Application of Optical Remote Sensing in Harmful Algal Blooms in Lakes: A Review
Abstract
:1. Introduction
2. Bibliometric Characteristics
3. Data and Methods for HAB Retrieval
3.1. Evolution of Satellite Technologies
3.1.1. Early Multispectral Sensors (1970s–1990s)
3.1.2. Multispectral Sensors with Better Features (2000s–2010s)
3.1.3. Hyperspectral Sensors (2010s–Present)
3.1.4. Complementary Approaches
3.2. Spectral-Based Methods and Applications
3.2.1. Spectral-Based Methods for Pigment Detection
3.2.2. Machine Learning and Deep Learning Methods for HABs Retrieval
3.2.3. Applications in Different Environments
4. Challenges and Future Opportunities
4.1. Limitation of ORS
4.1.1. Atmospheric Correction
4.1.2. Cloud Coverage and Shadow
4.1.3. Turbid Water
4.1.4. Wind and Lake Topography
4.1.5. Aquatic Vegetation and Vegetation on the Lakeshore
4.1.6. Validation
4.2. Current Directions
4.3. Future Opportunities
4.3.1. Defining Operational Thresholds for HABs Detection
4.3.2. Multi-Sensor Synergies and Data Fusion
4.3.3. Machine Learning
4.3.4. Future of HABs Detection
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Postel, S.L. Entering an Era of Water Scarcity: The Challenges Ahead. Ecol. Appl. 2000, 10, 941–948. [Google Scholar] [CrossRef]
- Pekel, J.-F.; Cottam, A.; Gorelick, N.; Belward, A.S. High-resolution Mapping of Global Surface Water and Its Long-term Changes. Nature 2016, 540, 418–422. [Google Scholar] [CrossRef]
- Yao, F.; Livneh, B.; Rajagopalan, B.; Wang, J.; Crétaux, J.-F.; Wada, Y.; Berge-Nguyen, M. Satellites Reveal Widespread Decline in Global Lake Water Storage. Science 2023, 380, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A Global Inventory of Lakes Based on High-resolution Satellite Imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Corman, J. Cleaner Chinese Lakes. Nat. Geosci. 2017, 10, 469–470. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, S.; Zhao, D.; Hu, C.; Xu, W.; Anderson, D.M.; Li, Y.; Song, X.-P.; Boyce, D.G.; Gibson, L.; et al. Coastal Phytoplankton Blooms Expand and Intensify in the 21st Century. Nature 2023, 615, 280–284. [Google Scholar] [CrossRef]
- Haig, H.A.; Chegoonian, A.M.; Davies, J.-M.; Bateson, D.; Leavitt, P.R. Marked Blue Discoloration of Late Winter Ice and Water Due to Autumn Blooms of Cyanobacteria. Lake Reserv. Manag. 2022, 38, 1–15. [Google Scholar] [CrossRef]
- Vilmin, L.; Bouwman, A.F.; Beusen, A.H.W.; van Hoek, W.J.; Mogollón, J.M. Past Anthropogenic Activities Offset Dissolved Inorganic Phosphorus Retention in the Mississippi River Basin. Biogeochemistry 2022, 161, 157–169. [Google Scholar] [CrossRef]
- Beusen, A.H.W.; Bouwman, A.F.; Van Beek, L.P.H.; Mogollón, J.M.; Middelburg, J.J. Global Riverine N and P Transport to Ocean Increased During the 20th Century Despite Increased Retention Along the Aquatic Continuum. Biogeosciences 2016, 13, 2441–2451. [Google Scholar] [CrossRef]
- Woolway, R.I.; Sharma, S.; Weyhenmeyer, G.A.; Debolskiy, A.; Golub, M.; Mercado-Bettín, D.; Perroud, M.; Stepanenko, V.; Tan, Z.; Grant, L.; et al. Phenological Shifts in Lake Stratification Under Climate Change. Nat. Commun. 2021, 12, 2318. [Google Scholar] [CrossRef]
- Hou, X.; Feng, L.; Dai, Y.; Hu, C.; Gibson, L.; Tang, J.; Lee, Z.; Wang, Y.; Cai, X.; Liu, J.; et al. Global Mapping Reveals Increase in Lacustrine Algal Blooms Over the Past Decade. Nat. Geosci. 2022, 15, 130–134. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread Global Increase in Intense Lake Phytoplankton Blooms Since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Schluchter, W.M. A Novel Algorithm for Predicting Phycocyanin Concentrations in Cyanobacteria: A Proximal Hyperspectral Remote Sensing Approach. Remote Sens. 2009, 1, 758–775. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial Blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. A Review of Harmful Algal Blooms and Their Apparent Global Increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baurès, E.; Thomas, O. State of Knowledge and Concerns on Cyanobacterial Blooms and Cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial Toxins: Risk Management for Health Protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Qin, B.; Paerl, H.W.; Brookes, J.D.; Liu, J.; Jeppesen, E.; Zhu, G.; Zhang, Y.; Xu, H.; Shi, K.; Deng, J. Why Lake Taihu Continues to Be Plagued with Cyanobacterial Blooms Through 10 Years (2007–2017) Efforts. Sci. Bull. 2019, 64, 354–356. [Google Scholar] [CrossRef]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins Produced in Cyanobacterial Water Blooms—Toxicity and Risks. Interdiscip. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M. Harmful Algal Blooms and Climate Change: Learning from the Past and Present to Forecast the Future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic Conditions Trigger Record Harmful Algal Bloom in Western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Jang, W.; Park, Y.; Pyo, J.; Park, S.; Kim, J.; Kim, J.H.; Cho, K.H.; Shin, J.-K.; Kim, S. Optimal Band Selection for Airborne Hyperspectral Imagery to Retrieve a Wide Range of Cyanobacterial Pigment Concentration Using a Data-driven Approach. Remote Sens. 2022, 14, 1754. [Google Scholar] [CrossRef]
- Reinl, K.L.; Brookes, J.D.; Carey, C.C.; Harris, T.D.; Ibelings, B.W.; Morales-Williams, A.M.; De Senerpont Domis, L.N.; Atkins, K.S.; Isles, P.D.F.; Mesman, J.P.; et al. Cyanobacterial Blooms in Oligotrophic Lakes: Shifting the High-nutrient Paradigm. Freshw. Biol. 2021, 66, 1846–1859. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Qin, B.; Zhou, B. Remote Sensing of Cyanobacterial Blooms in Inland Waters: Present Knowledge and Future Challenges. Sci. Bull. 2019, 64, 1540–1556. [Google Scholar] [CrossRef]
- Murray, C. Remote Sensing of Water Color to Assess Water Quality in a Changing Climate. Master’s thesis, University of Rhode Island, Kingston, RI, USA, 2022. [Google Scholar]
- Strong, A.E. Remote Sensing of Algal Blooms by Aircraft and Satellite in Lake Erie and Utah Lake. Remote Sens. Environ. 1974, 3, 99–107. [Google Scholar] [CrossRef]
- Clark, J.M.; Schaeffer, B.A.; Darling, J.A.; Urquhart, E.A.; Johnston, J.M.; Ignatius, A.R.; Myer, M.H.; Loftin, K.A.; Werdell, P.J.; Stumpf, R.P. Satellite Monitoring of Cyanobacterial Harmful Algal Bloom Frequency in Recreational Waters and Drinking Water Sources. Ecol. Indic. 2017, 80, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Wynne, T.T.; Stumpf, R.P. Spatial and Temporal Patterns in the Seasonal Distribution of Toxic Cyanobacteria in Western Lake Erie from 2002–2014. Toxins 2015, 7, 1649–1663. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Hou, X.; Qin, B.; Kuster, T.; Qu, F.; Chen, N.; Paerl, H.W.; Zheng, C. Harmful Algal Blooms in Inland Waters. Nat. Rev. Earth Environ. 2024, 5, 631–644. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Simis, S.G.H.; Kauko, H.M. In Vivo Mass-specific Absorption Spectra of Phycobilipigments Through Selective Bleaching. Limnol. Oceanogr. Methods 2012, 10, 214–226. [Google Scholar] [CrossRef]
- Yacobi, Y.Z.; Köhler, J.; Leunert, F.; Gitelson, A. Phycocyanin-specific Absorption Coefficient: Eliminating the Effect of Chlorophylls Absorption. Limnol. Oceanogr. Methods 2015, 13, 157–168. [Google Scholar] [CrossRef]
- Woźniak, M.; Bradtke, K.M.; Darecki, M.; Krężel, A. Empirical Model for Phycocyanin Concentration Estimation As an Indicator of Cyanobacterial Bloom in the Optically Complex Coastal Waters of the Baltic Sea. Remote Sens. 2016, 8, 212. [Google Scholar] [CrossRef]
- Jensen, J.R. Remote Sensing of the Environment: An Earth Resource Perspective 2/e; Pearson Education India: Chennai, India, 2009. [Google Scholar]
- Thiemann, S. The origin of the reflectance peak near 700 nm in chlorophyll-a laden waters—An experiment. In Proceedings of the IEEE 1999 International Geoscience and Remote Sensing Symposium (IGARSS’99), Hamburg, Germany, 28 June–2 July 1999; Volume 2, pp. 1146–1148. [Google Scholar]
- Zhao, D.; Xing, X.; Liu, Y.; Yang, J.; Wang, L. The Relation of Chlorophyll-a Concentration with the Reflectance Peak Near 700 Nm in Algae-dominated Waters and Sensitivity of Fluorescence Algorithms for Detecting Algal Bloom. Int. J. Remote Sens. 2010, 31, 39–48. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Robertson, L. An Algorithm for Detecting Trophic Status (chlorophyll-a), Cyanobacterial-dominance, Surface Scums and Floating Vegetation in Inland and Coastal Waters. Remote Sens. Environ. 2012, 124, 637–652. [Google Scholar] [CrossRef]
- Sayers, M.J.; Grimm, A.G.; Shuchman, R.A.; Bosse, K.R.; Fahnenstiel, G.L.; Ruberg, S.A.; Leshkevich, G.A. Satellite Monitoring of Harmful Algal Blooms in the Western Basin of Lake Erie: A 20-year Time-series. J. Great Lakes Res. 2019, 45, 508–521. [Google Scholar] [CrossRef]
- Sultan, M.; Becker, R. Monitoring Algal Blooms in Inland Waters from Space-Borne Observation; A Case Study from Northern Africa, Lake Nasser. 2004, p. B41A-11. Available online: https://ui.adsabs.harvard.edu/abs/2004AGUSM.B41A..11S/abstract (accessed on 23 March 2024).
