Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
| Date | Water Condition | Water Consumption (m3/per barrel) | |||
|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||
| 2 July | The gradient watering | 0 | 1.25×10−3 | 2.5×10−3 | 5×10−3 |
| 5 July | The filled watering | 5×10−3 | 3.75×10−3 | 2.5×10−3 | 0 |
| Date | Stage | Water | Measure Parameters | Time |
|---|---|---|---|---|
| 2July | V12 | at night | Leaf spectrum, chlorophyll fluorescence, PAR and soil water content | 9:00 am |
| 11:40 am | ||||
| 14:30 pm | ||||
| 16:40 pm | ||||
| 5 July | V12 | at night | Leaf spectrum, chlorophyll fluorescence, PAR and soil water content leaf temperature | 9:30 am |
| 11:30 am | ||||
| 14:30 pm | ||||
| 16:00 pm | ||||
| 6 July | V12 | - | Leaf spectrum, chlorophyll fluorescence, PAR and soil water content leaf temperature | 10:00 am |
2.2. Data Collection
2.2.1. PAR Data and Soil Water Content Data Collection
2.2.2. Leaf Spectral Data Collection
2.2.3. Leaf Fluorescence Measurements
2.2.4. Leaf Temperature Measurement
2.3. FLD and 3FLD Methods
2.4. SCOPE Model
- (i)
- PROSPECT parameters, such as the chlorophyll a + b content, dry matter content, leaf water equivalent layer, senescent material fraction, leaf thickness parameters and thermal reflectance and transmittance;
- (ii)
- Leaf Biochemical parameters, including the maximum carboxylation capacity, stomatal conductance parameter, photochemical pathway, extinction coefficient, respiration and temperature response;
- (iii)
- Fluorescence quantum yield efficiency at the photosystem level;
- (iv)
- Soil parameters, including soil spectrum, soil resistance, soil reflectance in the thermal range, heat capacity of the soil, specific mass of the soil and soil moisture content;
- (v)
- Canopy geometry parameters, including leaf area index, vegetation height, canopy structure and leaf width;
- (vi)
- Meteorological data, including measurement height, incoming shortwave and longwave radiation, air temperature, air pressure, atmospheric vapor pressure, wind speed, and atmospheric CO2 and O2 concentrations;
- (vii)
- Aerodynamic data, including roughness length for the momentum of the canopy, height, leaf drag coefficient, and resistance;
- (viii)
- Time series information (only for daily simulation); and
- (ix)
- Three observation angles (solar zenith angle, observation zenith angle, and the azimuthal difference between solar and observation angles).
3. Results and Discussion
3.1. The Results of the SCOPE Simulation
3.1.1. Variations in Chlorophyll Fluorescence and Canopy Temperature under Stress Conditions

3.1.2. Relationships between Chlorophyll Fluorescence and Canopy Temperatures on the Canopy

3.2. Analysis of the Field Data
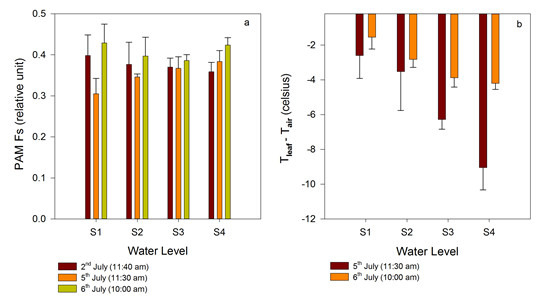
3.2.1. Time Series of Chlorophyll Fluorescence and Leaf Temperature at Different Water Levels


3.2.2. Comparison between Gradient Watering and Filled Watering

3.2.3. Fluorescence Computed Using Passive Measurements

