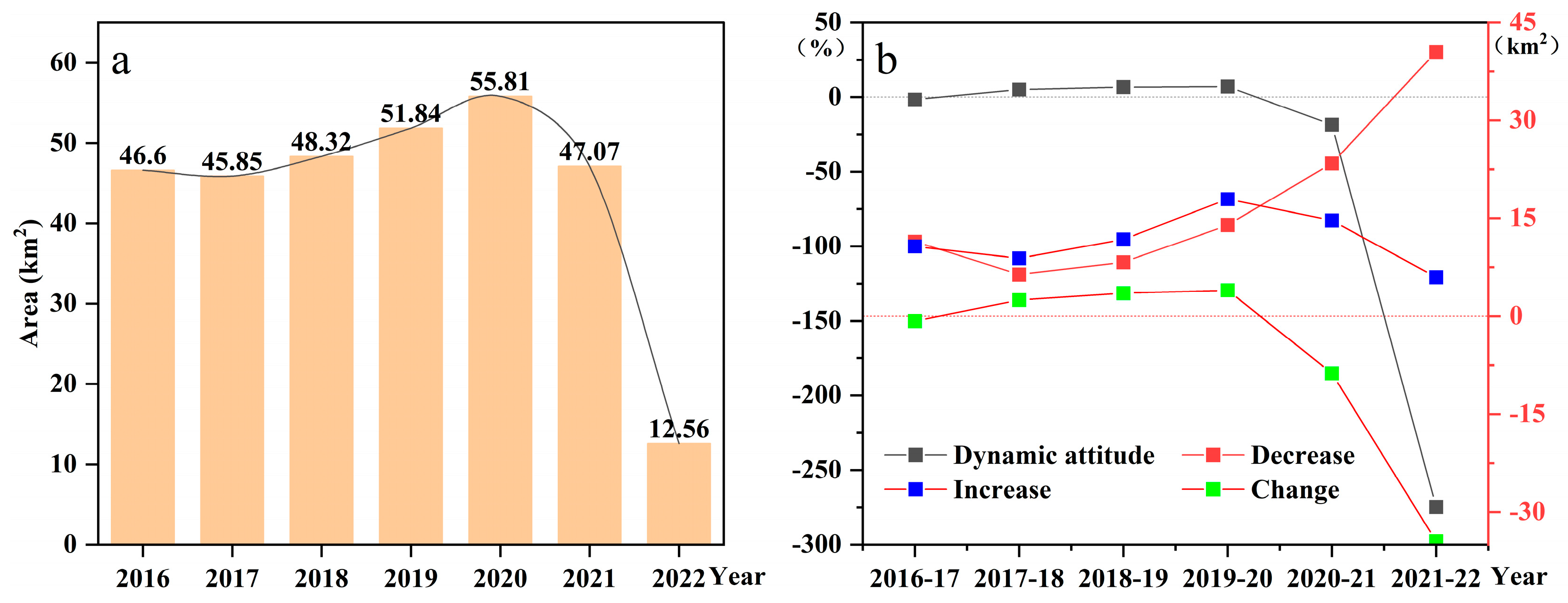
| Variables | Dataset | Source | Count |
|---|---|---|---|
| Bio1–19 | Climate factor | BIOCLIM WorldClim 2.1 dataset within the WorldClim database (https://worldclim.org/, accessed on 15 April 2024) | 19 |
| Dem Slope | Terrain factor | ASTER GDEM digital elevation data (https://www.gscloud.cn/, accessed on 18 April 2024) | 2 |
| Cec/pH_H2O/Nitrogen/Ocd/Ocs/Clay/Sand/Soc/Silt/Soiltype | Soil factor | SoilGrids global soil dataset (https://soilgrids.org/, accessed on 26 April 2024) | 9 |
| Seatemp | Marine factor | Sentinel-3 SLSTR (https://earthengine.google.com/, accessed on 26 April 2024) ImageCollection (“COPERNICUS/S3/OLCI”) | 2 |
| Seasalt | Global Ocean Data Assimilation Experiment (GODAE) ImageCollectio (“HYCOM/sea_temp_salinity”) |
| Index | Abbreviation | Equation | Source |
|---|---|---|---|
| Spectral Bands | B | B2, B3, B4, B5, B6, B7, B8 | Sentinel-2 |
| SAR Difference Index | SAR_D | VV − VH | Sentinel-1 |
| SAR Sum Index | SAR_S | VV + VH | |
| SAR Nominalized Difference Vegetation Index | SAR_N | (VV − VH)/(VV + VH) | |
| Nominalized Difference Vegetation Index | NDVI | Sentinel-2 | |
| Difference Vegetation Index | DVI | ||
| Enhanced Vegetation Index | EVI | ||
| Ratio Vegetation Index | RVI | ||
| Green Chlorophyll Vegetation Index | GCI | ||
| Red Edge Index | CIre | ||
| NDre1 | |||
| NDre2 |
| Variables | Environmental Variables | Dataset | Unit |
|---|---|---|---|
| Bio3 | Isothermality (Bio2/Bio7) (×100) | Climate factor | |
| Bio5 | Max temperature of warmest month | Climate factor | °C |
| Bio8 | Mean temperature of wettest quarter | Climate factor | °C |
| Bio10 | Mean temperature of warmest quarter | Climate factor | °C |
| Bio13 | Precipitation of wettest month | Climate factor | mm |
| Bio2 | Mean diurnal range | Climate factor | |
| Soc | Soil organic carbon content in the fine earth fraction | Soil factor | g/kg |
| Soiltype | Soil type | Soil factor | |
| Seatemp | Sea surface temperatures | Marine factor | °C |
| Year | Class | Non-SA | SA | Total | UA | Kappa | OA |
|---|---|---|---|---|---|---|---|
| 2016 | Non-SA | 269 | 2 | 271 | 0.993 | 0.933 | 0.975 |
| SA | 7 | 85 | 92 | 0.924 | |||
| Total | 276 | 87 | 363 | - | |||
| PA | 0.975 | 0.977 | - | - | |||
| 2017 | Non-SA | 223 | 4 | 227 | 0.982 | 0.978 | 0.947 |