- Binding, C.E.; Jerome, J.H.; Bukata, R.P.; Booty, W.G. Trends in Water Clarity of the Lower Great Lakes from Remotely Sensed Aquatic Color. J. Great Lakes Res. 2007, 33, 828–841. [Google Scholar] [CrossRef]
- Shuchman, R.; Korosov, A.; Hatt, C.; Pozdnyakov, D.; Means, J.; Meadows, G. Verification and Application of a Bio-optical Algorithm for Lake Michigan Using Seawifs: A 7-year Inter-annual Analysis. J. Great Lakes Res. 2006, 32, 258–279. [Google Scholar] [CrossRef]
- Budd, J.W.; Beeton, A.M.; Stumpf, R.P.; Culver, D.A.; Charles Kerfoot, W. Satellite Observations of Microcystis Blooms in Western Lake Erie. Sil Proc. 1922–2010 2001, 27, 3787–3793. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Xie, Z.; Xie, X. Integrated Application of Multi-sources Remotely Sensed Imagery in Cyanobacteria Bloom Monitoring. Remote Sens. Technol. Appl. 2013, 28, 569–575. [Google Scholar] [CrossRef]
- Bao, Y.; Tian, Q. Spatial Scale of Chlorophyll-a Concentration in Lake Taihu by Using Remote Sensing Images. In Proceedings of the Seventeenth China Symposium on Remote Sensing, Hangzhou, China, 27–31 August 2011; p. 820314. [Google Scholar]
- Sheng, Y.; Su, Y.; Xiao, Q. Challenging the Cloud-contamination Problem in Flood Monitoring with NOAA/AVHRR Imagery. Photogramm. Eng. Remote Sens. 1998, 64, 191–198. [Google Scholar] [CrossRef]
- Wulder, M.A.; Loveland, T.R.; Roy, D.P.; Crawford, C.J.; Masek, J.G.; Woodcock, C.E.; Allen, R.G.; Anderson, M.C.; Belward, A.S.; Cohen, W.B.; et al. Current Status of Landsat Program, Science, and Applications. Remote Sens. Environ. 2019, 225, 127–147. [Google Scholar] [CrossRef]
- Ho, J.C.; Stumpf, R.P.; Bridgeman, T.B.; Michalak, A.M. Using Landsat to Extend the Historical Record of Lacustrine Phytoplankton Blooms: A Lake Erie Case Study. Remote Sens. Environ. 2017, 191, 273–285. [Google Scholar] [CrossRef]
- Qing, S.; Runa, A.; Shun, B.; Zhao, W.; Bao, Y.; Hao, Y. Distinguishing and Mapping of Aquatic Vegetations and Yellow Algae Bloom with Landsat Satellite Data in a Complex Shallow Lake, China During 1986–2018. Ecol. Indic. 2020, 112, 106073. [Google Scholar] [CrossRef]
- Liu, D.; Ding, H.; Han, X.; Lang, Y.; Chen, W. Mapping Algal Blooms in Aquatic Ecosystems Using Long-term Landsat Data: A Case Study of Yuqiao Reservoir from 1984–2022. Remote Sens. 2023, 15, 4317. [Google Scholar] [CrossRef]
- Isenstein, E.M.; Kim, D.; Park, M.-H. Modeling for Multi-temporal Cyanobacterial Bloom Dominance and Distributions Using Landsat Imagery. Ecol. Inform. 2020, 59, 101119. [Google Scholar] [CrossRef]
- Ma, J.; Loiselle, S.; Cao, Z.; Qi, T.; Shen, M.; Luo, J.; Song, K.; Duan, H. Unbalanced Impacts of Nature and Nurture Factors on the Phenology, Area and Intensity of Algal Blooms in Global Large Lakes: Modis Observations. Sci. Total Environ. 2023, 880, 163376. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Zhou, Y.; Liu, X.; Zhu, G.; Qin, B.; Gao, G. Long-term Modis Observations of Cyanobacterial Dynamics in Lake Taihu: Responses to Nutrient Enrichment and Meteorological Factors. Sci. Rep. 2017, 7, 40326. [Google Scholar] [CrossRef]
- Lai, L.; Liu, Y.; Zhang, Y.; Cao, Z.; Yang, Q.; Chen, X. Modis Terra and Aqua Images Bring Non-negligible Effects to Phytoplankton Blooms Derived from Satellites in Eutrophic Lakes. Water Res. 2023, 246, 120685. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, M.; Kutser, T.; Liu, M.; Qi, T.; Ma, J.; Ma, R.; Duan, H. What Water Color Parameters Could Be Mapped Using Modis Land Reflectance Products: A Global Evaluation Over Coastal and Inland Waters. Earth-Sci. Rev. 2022, 232, 104154. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S.; Winter, K. Remote Sensing of Cyanobacteria-dominant Algal Blooms and Water Quality Parameters in Zeekoevlei, a Small Hypertrophic Lake, Using Meris. Remote Sens. Environ. 2010, 114, 2070–2087. [Google Scholar] [CrossRef]
- Wheeler, S.M.; Morrissey, L.A.; Levine, S.N.; Livingston, G.P.; Vincent, W.F. Mapping Cyanobacterial Blooms in Lake Champlain’s Missisquoi Bay Using Quickbird and Meris Satellite Data. J. Great Lakes Res. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Tyler, A.N.; Hunter, P.D.; Spyrakos, E.; Groom, S.; Constantinescu, A.M.; Kitchen, J. Developments in Earth Observation for the Assessment and Monitoring of Inland, Transitional, Coastal and Shelf-sea Waters. Sci. Total Environ. 2016, 572, 1307–1321. [Google Scholar] [CrossRef] [PubMed]
- Kutser, T.; Metsamaa, L.; Strömbeck, N.; Vahtmäe, E. Monitoring Cyanobacterial Blooms by Satellite Remote Sensing. Estuar. Coast. Shelf Sci. 2006, 67, 303–312. [Google Scholar] [CrossRef]
- Soomets, T.; Uudeberg, K.; Jakovels, D.; Zagars, M.; Reinart, A.; Brauns, A.; Kutser, T. Comparison of Lake Optical Water Types Derived from Sentinel-2 and Sentinel-3. Remote Sens. 2019, 11, 2883. [Google Scholar] [CrossRef]
- Masoud, A.A. On the Retrieval of the Water Quality Parameters from Sentinel-3/2 and Landsat-8 Oli in the Nile Delta’s Coastal and Inland Waters. Water 2022, 14, 593. [Google Scholar] [CrossRef]
- Maltese, A.; Capodici, F.; Ciraolo, G.; Corbari, C.; Granata, A.; La Loggia, G. Planktothrix rubescens in Freshwater Reservoirs: The Sentinel-2 Potentiality for Mapping Phycocyanin Concentration. In Proceedings of the First Sentinel-2 Preparatory Symposium, Frascati, Italy, 23–27 April 2012; Volume 707, p. 37. [Google Scholar]
- Akbarnejad Nesheli, S.; Quackenbush, L.J.; McCaffrey, L. Estimating Chlorophyll-a and Phycocyanin Concentrations in Inland Temperate Lakes Across New York State Using Sentinel-2 Images: Application of Google Earth Engine for Efficient Satellite Image Processing. Remote Sens. 2024, 16, 3504. [Google Scholar] [CrossRef]
- Ma, J.; Qin, B.; Paerl, H.W.; Brookes, J.D.; Hall, N.S.; Shi, K.; Zhou, Y.; Guo, J.; Li, Z.; Xu, H.; et al. The Persistence of Cyanobacterial (Microcystis spp.) Blooms Throughout Winter in Lake Taihu, China. Limnol. Oceanogr. 2016, 61, 711–722. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Y.; Tuo, Y.; Cao, R.; Zhou, Z.; Xiao, Y. Study on the Temporal and Spatial Distribution of Chlorophyll a in Erhai Lake Based on Multispectral Data from Environmental Satellites. Ecol. Inform. 2021, 61, 101201. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Ma, R.; Li, F.; Duan, H.; Hu, W.; Qin, B.; Huang, W. Applying Remote Sensing Techniques to Monitoring Seasonal and Interannual Changes of Aquatic Vegetation in Taihu Lake, China. Ecol. Indic. 2016, 60, 503–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Zhang, Y.; Moreno-Madriñán, M.J.; Zhu, G.; Zhou, Y.; Yao, X. Long-term Change of Total Suspended Matter in a Deep-valley Reservoir with HJ-1a/b: Implications for Reservoir Management. Environ. Sci. Pollut. Res. 2019, 26, 3041–3054. [Google Scholar] [CrossRef]
- Qi, L.; Hu, C.; Visser, P.M.; Ma, R. Diurnal Changes of Cyanobacteria Blooms in Taihu Lake As Derived from Goci Observations. Limnol. Oceanogr. 2018, 63, 1711–1726. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Xu, X.; Kong, F.; Zhang, S.; Kong, W.; Hao, J.; Shang, L. Two-decade Reconstruction of Algal Blooms in China’s Lake Taihu. Environ. Sci. Technol. 2009, 43, 3522–3528. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, C.S.; Gregg, W.W.; Ott, L. Assessing the Skills of a Seasonal Forecast of Chlorophyll in the Global Pelagic Oceans. Remote Sens. 2021, 13, 1051. [Google Scholar] [CrossRef]
- Bhatnagar, R.; Raman, M. Comparative Analysis of Chlorophyll-a Measurements of Oceansat-2 Ocm and Suomi Npp-Viirs Over Arabian Sea. In Proceedings of the 2021 IEEE International India Geoscience and Remote Sensing Symposium (InGARSS), Ahmedabad, India, 6–10 December 2021; pp. 94–97. [Google Scholar]
- Cao, M.; Qing, S.; Jin, E.; Hao, Y.; Zhao, W. A Spectral Index for the Detection of Algal Blooms Using Sentinel-2 Multispectral Instrument (msi) Imagery: A Case Study of Hulun Lake, China. Int. J. Remote Sens. 2021, 42, 4514–4535. [Google Scholar] [CrossRef]
- Hou, X.; Feng, L. High-resolution Satellite Observations Reveal Extensive Algal Blooms in Both Small and Large Lakes in China. Sustain. Horiz. 2023, 6, 100054. [Google Scholar] [CrossRef]
- Mbuh, M. Use of Hyperspectral Remote Sensing to Estimate Water Quality. In Processing and Analysis of Hyperspectral Data; Chen, J., Song, Y., Li, H., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Flores, A.; Griffin, R.; Dix, M.; Romero-Oliva, C.S.; Ochaeta, G.; Skinner-Alvarado, J.; Moran, M.V.R.; Hernandez, B.; Cherrington, E.; Page, B.; et al. Hyperspectral Satellite Remote Sensing of Water Quality in Lake Atitlán, Guatemala. Front. Environ. Sci. 2020, 8, 7. [Google Scholar] [CrossRef]
- Giardino, C.; Brando, V.E.; Dekker, A.G.; Strömbeck, N.; Candiani, G. Assessment of Water Quality in Lake Garda (Italy) Using Hyperion. Remote Sens. Environ. 2007, 109, 183–195. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Pan, D.; Yang, S.X.; Gharabaghi, B. Review of Recent Advances in Remote Sensing and Machine Learning Methods for Lake Water Quality Management. Remote Sens. 2024, 16, 4196. [Google Scholar] [CrossRef]
- Amani, M.; Ghorbanian, A.; Ahmadi, S.A.; Kakooei, M.; Moghimi, A.; Mirmazloumi, S.M.; Moghaddam, S.H.A.; Mahdavi, S.; Ghahremanloo, M.; Parsian, S.; et al. Google Earth Engine Cloud Computing Platform for Remote Sensing Big Data Applications: A Comprehensive Review. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 5326–5350. [Google Scholar] [CrossRef]
- Legleiter, C.J.; King, T.V.; Carpenter, K.D.; Hall, N.C.; Mumford, A.C.; Slonecker, T.; Graham, J.L.; Stengel, V.G.; Simon, N.; Rosen, B.H. Spectral Mixture Analysis for Surveillance of Harmful Algal Blooms (smash): A Field-, Laboratory-, and Satellite-based Approach to Identifying Cyanobacteria Genera from Remotely Sensed Data. Remote Sens. Environ. 2022, 279, 113089. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L.; Lee, Z.; Davis, C.O.; Mannino, A.; McClain, C.R.; Franz, B.A. Dynamic Range and Sensitivity Requirements of Satellite Ocean Color Sensors: Learning from the Past. Appl. Opt. 2012, 51, 6045–6062. [Google Scholar] [CrossRef]
- Hu, C. Hyperspectral reflectance spectra of floating matters derived from Hyperspectral Imager for the Coastal Ocean (HICO) observations. Earth Syst. Sci. Data 2022, 14, 1183–1192. [Google Scholar] [CrossRef]
- Amieva, J.F.; Oxoli, D.; Brovelli, M.A. Machine and Deep Learning Regression of Chlorophyll-a Concentrations in Lakes Using Prisma Satellite Hyperspectral Imagery. Remote Sens. 2023, 15, 5385. [Google Scholar] [CrossRef]
- Ruddick, K.; Vanhellemont, Q.; Dogliotti, A.; Nechad, B.; Pringle, N.; Van der Zande, D. New Opportunities and Challenges for High Resolution Remote Sensing of Water Colour. In Proceedings of the Ocean Optics XXIII Conference, Victoria, BC, Canada, 23–28 October 2016; Available online: https://www.researchgate.net/publication/309592981_New_opportunities_and_challenges_for_high_resolution_remote_sensing_of_water_colour (accessed on 10 April 2024).