3.2.4. Comparison of the Active and Passive Measurements

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Qu, J.J. Satellite remote sensing applications for surface soil moisture monitoring: A review. Front. Earth Sci. China 2009, 3, 237–247. [Google Scholar] [CrossRef]
- Peters, A.J.; Walter-Shea, E.A.; Ji, L.; Vina, A.; Hayes, M.; Svoboda, M.D. Drought monitoring with NDVI-based standardized vegetation index. Photogramm. Eng. Remote Sens. 2002, 68, 71–75. [Google Scholar]
- Fuchs, M.; Tanner, C. Infrared thermometry of vegetation. Agron. J. 1966, 58, 597–601. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.; Reginato, R.; Pinter, P. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Paloscia, S.; Pampaloni, P. Microwave remote sensing of plant water stress. Remote Sens. Environ. 1984, 16, 249–255. [Google Scholar] [CrossRef]
- Hunt Jr, E.R.; Rock, B.N. Detection of changes in leaf water content using Near- and Middle-Infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar] [CrossRef]
- Gao, B.-C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Walker, J.P. Estimating Soil Moisture Profile Dynamics from Near-Surface Soil Moisture Measurements and Standard Meteorological Data. Ph.D. Thesis, the University of Newcastle, New South Wales, Austrilia, 1999. [Google Scholar]
- Sandholt, I.; Rasmussen, K.; Andersen, J. A simple interpretation of the surface temperature/vegetation index space for assessment of surface moisture status. Remote Sens. Environ. 2002, 79, 213–224. [Google Scholar] [CrossRef]
- Vereecken, H.; Weihermüller, L.; Jonard, F.; Montzka, C. Characterization of crop canopies and water stress related phenomena using microwave remote sensing methods: A review. Vadose Zone J. 2012, 11. [Google Scholar] [CrossRef]
- Bellvert, J.; Marsal, J.; Girona, J.; Zarco-Tejada, P.J. Seasonal evolution of crop water stress index in grapevine varieties determined with high-resolution remote sensing thermal imagery. Irrigation Sci. 2014, 33, 1–13. [Google Scholar]
- Zarco-Tejada, P.J.; González-Dugo, V.; Berni, J.A. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2012, 117, 322–337. [Google Scholar] [CrossRef]
- Méthy, M.; Olioso, A.; Trabaud, L. Chlorophyll fluorescence as a tool for management of plant resources. Remote Sens. Environ. 1994, 47, 2–9. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. Ecophysiol. Photosynth. 1994, 100, 49–70. [Google Scholar]
- Razavi, F.; Pollet, B.; Steppe, K.; Van Labeke, M.-C. Chlorophyll fluorescence as a tool for evaluation of drought stress in strawberry. Photosynthetica 2008, 46, 631–633. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Tyystjärvi, E.; Atherton, J.; van der Tol, C.; Flexas, J.; Pfündel, E.E.; Moreno, J.; Frankenberg, C.; Berry, J.A. Linking Chlorophyll a Fluorescence to Photosynthesis for Remote Sensing Applications: Mechanisms and Challenges. Available online: http://jxb.oxfordjournals.org/content/early/2014/05/26/jxb.eru191.full (accessed on 31 December 2014).