| SA | 3 | 90 | 93 | 0.968 | |||
| Total | 226 | 94 | 320 | - | |||
| PA | 0.987 | 0.957 | - | - | |||
| 2018 | SA | 248 | 5 | 253 | 0.98 | 0.879 | 0.952 |
| Non-SA | 12 | 88 | 100 | 0.88 | |||
| Total | 260 | 93 | 353 | - | |||
| PA | 0.954 | 0.946 | - | - | |||
| 2019 | SA | 184 | 13 | 197 | 0.934 | 0.82 | 0.921 |
| Non-SA | 10 | 83 | 93 | 0.892 | |||
| Total | 194 | 96 | 290 | - | |||
| PA | 0.948 | 0.866 | - | - | |||
| 2020 | SA | 231 | 8 | 239 | 0.966 | 0.854 | 0.942 |
| Non-SA | 11 | 80 | 91 | 0.879 | |||
| Total | 241 | 88 | 330 | - | |||
| PA | 0.955 | 0.909 | - | - | |||
| 2021 | SA | 220 | 11 | 231 | 0.952 | 0.885 | 0.951 |
| Non-SA | 5 | 92 | 97 | 0.948 | |||
| Total | 225 | 103 | 328 | - | |||
| PA | 0.978 | 0.893 | - | - | |||
| 2022 | SA | 231 | 12 | 243 | 0.951 | 0.888 | 0.953 |
| Non-SA | 4 | 94 | 98 | 0.959 | |||
| Total | 235 | 106 | 341 | - | |||
| PA | 0.983 | 0.887 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Cui, G.; Liu, H.; Huang, X.; Xiao, X.; Wang, M.; Jia, M.; Mao, D.; Li, X.; Xiao, Y.; et al. Spatiotemporal Dynamics and Potential Distribution Prediction of Spartina alterniflora Invasion in Bohai Bay Based on Sentinel Time-Series Data and MaxEnt Modeling. Remote Sens. 2025, 17, 975. https://doi.org/10.3390/rs17060975
Wang Q, Cui G, Liu H, Huang X, Xiao X, Wang M, Jia M, Mao D, Li X, Xiao Y, et al. Spatiotemporal Dynamics and Potential Distribution Prediction of Spartina alterniflora Invasion in Bohai Bay Based on Sentinel Time-Series Data and MaxEnt Modeling. Remote Sensing. 2025; 17(6):975. https://doi.org/10.3390/rs17060975
Chicago/Turabian StyleWang, Qi, Guoli Cui, Haojie Liu, Xiao Huang, Xiangming Xiao, Ming Wang, Mingming Jia, Dehua Mao, Xiaoyan Li, Yihua Xiao, and et al. 2025. "Spatiotemporal Dynamics and Potential Distribution Prediction of Spartina alterniflora Invasion in Bohai Bay Based on Sentinel Time-Series Data and MaxEnt Modeling" Remote Sensing 17, no. 6: 975. https://doi.org/10.3390/rs17060975
APA StyleWang, Q., Cui, G., Liu, H., Huang, X., Xiao, X., Wang, M., Jia, M., Mao, D., Li, X., Xiao, Y., & Li, H. (2025). Spatiotemporal Dynamics and Potential Distribution Prediction of Spartina alterniflora Invasion in Bohai Bay Based on Sentinel Time-Series Data and MaxEnt Modeling. Remote Sensing, 17(6), 975. https://doi.org/10.3390/rs17060975