- Saberioon, M.; Khosravi, V.; Brom, J.; Gholizadeh, A.; Segl, K. Examining the Sensitivity of Simulated Enmap Data for Estimating Chlorophyll-a and Total Suspended Solids in Inland Waters. Ecol. Inform. 2023, 75, 102058. [Google Scholar] [CrossRef]
- Hu, C.; Feng, L. GOES Imager Shows Diurnal Changes of a Trichodesmium erythraeum Bloom on the West Florida Shelf. IEEE Geosci. Remote Sens. Lett. 2014, 11, 1428–1432. [Google Scholar] [CrossRef]
- Uz, S.S.; Kim, G.E.; Mannino, A.; Werdell, P.J.; Tzortziou, M. Developing a Community of Practice for Applied Uses of Future PACE Data to Address Marine Food Security Challenges. Front. Earth Sci. 2019, 7, 283. [Google Scholar] [CrossRef]
- Fang, C.; Song, K.; Yan, Z.; Liu, G. Monitoring Phycocyanin in Global Inland Waters by Remote Sensing: Progress and Future Developments. Water Res. 2025, 275, 123176. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fazli, S.; Maharjan, S.; El-Askary, H. Deciphering Water Quality and Algal Dynamics in Clear Lake Through Hyperspectral Analysis Using Emit Data. In Proceedings of the IGARSS 2024—2024 IEEE International Geoscience and Remote Sensing Symposium, Athens, Greece, 7–12 July 2024; pp. 9302–9306. [Google Scholar]
- Cao, Z.; Ma, R.; Duan, H.; Xue, K. Effects of Broad Bandwidth on the Remote Sensing of Inland Waters: Implications for High Spatial Resolution Satellite Data Applications. ISPRS J. Photogramm. Remote Sens. 2019, 153, 110–122. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, C.; Li, Y.; Lyu, H.; Huang, C.; Chen, N.; Liu, G.; Guo, Y.; Lei, S.; Zhang, R.; et al. A Semi-analytical Model to Estimate Chlorophyll-a Spatial-temporal Patterns from Orbita Hyperspectral Image in Inland Eutrophic Waters. Sci. Total Environ. 2023, 904, 166785. [Google Scholar] [CrossRef]
- Toth, C.; Jóźków, G. Remote Sensing Platforms and Sensors: A Survey. ISPRS J. Photogramm. Remote Sens. 2016, 115, 22–36. [Google Scholar] [CrossRef]
- Kislik, C.; Dronova, I.; Kelly, M. Uavs in Support of Algal Bloom Research: A Review of Current Applications and Future Opportunities. Drones 2018, 2, 35. [Google Scholar] [CrossRef]
- Arias, F.; Zambrano, M.; Galagarza, E.; Broce, K. Mapping Harmful Algae Blooms: The Potential of Hyperspectral Imaging Technologies. Remote Sens. 2025, 17, 608. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, C.; Qiu, Y.; Jia, W.; Gao, C.; Duan, H. A Three-step Machine Learning Approach for Algal Bloom Detection Using Stationary Rgb Camera Images. Int. J. Appl. Earth Obs. Geoinf. 2023, 122, 103421. [Google Scholar] [CrossRef]
- Cook, K.V.; Beyer, J.E.; Xiao, X.; Hambright, K.D. Ground-based Remote Sensing Provides Alternative to Satellites for Monitoring Cyanobacteria in Small Lakes. Water Res. 2023, 242, 120076. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, K.; Zhang, Y.; Li, N.; Sun, X.; Zhang, D.; Zhang, Y.; Qin, B.; Zhu, G. A Ground-based Remote Sensing System for High-frequency and Real-time Monitoring of Phytoplankton Blooms. J. Hazard. Mater. 2022, 439, 129623. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, J.; Luo, J.; Xiao, Q.; Shen, M.; Xiao, P.; Peng, Z.; Jiao, Y.; Duan, H. Monitoring, Simulation and Early Warning of Cyanobacterial Harmful Algal Blooms: An Upgraded Framework for Eutrophic Lakes. Environ. Res. 2025, 264, 120296. [Google Scholar] [CrossRef]
- Kutser, T. Quantitative Detection of Chlorophyll in Cyanobacterial Blooms by Satellite Remote Sensing. Limnol. Oceanogr. 2004, 49, 2179–2189. [Google Scholar] [CrossRef]
- Song, K.; Fang, C.; Jacinthe, P.-A.; Wen, Z.; Liu, G.; Xu, X.; Shang, Y.; Lyu, L. Climatic Versus Anthropogenic Controls of Decadal Trends (1983–2017) in Algal Blooms in Lakes and Reservoirs Across China. Environ. Sci. Technol. 2021, 55, 2929–2938. [Google Scholar] [CrossRef]
- Sakuno, Y.; Kunii, H. Cyanobacteria Bloom Mapping Using Satellite Data in Brackish Lake Shinji and Lake Nakaumi. In Proceedings of the 2011 IEEE International Geoscience and Remote Sensing Symposium, Vancouver, BC, Canada, 24–29 July 2011; pp. 2029–2032. [Google Scholar]
- Mishra, S.; Stumpf, R.P.; Schaeffer, B.A.; Werdell, P.J.; Loftin, K.A.; Meredith, A. Measurement of Cyanobacterial Bloom Magnitude Using Satellite Remote Sensing. Sci. Rep. 2019, 9, 18310. [Google Scholar] [CrossRef]
- Binding, C.E.; Zastepa, A.; Zeng, C. The Impact of Phytoplankton Community Composition on Optical Properties and Satellite Observations of the 2017 Western Lake Erie Algal Bloom. J. Great Lakes Res. 2019, 45, 573–586. [Google Scholar] [CrossRef]
- Hu, C. A Novel Ocean Color Index to Detect Floating Algae in the Global Oceans. Remote Sens. Environ. 2009, 113, 2118–2129. [Google Scholar] [CrossRef]
- Ma, J.; Jin, S.; Li, J.; He, Y.; Shang, W. Spatio-Temporal Variations and Driving Forces of Harmful Algal Blooms in Chaohu Lake: A Multi-Source Remote Sensing Approach. Remote Sens. 2021, 13, 427. [Google Scholar] [CrossRef]
- Das, S.; Nandi, D.; Thakur, R.R.; Bera, D.K.; Behera, D.; Đurin, B.; Cetl, V. A Novel Approach for Ex Situ Water Quality Monitoring Using the Google Earth Engine and Spectral Indices in Chilika Lake, Odisha, India. ISPRS Int. J. Geo-Inf. 2024, 13, 381. [Google Scholar] [CrossRef]
- Gower, J.; King, S.; Borstad, G.; Brown, L. Detection of Intense Plankton Blooms Using the 709 nm Band of the Meris Imaging Spectrometer. Int. J. Remote Sens. 2005, 26, 2005–2012. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Gu, K.-J.; Zhang, S.-M. Extracting Temporal and Spatial Distribution Features of Lake Taihu from MODIS-EVI Data by Empirical Orthogonal Function Analysis. Chin. J. Ecol. 2018, 37, 3802–3808. [Google Scholar] [CrossRef]
- Fang, C.; Song, K.; Shang, Y.; Jianhang, M.; Wen, Z.; Du, J. Remote Sensing of Harmful Algal Blooms Variability for Lake Hulun Using Adjusted FAI (AFAI) Algorithm. J. Environ. Inform. 2018, 34, 108–122. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Warner, R.A.; Tester, P.A.; Dyble, J.; Fahnenstiel, G.L. Relating Spectral Shape to Cyanobacterial Blooms in the Laurentian Great Lakes. Int. J. Remote Sens. 2008, 29, 3665–3672. [Google Scholar] [CrossRef]
- Zhou, B.; Shang, M.; Wang, G.; Zhang, S.; Feng, L.; Liu, X.; Wu, L.; Shan, K. Distinguishing Two Phenotypes of Blooms Using the Normalised Difference Peak-valley Index (NDPI) and Cyano-Chlorophyta index (CCI). Sci. Total Environ. 2018, 628–629, 848–857. [Google Scholar] [CrossRef]
- Xing, Q.G.; Hu, C.M. Mapping Macroalgal Blooms in the Yellow Sea and East China Sea Using HJ-1 and Landsat Data: Application of a Virtual Baseline Reflectance Height Technique. Remote Sens. Environ. 2016, 178, 113–126. [Google Scholar] [CrossRef]
- Gower, J.F.R.; Doerffer, R.; Borstad, G.A. Interpretation of the 685nm Peak in Water-leaving Radiance Spectra in Terms of Fluorescence, Absorption and Scattering, and Its Observation by Meris. Int. J. Remote Sens. 1999, 20, 1771–1786. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Peters, S.W.M.; Gons, H.J. Remote Sensing of the Cyanobacterial Pigment Phycocyanin in Turbid Inland Water. Limnol. Oceanogr. 2005, 50, 237–245. [Google Scholar] [CrossRef]
- Shi, K.; Li, Y.; Zhang, Y.; Li, L.; Lv, H.; Song, K. Classification of Inland Waters Based on Bio-optical Properties. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 543–561. [Google Scholar] [CrossRef]
- Ueno, Y.; Aikawa, S.; Kondo, A.; Akimoto, S. Adaptation of Light-harvesting Functions of Unicellular Green Algae to Different Light Qualities. Photosynth. Res. 2019, 139, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Stauber, J.L.; Jeffrey, S.W. Photosynthetic Pigments in Fifty-one Species of Marine Diatoms. J. Phycol. 1988, 24, 158–172. [Google Scholar] [CrossRef]
- Aizaki, M.; Fukushima, T.; Takagi, H.; Kitamura, H. Evaluation of Lake Kasumigaura, Japan, Using a Landscape Index for Cyanobacterial Bloom. Aoko (Water-Blooms of Blue-Green Algae), Measurement, Occurrence, and Factors on Its Growth; National Institute for Environmental Studies: Tsukuba, Japan, 1995; pp. 33–39.