- Lichtenthaler, H.K.; Miehe, J.A. Fluorescence imaging as a diagnostic tool for plant stress. Trends Plant Sci. 1997, 2, 316–320. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Van der Tol, C.; Verhoef, W.; Timmermans, J.; Verhoef, A.; Su, Z. An integrated model of soil-canopy spectral radiance observations, photosynthesis, fluorescence, temperature and energy balance. Biogeosci. Discussion 2009, 6, 6025–6075. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.; Dobrowski, S.; Ustin, S. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Dobrowski, S.; Pushnik, J.; Zarco-Tejada, P.J.; Ustin, S. Simple reflectance indices track heat and water stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar] [CrossRef]
- Pérez-Priego, O.; Zarco-Tejada, P.J.; Miller, J.R.; Sepulcre-Cantó, G.; Fereres, E. Detection of water stress in orchard trees with a high-resolution spectrometer through chlorophyll fluorescence in-filling of the O2-A band. IEEE Trans. Geosci. Remote Sens. 2005, 43, 2860–2869. [Google Scholar] [CrossRef]
- Campbell, P.; Middleton, E.; McMurtrey, J.; Chappelle, E. Assessment of vegetation stress using reflectance or fluorescence measurements. J. Environ. Qual. 2007, 36, 832–845. [Google Scholar] [CrossRef] [PubMed]
- Chaerle, L.; Leinonen, I.; Jones, H.G.; van Der Straeten, D. Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J. Exp. Bot. 2007, 58, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Berni, J.A.J.; Suárez, L.; Sepulcre-Cantó, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- McFarlane, J.; Watson, R.; Theisen, A.; Jackson, R.D.; Ehrler, W.; Pinter, P., Jr.; Idso, S.B.; Reginato, R. Plant stress detection by remote measurement of fluorescence. Appl. Optics 1980, 19, 3287–3289. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. In vivo chlorophyll fluorescence as a tool for stress detection in plants. In Applications of Chlorophyll Fluorescene in Photosynthesis Research, Stress Physiology, Hydrobiology and Remote Sensing; Springer Netherlands: Berlin, Germany, 1988; pp. 129–142. [Google Scholar]
- Guanter, L.; Frankenberg, C.; Dudhia, A.; Lewis, P.E.; Góez-Dans, J.; Kuze, A.; Suto, H.; Grainger, R.G. Retrieval and global assessment of terrestrial chlorophyll fluorescence from GOSAT space measurements. Remote Sens. Environ. 2012, 121, 236–251. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.; Mohammed, G.; Noland, T.; Sampson, P. Vegetation stress detection through chlorophyll+ estimation and fluorescence effects on hyperspectral imagery. J. Environ. Qual. 2002, 31, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Marcassa, L.; Gasparoto, M.; Belasque, J., Jr.; Lins, E.; Nunes, F.D.; Bagnato, V. Fluorescence spectroscopy applied to orange trees. Laser Phys. 2006, 16, 884–888. [Google Scholar] [CrossRef]
- Meroni, M.; Panigada, C.; Rossini, M.; Picchi, V.; Cogliati, S.; Colombo, R. Using optical remote sensing techniques to track the development of ozone-induced stress. Environ. Pollut. 2009, 157, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Yu, Zhenwen. Overview of Crop Cultivation; China Agriculture Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Edner, H.; Johansson, J.; Ragnarsson, P.; Svanberg, S.; Wallinder, E. Remote monitoring of vegetation using a fluorescence lidar system in spectrally resolving and multi-spectral imaging modes. EARSeL Adv. Remote Sens. 1995, 3, 198–206. [Google Scholar]
- Zarco-Tejada, P.J.; Morales, A.; Testi, L.; Villalobos, F. Spatio-temporal patterns of chlorophyll fluorescence and physiological and structural indices acquired from hyperspectral imagery as compared with carbon fluxes measured with eddy covariance. Remote Sens. Environ. 2013, 133, 102–115. [Google Scholar] [CrossRef]
- Lee, J.-E.; Frankenberg, C.; van der Tol, C.; Berry, J.A.; Guanter, L.; Boyce, C.K.; Fisher, J.B.; Morrow, E.; Worden, J.R.; Asefi, S.; et al. Forest Productivity and Water Stress in Amazonia: Observations from GOSAT Chlorophyll Fluorescence. Available online: http://rspb.royalsocietypublishing.org/content/280/1761/20130171.short (accessed on 31 December 2014).