- Fang, C.; Song, K.; Li, L.; Wen, Z.; Liu, G.; Du, J.; Shang, Y.; Zhao, Y. Spatial Variability and Temporal Dynamics of Habs in Northeast China. Ecol. Indic. 2018, 90, 280–294. [Google Scholar] [CrossRef]
- Kiage, L.M.; Walker, N.D. Using Ndvi from Modis to Monitor Duckweed Bloom in Lake Maracaibo, Venezuela. Water Resour. Manag. 2009, 23, 1125–1135. [Google Scholar] [CrossRef]
- Duan, H.; Ma, R.; Zhang, Y.; Loiselle, S.A. Are Algal Blooms Occurring Later in Lake Taihu? Climate Local Effects Outcompete Mitigation Prevention. J. Plankton Res. 2014, 36, 866–871. [Google Scholar] [CrossRef]
- Hu, C.; Lee, Z.; Ma, R.; Yu, K.; Li, D.; Shang, S. Moderate Resolution Imaging Spectroradiometer (modis) Observations of Cyanobacteria Blooms in Taihu Lake, China. J. Geophys. Res. Ocean. 2010, 115, C04002. [Google Scholar] [CrossRef]
- Li, Y.; Tao, J.; Zhang, Y.; Shi, K.; Chang, J.; Pan, M.; Song, L.; Jeppesen, E.; Zhou, Q. Urbanization Shifts Long-term Phenology and Severity of Phytoplankton Blooms in an Urban Lake Through Different Pathways. Glob. Change Biol. 2023, 29, 4983–4999. [Google Scholar] [CrossRef]
- Gower, J.; King, S. A Global Survey of Intense Surface Plankton Blooms and Floating Vegetation Using MERIS MCI. In Remote Sensing of the Changing Oceans; Springer: Berlin/Heidelberg, Germany, 2011; pp. 99–121. [Google Scholar] [CrossRef]
- O’Shea, R.E.; Pahlevan, N.; Smith, B.; Bresciani, M.; Egerton, T.; Giardino, C.; Li, L.; Moore, T.; Ruiz-Verdu, A.; Ruberg, S.; et al. Advancing Cyanobacteria Biomass Estimation from Hyperspectral Observations: Demonstrations with Hico and Prisma Imagery. Remote Sens. Environ. 2021, 266, 112693. [Google Scholar] [CrossRef]
- Yuan, D.; Tang, B.H.; Wang, D.; Chen, J.; Zhang, Z.; Fu, Z. An Enhanced Three-band Algorithm for Retrieving Phycocyanin Concentration from Satellite Data in Plateau Inland Lake. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2025, 18, 3654–3670. [Google Scholar] [CrossRef]
- Laneve, G.; Téllez, A.; Kallikkattil Kuruvila, A.; Bruno, M.; Messineo, V. Eutrophication and Hab Occurrence Control in Lakes of Different Origins: A Multi-source Remote Sensing Detection Strategy. Remote Sens. 2024, 16, 1792. [Google Scholar] [CrossRef]
- Hill, P.R.; Kumar, A.; Temimi, M.; Bull, D.R. Habnet: Machine Learning, Remote Sensing-based Detection of Harmful Algal Blooms. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2020, 13, 3229–3239. [Google Scholar] [CrossRef]
- Lin, S.; Pierson, D.C.; Mesman, J.P. Prediction of Algal Blooms Via Data-driven Machine Learning Models: An Evaluation Using Data from a Well-monitored Mesotrophic Lake. Geosci. Model Dev. 2023, 16, 35–46. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ma, R.; Duan, H.; Loiselle, S.; Xue, K.; Liang, Q. Satellite-Based Estimation of Column-Integrated Algal Biomass in Non-algae Bloom Conditions: A Case Study of Lake Chaohu, China. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 450–462. [Google Scholar] [CrossRef]
- Liu, D.; Yu, S.; Cao, Z.; Qi, T.; Duan, H. Process-oriented Estimation of Column-integrated Algal Biomass in Eutrophic Lakes by MODIS/Aqua. Int. J. Appl. Earth Obs. Geoinf. 2021, 99, 10232. [Google Scholar] [CrossRef]
- Shin, J.; Khim, B.-K.; Jang, L.-H.; Lim, J.; Jo, Y.-H. Convolutional Neural Network Model for Discrimination of Harmful Algal Bloom (hab) from Non-habs Using Sentinel-3 OLCI Imagery. ISPRS J. Photogramm. Remote Sens. 2022, 191, 250–262. [Google Scholar] [CrossRef]
- Sun, Z.; Chang, N.-B.; Chen, C.-F.; Gao, W. Lake Algal Bloom Monitoring Via Remote Sensing with Biomimetic and Computational Intelligence. Int. J. Appl. Earth Obs. Geoinf. 2022, 113, 102991. [Google Scholar] [CrossRef]
- Yan, K.; Li, J.; Zhao, H.; Wang, C.; Hong, D.; Du, Y.; Mu, Y.; Tian, B.; Xie, Y.; Yin, Z.; et al. Deep Learning-based Automatic Extraction of Cyanobacterial Blooms from Sentinel-2 MSI Satellite Data. Remote Sens. 2022, 14, 4763. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Z.; Li, Y.; Shen, M.; Duan, H. A Comparative Analysis of Machine Learning Methods for Algal Bloom Detection Using Remote Sensing Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2023, 16, 7953–7967. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Huang, J.; Wang, P.; Sun, C.; Zhang, Z.; Jiang, S. Explainable Deep Learning Identifies Patterns and Drivers of Freshwater Harmful Algal Blooms. Environ. Sci. Ecotechnol. 2025, 23, 100522. [Google Scholar] [CrossRef] [PubMed]
- Reinoso, N.L. Forecasting Harmful Algal Blooms for Western Lake Erie Using Data Driven Machine Learning Techniques. Master’s Thesis, Cleveland State University, Cleveland, OH, USA, 2017. [Google Scholar]
- Pham, T.L.; Dao, T.S.; Tran, N.D.; Nimptsch, J.; Wiegand, C.; Motoo, U. Influence of Environmental Factors on Cyanobacterial Biomass and Microcystin Concentration in the Dau Tieng Reservoir, a Tropical Eutrophic Water Body in Vietnam. Ann. Limnol.-Int. J. Limnol. 2017, 53, 89–100. [Google Scholar] [CrossRef]
- Fang, C.; Song, K.; Paerl, H.W.; Jacinthe, P.-A.; Wen, Z.; Liu, G.; Tao, H.; Xu, X.; Kutser, T.; Wang, Z.; et al. Global Divergent Trends of Algal Blooms Detected by Satellite During 1982–2018. Glob. Change Biol. 2022, 28, 2327–2340. [Google Scholar] [CrossRef] [PubMed]
- Vadadi Fülöp, C.; Hufnagel, L. Climate Change and Plankton Phenology in Freshwater: Current Trends and Future Commitments. J. Limnol. 2014, 73, 1–16. [Google Scholar] [CrossRef]
- Huo, D.; Gan, N.; Geng, R.; Cao, Q.; Song, L.; Yu, G.; Li, R. Cyanobacterial Blooms in China: Diversity, Distribution, and Cyanotoxins. Harmful Algae 2021, 109, 102106. [Google Scholar] [CrossRef]
- Binding, C.E.; Zeng, C.; Pizzolato, L.; Booth, C.; Valipour, R.; Fong, P.; Zastepa, A.; Pascoe, T. Reporting on the Status, Trends, and Drivers of Algal Blooms on Lake of the Woods Using Satellite-derived Bloom Indices (2002–2021). J. Great Lakes Res. 2023, 49, 32–43. [Google Scholar] [CrossRef]
- Reinl, K.L.; Harris, T.D.; North, R.L.; Almela, P.; Berger, S.A.; Bizic, M.; Burnet, S.H.; Grossart, H.-P.; Ibelings, B.W.; Jakobsson, E.; et al. Blooms Also Like It Cold. Limnol. Oceanogr. Lett. 2023, 8, 546–564. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, K.H.; Hwang, S.J.; Lee, T.K. Role of Algal Community Stability in Harmful Algal Blooms in River-connected Lakes. Microb. Ecol. 2021, 82, 309–318. [Google Scholar] [CrossRef]
- Derot, J.; Yajima, H.; Jacquet, S. Advances in Forecasting Harmful Algal Blooms Using Machine Learning Models: A Case Study with Planktothrix Rubescens in Lake Geneva. Harmful Algae 2020, 99, 101906. [Google Scholar] [CrossRef]
- Antonino, M.; Fulvio, C.; Giuseppe, C.; Goffredo La, L.; Antonio, G.; Chiara, C. Planktothrix Rubescens in Freshwater Reservoirs: Remote Sensing Potentiality for Mapping Cell Density. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XIV, Edinburgh, UK, 24–27 September 2012; Volume 8531. [Google Scholar] [CrossRef]
- Vilhena, L.C.; Hillmer, I.; Imberger, J.r. The Role of Climate Change in the Occurrence of Algal Blooms: Lake Burragorang, Australia. Limnol. Oceanogr. 2010, 55, 1188–1200. [Google Scholar] [CrossRef]
- Li, J.; Qin, B.; Brookes, J.D.; Shi, K.; Deng, J.; Zhou, J.; Wu, Y. Teleconnection Between Early Winter Monsoon System and Harmful Algal Blooms in Shallow Lake Taihu. Water Resour. Res. 2024, 60, e2023WR036931. [Google Scholar] [CrossRef]
- Gidudu, A.; Banura, C.; Angella, N. Monitoring Water Quality on Lake Victoria Using Modis Imagery. Int. J. Technosci. Dev. 2016, 3, 10. [Google Scholar]
- Makwinja, R.; Inagaki, Y.; Tesfamichael, S.G.; Curtis, C.J. Novel Methods for Monitoring Low Chlorophyll-a Concentrations in the Large, Oligotrophic Lake Malawi/nyasa/niassa. J. Environ. Manag. 2024, 364, 121462. [Google Scholar] [CrossRef]
- Pan, X.; Yuan, J.; Yang, Z.; Tansey, K.; Xie, W.; Song, H.; Wu, Y.; Yang, Y. Spatio-temporal Variation of Cyanobacteria Blooms in Taihu Lake Using Multiple Remote Sensing Indices and Machine Learning. Remote Sens. 2024, 16, 889. [Google Scholar] [CrossRef]
- Li, J.; Ma, R.; Xue, K.; Zhang, Y.; Loiselle, S. A Remote Sensing Algorithm of Column-integrated Algal Biomass Covering Algal Bloom Conditions in a Shallow Eutrophic Lake. ISPRS Int. J. Geo-Inf. 2018, 7, 466. [Google Scholar] [CrossRef]
- Yadav, S.; Yamashiki, Y.; Susaki, J.; Yamashita, Y.; Ishikawa, K. Chlorophyll Estimation of Lake Water and Coastal Water Using Landsat-8 and Sentinel-2a Satellite. Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2019, 42, 77–82. [Google Scholar] [CrossRef]
- Arias-Rodriguez, L.F.; Duan, Z.; Díaz-Torres, J.D.; Basilio Hazas, M.; Huang, J.; Kumar, B.U.; Tuo, Y.; Disse, M. Integration of Remote Sensing and Mexican Water Quality Monitoring System Using an Extreme Learning Machine. Sensors 2021, 21, 4118. [Google Scholar] [CrossRef]
- Ammarin, M.; Chi-Han, C.; Ni-Bin, C. Use of Modis Satellite Images to Investigate the Chlorophyll-a Concentrations in Lake Okeechobee, Florida. In Proceedings of the Remote Sensing and Modeling of Ecosystems for Sustainability V, San Diego, CA, USA, 10–14 August 2008; p. 708309. [Google Scholar]
- Fabbretto, A.; Pellegrino, A.; Giardino, C.; Bresciani, M.; Alikas, K.; Braga, F.; Vaičiūtė, D.; Lima, T.M.A.D.; Mangano, S.; Ghirardi, N.; et al. Hyperspectral Prisma Data Processing for Water Quality Research and Applications. In Proceedings of the IGARSS 2023—2023 IEEE International Geoscience and Remote Sensing Symposium, Pasadena, CA, USA, 16–21 July 2023; pp. 1744–1747. [Google Scholar]
- Palmer, S.C.J.; Odermatt, D.; Hunter, P.D.; Brockmann, C.; Présing, M.; Balzter, H.; Tóth, V.R. Satellite Remote Sensing of Phytoplankton Phenology in Lake Balaton Using 10 years of Meris Observations. Remote Sens. Environ. 2015, 158, 441–452. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Yan, X.; Fang, S.; Du, Y.; Xue, B.; Yu, K.; Wang, C. Monitoring Cyanobacteria Bloom in Dianchi Lake Based on Ground-based Multispectral Remote-sensing Imaging: Preliminary Results. Remote Sens. 2021, 13, 3970. [Google Scholar] [CrossRef]
- Irani Rahaghi, A.A.-O.; Odermatt, D.A.-O.; Anneville, O.A.-O.; Sepúlveda Steiner, O.A.-O.; Reiss, R.A.-O.; Amadori, M.A.-O.; Toffolon, M.A.-O.; Jacquet, S.A.-O.; Harmel, T.A.-O.; Werther, M.; et al. Combined Earth observations reveal the sequence of conditions leading to a large algal bloom in Lake Geneva. Commun. Earth Environ. 2024, 5, 229. [Google Scholar] [CrossRef]
- Binding, C.E.; Greenberg, T.A.; McCullough, G.; Watson, S.B.; Page, E. An Analysis of Satellite-derived Chlorophyll and Algal Bloom Indices on Lake Winnipeg. J. Great Lakes Res. 2018, 44, 436–446. [Google Scholar] [CrossRef]
- Molkov, A.A.; Fedorov, S.V.; Pelevin, V.V.; Korchemkina, E.N. Regional Models for High-resolution Retrieval of Chlorophyll a and Tsm Concentrations in the Gorky Reservoir by Sentinel-2 Imagery. Remote Sens. 2019, 11, 1215. [Google Scholar] [CrossRef]
- Song, C.; Woodcock, C.E.; Seto, K.C.; Lenney, M.P.; Macomber, S.A. Classification and Change Detection Using Landsat Tm Data: When and How to Correct Atmospheric Effects? Remote Sens. Environ. 2001, 75, 230–244. [Google Scholar] [CrossRef]
- Gordon, H.R. Normalized Water-leaving Radiance: Revisiting the Influence of Surface Roughness. Appl. Opt. 2005, 44, 241–248. [Google Scholar] [CrossRef]
- Vermote, E.F.; Tanre, D.; Deuze, J.L.; Herman, M.; Morcette, J.J. Second Simulation of the Satellite Signal in the Solar Spectrum, 6s: An Overview. IEEE Trans. Geosci. Remote Sens. 1997, 35, 675–686. [Google Scholar] [CrossRef]
- Gordon, H.R. Atmospheric Correction of Ocean Color Imagery in the Earth Observing System Era. J. Geophys. Res. Atmos. 1997, 102, 17081–17106. [Google Scholar] [CrossRef]
- Zhao, W.; Tamura, M.; Takahashi, H. Atmospheric and Spectral Corrections for Estimating Surface Albedo from Satellite Data Using 6S Code. Remote Sens. Environ. 2001, 76, 202–212. [Google Scholar] [CrossRef]
- Tachiiri, K. Calculating NDVI for NOAA/AVHRR Data After Atmospheric Correction for Extensive Images Using 6S Code: A Case Study in the Marsabit District, Kenya. ISPRS J. Photogramm. Remote Sens. 2005, 59, 103–114. [Google Scholar] [CrossRef]
- Ouaidrari, H.; Vermote, E.F. Operational Atmospheric Correction of Landsat TM Data. Remote Sens. Environ. 1999, 70, 4–15. [Google Scholar] [CrossRef]
- Moses, W.J.; Sterckx, S.; Montes, M.J.; De Keukelaere, L.; Knaeps, E. Chapter 3—Atmospheric Correction for Inland Waters. In Bio-Optical Modeling and Remote Sensing of Inland Waters; Mishra, D.R., Ogashawara, I., Gitelson, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–100. [Google Scholar]
- Vanhellemont, Q. Adaptation of the Dark Spectrum Fitting Atmospheric Correction for Aquatic Applications of the Landsat and Sentinel-2 Archives. Remote Sens. Environ. 2019, 225, 175–192. [Google Scholar] [CrossRef]
- Vanhellemont, Q.; Ruddick, K. Atmospheric Correction of Metre-scale Optical Satellite Data for Inland and Coastal Water Applications. Remote Sens. Environ. 2018, 216, 586–597. [Google Scholar] [CrossRef]
- Ogashawara, I.; Kiel, C.; Jechow, A.; Kohnert, K.; Ruhtz, T.; Grossart, H.-P.; Hölker, F.; Nejstgaard, J.C.; Berger, S.A.; Wollrab, S. The Use of Sentinel-2 for Chlorophyll-a Spatial Dynamics Assessment: A Comparative Study on Different Lakes in Northern Germany. Remote Sens. 2021, 13, 1542. [Google Scholar] [CrossRef]
- Caballero, I.; Fernández, R.; Escalante, O.M.; Mamán, L.; Navarro, G. New Capabilities of Sentinel-2a/b Satellites Combined with in Situ Data for Monitoring Small Harmful Algal Blooms in Complex Coastal Waters. Sci. Rep. 2020, 10, 8743. [Google Scholar] [CrossRef]