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Ann. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Meroni, M.; Picchi, V.; Rossini, M.; Cogliati, S.; Panigada, C.; Nali, C.; Lorenzini, G.; Colombo, R. Leaf level early assessment of ozone injuries by passive fluorescence and photochemical reflectance index. Int. J. Remote Sens. 2008, 29, 5409–5422. [Google Scholar] [CrossRef]
- Suárez, L.; Zarco-Tejada, P.J.; Berni, J.A.; González-Dugo, V.; Fereres, E. Modelling PRI for water stress detection using radiative transfer models. Remote Sens. Environ. 2009, 113, 730–744. [Google Scholar] [CrossRef]
- Portable Chlorophyll Fluorometer PAM-2500 Handbook of Operation. Available online: http://www.walz.com/downloads/manuals/pam-2500/PAM_2500_03pp.pdf (accessed on 31 December 2014).
- Plascyk, J.A.; Gabriel, F.C. The Fraunhofer line discriminator MKII-an airborne instrument for precise and standardized ecological luminescence measurement. IEEE Trans. Instrum. Meas. 1975, 24, 306–313. [Google Scholar] [CrossRef]
- Plascyk, J.A. The MK II Fraunhofer line discriminator (FLD-II) for airborne and orbital remote sensing of solar-stimulated luminescence. Opt. Eng. 1975, 14, 339–330. [Google Scholar] [CrossRef]
- Meroni, M.; Rossini, M.; Guanter, L.; Alonso, L.; Rascher, U.; Colombo, R.; Moreno, J. Remote sensing of solar-induced chlorophyll fluorescence: Review of methods and applications. Remote Sens. Environ. 2009, 113, 2037–2051. [Google Scholar] [CrossRef]
- Timmermans, J. Coupling optical and Thermal Directional Radiative Transfer to Biophysical Processes in Vegetated Canopies. Ph.D. Thesis, University of Twente, Enchede, The Netherlands, 2011. [Google Scholar]
- El Baki, A.M.A.A. Estimation of Evapotranspiration from Airborne Hyperspectral Scanner Data Using the SCOPE Model. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2013. [Google Scholar]
- Zhao, F.; Guo, Y.; Verhoef, W.; Gu, X.; Liu, L.; Yang, G. A method to reconstruct the solar-induced canopy fluorescence spectrum from hyperspectral measurements. Remote Sens. 2014, 6, 10171–10192. [Google Scholar] [CrossRef]
- Zhang, Y.; Guanter, L.; Berry, J.A.; Joiner, J.; Tol, C.; Huete, A.; Gitelson, A.; Voigt, M.; Köhler, P. Estimation of vegetation photosynthetic capacity from space-based measurements of chlorophyll fluorescence for terrestrial biosphere models. Glob. Change Biol. 2014, 20. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R.; Mohammed, G.H.; Noland, T.L.; Sampson, P.H. Chlorophyll fluorescence effects on vegetation apparent reflectance: II. Laboratory and airborne canopy-level measurements with hyperspectral data. Remote Sens. Environ. 2000, 74, 596–608. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Z.; Liu, Z.; Huo, H.; Li, Z.-L.; Nerry, F.; Wang, Q.; Li, X. Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data. Remote Sens. 2015, 7, 3232-3249. https://doi.org/10.3390/rs70303232
Ni Z, Liu Z, Huo H, Li Z-L, Nerry F, Wang Q, Li X. Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data. Remote Sensing. 2015; 7(3):3232-3249. https://doi.org/10.3390/rs70303232
Chicago/Turabian StyleNi, Zhuoya, Zhigang Liu, Hongyuan Huo, Zhao-Liang Li, Françoise Nerry, Qingshan Wang, and Xiaowen Li. 2015. "Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data" Remote Sensing 7, no. 3: 3232-3249. https://doi.org/10.3390/rs70303232
APA StyleNi, Z., Liu, Z., Huo, H., Li, Z.-L., Nerry, F., Wang, Q., & Li, X. (2015). Early Water Stress Detection Using Leaf-Level Measurements of Chlorophyll Fluorescence and Temperature Data. Remote Sensing, 7(3), 3232-3249. https://doi.org/10.3390/rs70303232