- Chegoonian, A.M.; Leavitt, P.R.; Zolfaghari, K.; Davies, J.M.; Baulch, H.M.; Duguay, C.R. Regional Upscaling of Chlorophyll-a Retrieval from Small Eutrophic Lakes Via Sentinel-2. In Proceedings of the IGARSS 2022—2022 IEEE International Geoscience and Remote Sensing Symposium, Kuala Lumpur, Malaysia, 17–22 July 2022; pp. 5622–5625. [Google Scholar]
- Wang, M. Remote Sensing of the Ocean Contributions from Ultraviolet to Near-infrared Using the Shortwave Infrared Bands: Simulations. Appl. Opt. 2007, 46, 1535–1547. [Google Scholar] [CrossRef]
- Wang, M.; Shi, W.; Tang, J. Water Property Monitoring and Assessment for China’s Inland Lake Taihu from Modis-aqua Measurements. Remote Sens. Environ. 2011, 115, 841–854. [Google Scholar] [CrossRef]
- Konik, M.; Bradtke, K.; Stoń-Egiert, J.; Soja-Woźniak, M.; Śliwińska-Wilczewska, S.; Darecki, M. Cyanobacteria Index As a Tool for the Satellite Detection of Cyanobacteria Blooms in the Baltic Sea. Remote Sens. 2023, 15, 1601. [Google Scholar] [CrossRef]
- Luis, G.-C.; Julia, A.-L.; Gonzalo, M.-G.; Jordi, M.-M.; Gustau, C.-V. Cloud Masking and Removal in Remote Sensing Image Time Series. J. Appl. Remote Sens. 2017, 11, 015005. [Google Scholar] [CrossRef]
- Meng, Q.; Borders, B.E.; Cieszewski, C.J.; Madden, M. Closest Spectral Fit for Removing Clouds and Cloud Shadows. Photogramm. Eng. Remote Sens. 2009, 75, 569–576. [Google Scholar] [CrossRef]
- Mateo-García, G.; Gómez-Chova, L.; Amorós-López, J.; Muñoz-Marí, J.; Camps-Valls, G. Multitemporal Cloud Masking in the Google Earth Engine. Remote Sens. 2018, 10, 1079. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Woodcock, C.E. Improvement and Expansion of the Fmask Algorithm: Cloud, Cloud Shadow, and Snow Detection for Landsats 4–7, 8, and Sentinel 2 Images. Remote Sens. Environ. 2015, 159, 269–277. [Google Scholar] [CrossRef]
- Mercury, M.; Green, R.; Hook, S.; Oaida, B.; Wu, W.; Gunderson, A.; Chodas, M. Global Cloud Cover for Assessment of Optical Satellite Observation Opportunities: A Hyspiri Case Study. Remote Sens. Environ. 2012, 126, 62–71. [Google Scholar] [CrossRef]
- Moore, G.F.; Aiken, J.; Lavender, S.J. The Atmospheric Correction of Water Colour and the Quantitative Retrieval of Suspended Particulate Matter in Case Ii Waters: Application to Meris. Int. J. Remote Sens. 1999, 20, 1713–1733. [Google Scholar] [CrossRef]
- Volpe, V.; Silvestri, S.; Marani, M. Remote Sensing Retrieval of Suspended Sediment Concentration in Shallow Waters. Remote Sens. Environ. 2011, 115, 44–54. [Google Scholar] [CrossRef]
- Feng, L.; Hu, C.; Chen, X.; Tian, L.; Chen, L. Human Induced Turbidity Changes in Poyang Lake Between 2000 and 2010: Observations from Modis. J. Geophys. Res. Ocean. 2012, 117, C07006. [Google Scholar] [CrossRef]
- Franz, B.A.; Werdell, P.J.; Meister, G.; Kwiatkowska, E.J.; Bailey, S.W.; Ahmad, Z.; McClain, C.R. MODIS land bands for ocean remote sensing applications. In Proceedings of the Ocean Optics XVIII, Montreal, QC, Canada, 9–13 October 2006; pp. 9–13. [Google Scholar]
- Gordon, H.R.; Wang, M. Retrieval of Water-leaving Radiance and Aerosol Optical Thickness Over the Oceans with Seawifs: A Preliminary Algorithm. Appl. Opt. 1994, 33, 443–452. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, Y.; Ma, R.; Loiselle, S.; Li, J.; Hu, M. A Modis-based Novel Method to Distinguish Surface Cyanobacterial Scums and Aquatic Macrophytes in Lake Taihu. Remote Sens. 2017, 9, 133. [Google Scholar] [CrossRef]
- Soontiens, N.; Binding, C.; Fortin, V.; Mackay, M.; Rao, Y.R. Algal Bloom Transport in Lake Erie Using Remote Sensing and Hydrodynamic Modelling: Sensitivity to Buoyancy Velocity and Initial Vertical Distribution. J. Great Lakes Res. 2019, 45, 556–572. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, H.; Shi, X.; Yu, Y.; Kong, F. Contributions of Meteorology to the Phenology of Cyanobacterial Blooms: Implications for Future Climate Change. Water Res. 2012, 46, 442–452. [Google Scholar] [CrossRef]
- Moreno-Ostos, E.; Cruz-Pizarro, L.; Basanta, A.; George, D.G. The Influence of Wind-induced Mixing on the Vertical Distribution of Buoyant and Sinking Phytoplankton Species. Aquat. Ecol. 2009, 43, 271–284. [Google Scholar] [CrossRef]
- Chaffin, J.D.; Kane, D.D.; Johnson, A. Effectiveness of a Fixed-depth Sensor Deployed from a Buoy to Estimate Water-column Cyanobacterial Biomass Depends on Wind Speed. J. Environ. Sci. 2020, 93, 23–29. [Google Scholar] [CrossRef]
- Bridgeman, T.B.; Chaffin, J.D.; Filbrun, J.E. A Novel Method for Tracking Western Lake Erie Microcystis Blooms, 2002–2011. J. Great Lakes Res. 2013, 39, 83–89. [Google Scholar] [CrossRef]
- Wynne, T.T.; Stumpf, R.P.; Tomlinson, M.C.; Dyble, J. Characterizing a Cyanobacterial Bloom in Western Lake Erie Using Satellite Imagery and Meteorological Data. Limnol. Oceanogr. 2010, 55, 2025–2036. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, M.; Zhou, Y. High-frequency Optical Measurements in Shallow Lake Taihu, China: Determining the Relationships Between Hydrodynamic Processes and Inherent Optical Properties. Hydrobiologia 2014, 724, 187–201. [Google Scholar] [CrossRef]
- Wu, T.; Qin, B.; Zhu, G.; Luo, L.; Ding, Y.; Bian, G. Dynamics of Cyanobacterial Bloom Formation During Short-term Hydrodynamic Fluctuation in a Large Shallow, Eutrophic, and Wind-exposed Lake Taihu, China. Environ. Sci. Pollut. Res. 2013, 20, 8546–8556. [Google Scholar] [CrossRef]
- Urquhart, E.A.; Schaeffer, B.A.; Stumpf, R.P.; Loftin, K.A.; Werdell, P.J. A Method for Examining Temporal Changes in Cyanobacterial Harmful Algal Bloom Spatial Extent Using Satellite Remote Sensing. Harmful Algae 2017, 67, 144–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Liu, X.; Zhou, Y.; Qin, B. Lake Topography and Wind Waves Determining Seasonal-spatial Dynamics of Total Suspended Matter in Turbid Lake Taihu, China: Assessment Using Long-term High-resolution Meris Data. PLoS ONE 2014, 9, e98055. [Google Scholar] [CrossRef]
- Zhou, J.; Leavitt, P.R.; Zhang, Y.; Qin, B. Anthropog. Eutrophication Shallow Lakes: Is It Occas? Water Res. 2022, 221, 118728. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Bi, S.; Xu, J.; Guo, F.; Lyu, H.; Dong, X.; Cai, X. Utilization of Goci Data to Evaluate the Diurnal Vertical Migration of Microcystis Aeruginosa and the Underlying Driving Factors. J. Environ. Manag. 2022, 310, 114734. [Google Scholar] [CrossRef]
- Coops, H.; Hanganu, J.; Tudor, M.; Oosterberg, W. Classification of Danube Delta Lakes Based on Aquatic Vegetation and Turbidity. Hydrobiologia 1999, 415, 187–191. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Shi, K.; Zhou, Y.; Tang, X.; Zhu, G.; Qin, B. Mapping Aquatic Vegetation in a Large, Shallow Eutrophic Lake: A Frequency-based Approach Using Multiple Years of Modis Data. Remote Sens. 2015, 7, 10295–10320. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Gong, Z.; Wang, L.; Heino, J.; Qin, B. Unveiling the Influence of Specialists and Generalists on Macroinvertebrate Assemblage Heterogeneity in Lake Taihu. Ecol. Indic. 2023, 154, 110741. [Google Scholar] [CrossRef]
- Zhao, D.; Lv, M.; Jiang, H.; Cai, Y.; Xu, D.; An, S. Spatio-temporal Variability of Aquatic Vegetation in Taihu Lake Over the Past 30 Years. PLoS ONE 2013, 8, e66365. [Google Scholar] [CrossRef]
- Yang, X.; Pavelsky, T.M.; Bendezu, L.P.; Zhang, S. Simple Method to Extract Lake Ice Condition from Landsat Images. IEEE Trans. Geosci. Remote Sens. 2021, 60, 4202010. [Google Scholar] [CrossRef]
- Oyama, Y.; Matsushita, B.; Fukushima, T. Distinguishing Surface Cyanobacterial Blooms and Aquatic Macrophytes Using Landsat/tm and Etm+ Shortwave Infrared Bands. Remote Sens. Environ. 2015, 157, 35–47. [Google Scholar] [CrossRef]
- Pu, J.; Song, K.; Liu, G.; Wen, Z.; Fang, C.; Hou, J.; Lv, Y. Differentiation of Algal Blooms and Aquatic Vegetation in Chinese Lakes Using Modified Vegetation Presence Frequency Index Method. Chin. Geogr. Sci. 2022, 32, 792–807. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Zlinszky, A.; Balzter, H.; Nicolás-Perea, V.; Tóth, V.R. Copernicus Framework for Monitoring Lake Balaton Phytoplankton. In Earth Observation for Land and Emergency Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 173–191. [Google Scholar]
- Xu, D.; Pu, Y.; Zhu, M.; Luan, Z.; Shi, K. Automatic Detection of Algal Blooms Using Sentinel-2 Msi and Landsat Oli Images. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2021, 14, 8497–8511. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Wang, C.; Chen, N. Multivariable Integrated Risk Assessment for Cyanobacterial Blooms in Eutrophic Lakes and Its Spatiotemporal Characteristics. Water Res. 2023, 228, 119367. [Google Scholar] [CrossRef]
- Frau, D. Towards a quantitative definition of Cyanobacteria blooms. Environ. Rev. 2023, 31, 643–651. [Google Scholar] [CrossRef]
- Sotero-Santos, R.B.; Dellamano-Oliveira, M.J.; Carvalho, E.G.; Minillo, A.; Rocha, O. Phytoplanktonic Structure and Chemistry of the Water in the Monjolinho Reservoir (SP, Brazil) During a Cyanobacterial Bloom Episode. Ecotoxicol. Environ. Contam. 2010, 5, 63–70. [Google Scholar] [CrossRef]
- Le Vu, B.; Vinçon-Leite, B.; Lemaire, B.J.; Bensoussan, N.; Calzas, M.; Drezen, C.; Deroubaix, J.F.; Escoffier, N.; Dégrés, Y.; Freissinet, C.; et al. High-frequency Monitoring of Phytoplankton Dynamics Within the European Water Framework Directive: Application to Metalimnetic Cyanobacteria. Biogeochemistry 2011, 106, 229–242. [Google Scholar] [CrossRef]
- Eon, R.; Gerace, A.; Falcon, L.; Poole, E.; Kleynhans, T.; Raqueño, N.; Bauch, T. Validation of Landsat-9 and Landsat-8 Surface Temperature and Reflectance During the Underfly Event. Remote Sens. 2023, 15, 3370. [Google Scholar] [CrossRef]
- Reynolds, N.; Schaeffer, B.A.; Guertault, L.; Nelson, N.G. Satellite and in Situ Cyanobacteria Monitoring: Understanding the Impact of Monitoring Frequency on Management Decisions. J. Hydrol. 2023, 619, 129278. [Google Scholar] [CrossRef]
- Cannizzaro, J.P.; Hu, C.; English, D.C.; Carder, K.L.; Heil, C.A.; Müller-Karger, F.E. Detection of Karenia Brevis Blooms on the West Florida Shelf Using in Situ Backscattering and Fluorescence Data. Harmful Algae 2009, 8, 898–909. [Google Scholar] [CrossRef]
- Ma, J.; Duan, H.; He, L.; Tiffany, M.; Cao, Z.; Qi, T.; Shen, M.; Biggs, T.; Xu, X. Spatiotemporal Pattern of Gypsum Blooms in the Salton Sea, California, During 2000–2018. Int. J. Appl. Earth Obs. Geoinf. 2020, 89, 102090. [Google Scholar] [CrossRef]
- Lee, Z.; Jiang, M.; Davis, C.; Pahlevan, N.; Ahn, Y.-H.; Ma, R. Impact of Multiple Satellite Ocean Color Samplings in a Day on Assessing Phytoplankton Dynamics. Ocean Sci. J. 2012, 47, 323–329. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Ma, R.; Xue, K.; Cao, Z.; Chu, Q.; Jing, Y. Optimized Remote Sensing Estimation of the Lake Algal Biomass by Considering the Vertically Heterogeneous Chlorophyll Distribution: Study Case in Lake Chaohu of China. Sci. Total Environ. 2021, 771, 144811. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, X.; Xie, Q.; Jin, J.; Zhou, Y.; Luo, Y.; Liu, Y.; Tian, J.; Zhao, Y. Monitoring Nature’s Calendar from Space: Emerging Topics in Land Surface Phenology and Associated Opportunities for Science Applications. Glob. Change Biol. 2022, 28, 7186–7204. [Google Scholar] [CrossRef]
- Gao, F.; Masek, J.; Schwaller, M.; Hall, F. On the Blending of the Landsat and Modis Surface Reflectance: Predicting Daily Landsat Surface Reflectance. IEEE Trans. Geosci. Remote Sens. 2006, 44, 2207–2218. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, H.; Ma, X.; Zhang, Y. Long-term Spatiotemporal Variation and Environmental Driving Forces Analyses of Algal Blooms in Taihu Lake Based on Multi-source Satellite and Land Observations. Water 2020, 12, 1035. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, K.; Cao, Z.; Lai, L.; Geng, J.; Yu, K.; Zhan, P.; Liu, Z. Effects of Satellite Temporal Resolutions on the Remote Derivation of Trends in Phytoplankton Blooms in Inland Waters. ISPRS J. Photogramm. Remote Sens. 2022, 191, 188–202. [Google Scholar] [CrossRef]
- Shiyu, H.; Xiaoshuang, M.; Yanlan, W. Long Time Sequence Monitoring of Chaohu Algal Blooms Based on Multi-source Optical and Radar Remote Sensing. In Proceedings of the 2018 Fifth International Workshop on Earth Observation and Remote Sensing Applications (EORSA), Xi’an, China, 18–20 June 2018; pp. 1–5. [Google Scholar]
- Jung, J.; Maeda, M.; Chang, A.; Bhandari, M.; Ashapure, A.; Landivar-Bowles, J. The Potential of Remote Sensing and Artificial Intelligence as Tools to Improve the Resilience of Agriculture Production Systems. Curr. Opin. Biotechnol. 2021, 70, 15–22. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Xie, K.; Sun, J.; Zhu, S. Dongting Lake Algal Bloom Forecasting: Robustness and Accuracy Analysis of Deep Learning Models. J. Hazard. Mater. 2025, 485, 136804. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Feng, Y.; Fung, S.; Xomchuk, V.R.; Jiang, M.; Moore, T.; Beckler, J. Spatiotemporal Deep-learning-based Algal Bloom Prediction for Lake Okeechobee Using Multisource Data Fusion. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2022, 15, 8318–8331. [Google Scholar] [CrossRef]
- Wang, L.; Shan, K.; Yi, Y.; Yang, H.; Zhang, Y.; Xie, M.; Zhou, Q.; Shang, M. Employing Hybrid Deep Learning for Near-real-time Forecasts of Sensor-based Algal Parameters in a Microcystis Bloom-dominated Lake. Sci. Total Environ. 2024, 922, 171009. [Google Scholar] [CrossRef]
- Qian, J.; Qian, L.; Pu, N.; Bi, Y.; Wilhelms, A.; Norra, S. An Intelligent Early Warning System for Harmful Algal Blooms: Harnessing the Power of Big Data and Deep Learning. Environ. Sci. Technol. 2024, 58, 15607–15618. [Google Scholar] [CrossRef] [PubMed]
- Grendaitė, D.; Petkevičius, L. Identification of Algal Blooms in Lakes in the Baltic States Using Sentinel-2 Data and Artificial Neural Networks. IEEE Access 2024, 12, 27973–27988. [Google Scholar] [CrossRef]
- Wu, H.; Lin, Z.; Lin, B.; Li, Z.; Jin, N.; Zhu, X. Deep Learning to Model the Complexity of Algal Bloom. In Proceedings of the 2022 International Conference on Cyber-Enabled Distributed Computing and Knowledge Discovery (CyberC), Suzhou, China, 14–16 October 2022; pp. 114–122. [Google Scholar]
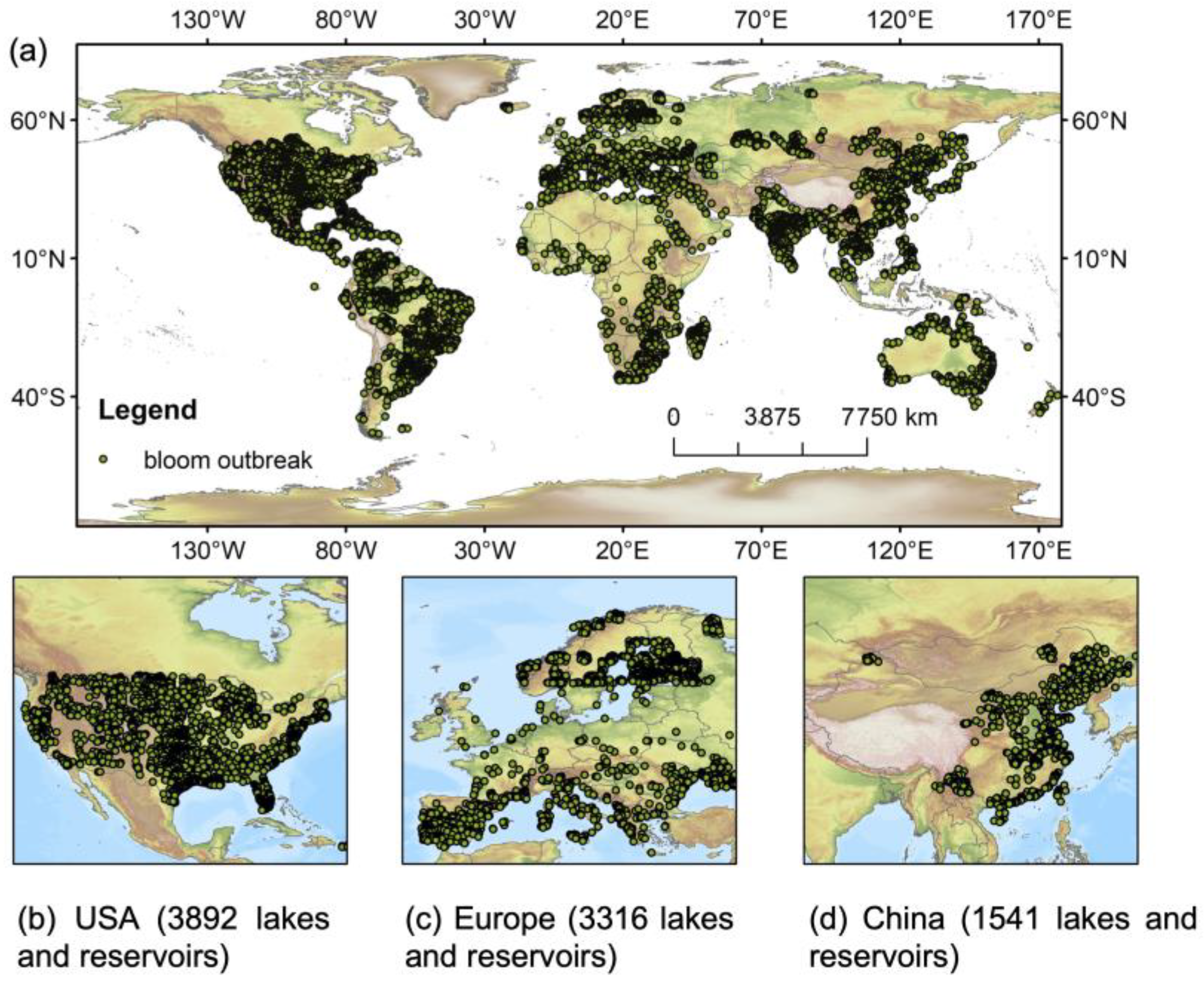


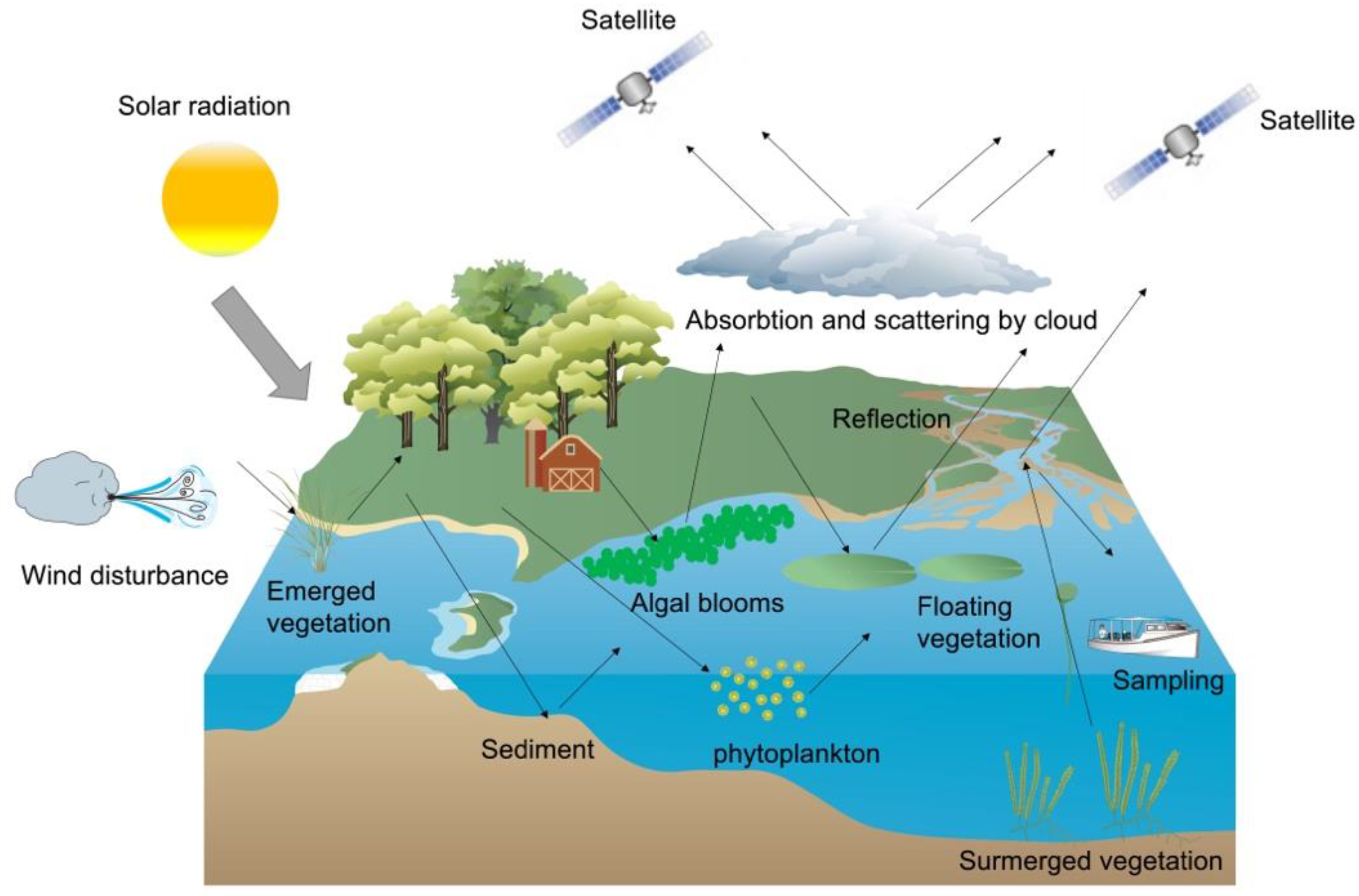


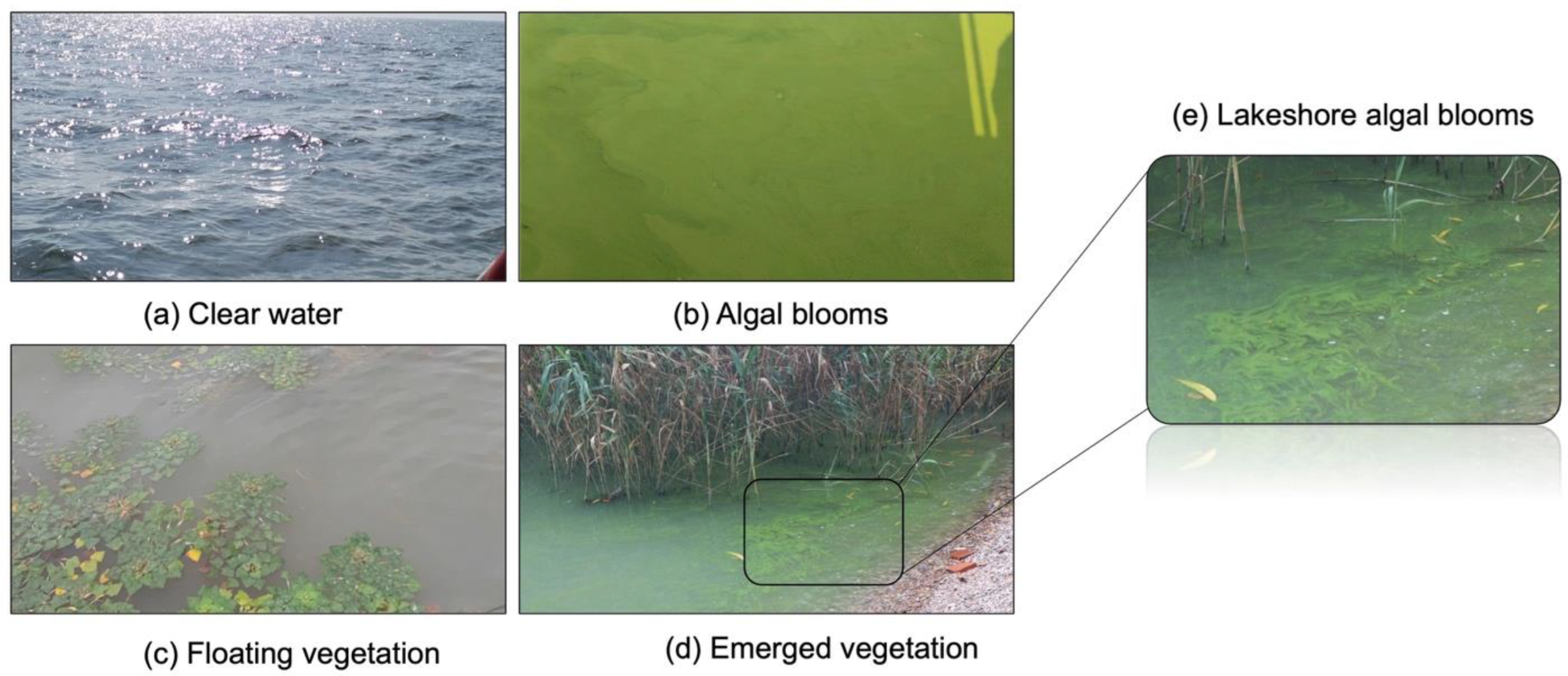
| Sensor Group | Satellite/Sensor | Spatial Resolution (m) | Temporal Resolution (Day) | Band Numbers | Data Availability | Operation | Country/Organization |
|---|---|---|---|---|---|---|---|
| Multispectral Sensors | AVHRR NOAA | 1100 | 1 | 5 | 1978–present | Normal | USA |
| MODIS Terra | 250–1000 | 1 | 36 | 1999–present | Normal | USA | |
| MODIS Aqua | 250–1000 | 1 | 36 | 2002–present | Normal | USA | |
| MERIS Envisat | 300 | 3 | 15 | 2002–2012 | Retired | ESA | |
| CZCS Nimbus-7 | 800 | 6 | 6 | 1978–1986 | Retired | USA | |
| SeaWiFS | 900 | 1 | 8 | 1997–2010 | Retired | USA | |
| Landsat 1–9 | 15–80 | 16 | 7–11 | 1972–present | Normal | USA | |
| Sentinel-2 MSI | 10–60 | 5 | 13 | 2015–present | Normal | ESA | |
| GOCI COMS | 500 | 0.5 | 8 | 2010–present | Normal | South Korea | |
| HJ-1A/1B CCD | 30 | 2 | 4 | 2008–present | Normal | China | |
| HJ-1C/D COCTS | 1100 | 1 | 8 | 2018–present | Normal | China | |
| Sentinel-3 OLCI | 300 | 1 | 21 | 2016–present | Normal | ESA | |
| GF-1/2/3/4 | 1–50 | 1–4 | 4 | 2013–present | Normal | China | |
| QuickBird | 0.65 | 1–1.3 | 5 | 2001–2015 | Retired | USA | |
| WorldView 1–4 | 0.31–0.50 | 1–4 | 8–16 | 2007–present | Normal | USA | |
| IKONOS | 1–4 | 1–3 | 4 | 2001–2015 | Retired | USA | |
| ADEOS-II GLI | 250–1000 | 4 | 36 | 2002–present | Normal | Japan | |
| GOES ABI | 500–2000 | 0.01 | 16 | 2017–present | Normal | USA | |
| VIIRS Suomi NPP/NOAA-20 | 375–750 | 1 | 22 | 2011–present | Normal | USA | |
| Himawari AHI | 500–2000 | 0.007 | 16 | 2015–present | Normal | Japan | |
| Zhuhai-1 OHS-01/02 | 2.5 | 5 | 256 | 2017–present | Normal | China | |
| Hyperspectral Sensors | Hyperion EO-1 | 30 | 16 | 242 | 2000–2017 | Retired | USA |
| HICO | 90 | 1–3 | 128 | 2009–2014 | Retired | USA | |
| EnMAP | 30 | 4 | 244 | 2022–present | Normal | Germany | |
| PRISMA HSI | 30 | 7 | 239 | 2019–present | Normal | Italy | |
| DESIS | 30 | 1 | 235 | 2018–present | Normal | Germany | |
| HySIS | 30 | 5 | 326 | 2018–present | Normal | India | |
| HISUI | 20 | 14 | 185 | 2021–present | Normal | Japan | |
| GF-5 | 30 | 4 | 330 | 2018–present | Normal | China | |
| EMIT | 60 | 3 | 285 | 2022–present | Normal | USA | |
| PACE OCI | 1000 | 1–2 | 250 | 2024–present | Normal | USA | |
| PROBA-1 CHRIS | 17/34 | 7 | 18/62 | 2001–present | Normal | ESA |
| Indices | Equation | Advantages | Limitations and Requirements | References |
|---|---|---|---|---|
| MPH | ρBRmax and λmax are the highest value bands at 681, 709 and 753 nm from MERIS | Detects chlorophyll peaks effectively | Sensor-specific (e.g., MERIS); insensitive to low-concentration blooms | [38] |
| ABDI | represent the central wavelengths of Red, Red Edge-2 and NIRn bands, respectively | Effectively avoids misclassifying thin cloud cover and turbid water as algal blooms, which improves the reliability of algal bloom detection in complex environments | Difficult for ABDI to distinguish between algal blooms and aquatic vegetation | [72] |
| FAI | Rrc,NIR − R’rc,NIR are the center wavelengths | Resists thin cloud interference | Misclassifies turbid water as algal blooms | [103] |
| NDVI | Simple, widely applicable | Sensitive to turbidity, overestimates bloom area in high-turbidity waters | [104] | |
| NDCI | represents the reflectance at the red wavelength | Relatively straightforward, allowing for rapid data analysis | Limited by sensor bands, influenced by high turbidity | [105] |
| MCI | Sensitive to cyanobacteria at high concentrations | Requires red-edge bands; ineffective for low concentrations | [106] | |
| EVI | Reduces atmospheric and background noise | Requires blue band; limited performance in optically complex waters | [107] | |
| AFAI | Enhances NIR reflection peaks for accurate cyanobacteria detection | Challenged by turbidity; struggles to distinguish turbid water from blooms | [108] | |
| CI | [(682 − 665)/(709 − 665)] | Exploits absorption peak at ~709 nm | Limited to high cyanobacteria concentrations | [109] |
| NDPI | (R820 − R690)/(R820 + R690) | Discriminates algae from suspended sediments | Sensitive to water optical complexity; requires NIR bands | [110] |
| VB-FAH | is the center wavelength of each satellite sensor | Compatible with sensors lacking SWIR (e.g., HJ/GF) | Reduced accuracy in turbid waters | [111] |
| FLH | Effective in clear waters | Affected by CDOM and non-algal particles | [112] |
| Climate Zone | Country | Lake | Key Parameters | Sensors | References |
|---|---|---|---|---|---|
| Tropical | Guatemala | Atitlán | Chla | Hyperion | [75] |
| Kenya Uganda Tanzania | Victoria | Chla | MODIS | [148] | |
| Nicaragua | Nicaragua | Chla | Sentinel-2 | [149] | |
| Sololá | Atitlán | Chla | Hyperion | [75] | |
| Subtropical | China | Taihu | Chla | MODIS | [150] |
| China | Chaohu | Chla | MODIS | [151] | |
| Japan | Biwa | Chla | Landsat, Sentinel-2 | [152] | |
| Mexico | Chapala | Chla | Landsat, MODIS | [153] | |
| USA | Okeechobee | Chla | MODIS | [154] | |
| Temperate | USA/Canada | Erie | PC | HICO, PRISMA | [124] |
| Estonia/Russia | Peipsi | Chla | PRISMA | [155] | |
| Hungary | Balaton | Chla | MERIS | [156] | |
| China | Dianchi | Chla | ground-basedimaging | [157] | |
| Switzerland | Geneva | Chla | Sentinel-2 | [158] | |
| Canada | Winnipeg | Chla | MERIS | [159] | |
| Italy/Switzerland | Lake Como, Lake Maggiore, and Lake Lugano | Chla | PRISMA, Sentinel-3 | [82] | |
| Russia | Lake Baikal | Chla | LiDAR | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Qin, B. Application of Optical Remote Sensing in Harmful Algal Blooms in Lakes: A Review. Remote Sens. 2025, 17, 1381. https://doi.org/10.3390/rs17081381
Wang S, Qin B. Application of Optical Remote Sensing in Harmful Algal Blooms in Lakes: A Review. Remote Sensing. 2025; 17(8):1381. https://doi.org/10.3390/rs17081381
Chicago/Turabian StyleWang, Simeng, and Boqiang Qin. 2025. "Application of Optical Remote Sensing in Harmful Algal Blooms in Lakes: A Review" Remote Sensing 17, no. 8: 1381. https://doi.org/10.3390/rs17081381
APA StyleWang, S., & Qin, B. (2025). Application of Optical Remote Sensing in Harmful Algal Blooms in Lakes: A Review. Remote Sensing, 17(8), 1381. https://doi.org/10.3390/rs17081381









