The reproduction of temporal and spatial dynamics of simulated crop growth is validated separately, mostly due to the lack of temporally continuous spatial validation data. In
Section 3.2 the model’s ability of reproducing the temporal dynamics of crop growth in comparison with temporally high-resolution point data is analyzed.
Section 3.3 studies the quality of spatial model results that were achieved by assimilating remote sensing data into the model system through a direct comparison of modelled and measured spatial yield maps. Since the quality of the EO-based greenLAI maps is crucial for the assimilation, validation results for greenLAI are given first in
Section 3.1.
3.2. Validation of Temporal Dynamics
For the analysis of the temporal model performance, the model was set up for the test site in southern Germany at a spatial resolution of 4 m. One pixel thus represents the spatial sampling unit of the in situ sample points. The average values (AV) of the five in situ measurements were directly compared to the daily mean of the model results, which equally was spatially averaged from the corresponding five locations in the test field. In addition to the average, also the extremes (EV) of measured and modelled values are analyzed, indicating the measured natural heterogeneity and the models’ capability of tracing this spatial variability.
The vertical bars in
Figure 5 and
Figure 6 represent the measured extremes and therefore should not be misinterpreted as error bars, although naturally the field data are not entirely free of error. In
Figure 5 and
Figure 6, average values of
in situ measurements are displayed as solid black dots, average model results as solid black lines, extremes of in-situ measurements as black whiskers, modelled extremes as grey whiskers in the scatterplots (right) or as grey surfaces in the line diagrams (left), linear regressions as yellow solid lines and the 1:1 lines as dashed black lines. In the respective figure captions, “N” is the number of validation data pairs corresponding to the number of available field measurements. Validation results for structural canopy variables (
Figure 5) and fractionized aboveground dry biomass accumulation (
Figure 6) are displayed for the winter wheat test site in southern Germany.
Figure 5.
Validation of structural canopy variables of winter wheat in southern Germany: Modelled values vs. in situ measurements: (a) leaf area index, (b) canopy height, (c) phenology.
Figure 5.
Validation of structural canopy variables of winter wheat in southern Germany: Modelled values vs. in situ measurements: (a) leaf area index, (b) canopy height, (c) phenology.
Figure 6.
Validation of aboveground dry biomass accumulation of winter wheat in southern Germany: Modelled values vs. in situ measurements: (a) leaf biomass, (b) stem biomass, (c) fruit biomass.
Figure 6.
Validation of aboveground dry biomass accumulation of winter wheat in southern Germany: Modelled values vs. in situ measurements: (a) leaf biomass, (b) stem biomass, (c) fruit biomass.
The rapid increase of the leaf area in spring, the peak around the middle of June as well as the steady decrease during the ripening phase is well reproduced by the model. The modelled decrease of leaf area during July is steeper compared to the measurements (
Figure 5a, left). This is due to the fact that the non-destructive field measurements represent the total plant area, while the model actually calculates the photosynthetically active LAI (greenLAI), which is characterized by the decomposition of chlorophyll pigments during senescence. From the 16 available measurements of LAI only the 10 dates that were measured before the crop had developed to the phenological stage of fruit development (BBCH stage 70) are included in the validation plot (
Figure 5a, right). Nonetheless, the spatial variation of both, the measured and the modelled values, has the same dimension of roughly one LAI unit during the summer months.
The height of the modelled canopy largely influences the energy balance of the leaf because the height of the plants determines the advection of air into the canopy. For winter wheat, the canopy height is modelled as a direct function of the LAI, applying a crop-specific ratio between LAI and canopy height (
LHrel in
Table 1). Compared to the measured data, the modelled canopy height develops approximately one week earlier, but the height of the fully developed canopy is closely met by the model (
Figure 5b, left).
The most determining factor, concerning the realistic representation of the carbon allocation to the different fractions of the canopy, is a correct representation of phenological progress. The simulation of phenological progress is mainly determined through the air temperature. The realistic representation of the air temperature through the spatial interpolation of meteorological station data consequently leads to a rather precise mapping of the phenological stages (
Figure 5c).
Figure 5c also indicates that the process of hibernation is well traced by the model, as well as the rapid phenological development that characterizes the month of June. For the aboveground canopy fractions of “leaf”, “stem” and “fruit,” the modelled values of dry biomass reasonably match the field data concerning magnitude and seasonal development (
Figure 6a–c).
While the average accumulation of leaf mass and leaf senescence is generally traced, the high heterogeneity of the measurements is not reproduced by the model (
Figure 6a). Both a large spatial heterogeneity of the measured data and a strong scattering of the average values can be observed. This clearly reveals the measured data to be the result of destructive measuring techniques (see
Section 2.4), manifested in jumps and steps that are most prominent in the
in situ data of leaf biomass (
Figure 6a) and lowest for the fruit dry weight (
Figure 6c). The rapid increase of the stem dry weight during the stem elongation phase is well reproduced by the model, while the maximum stem mass might be slightly underestimated (
Figure 6b, left).
Apart from some deviations, the model results for the three discerned canopy fractions of winter wheat are mostly within the variability range of the
in situ measurements. The accurate representation of the magnitude and course of biomass accumulation during the growing season results in high model efficiency indices, with Nash-Sutcliffe coefficients varying between 0.79 for the leaves (
Figure 6a, right), 0.93 for the fruit (
Figure 6c, right) and 0.97 for the weight of the stems (
Figure 6b, right). In addition, the regressions mostly stay close to the one-to-one line and the respective intercepts are low, both indicating a non-biased representation of the biomass development through the model.
Altogether, the spatially distributed modeling of photosynthesis controlled vegetation growth with PROMET shows high correlations concerning R2, RMSE as well as Nash-Sutcliffe coefficients for all of the observed variables. The good representation of the phenological progress contributes to a realistic simulation of assimilate allocation into aboveground canopy fractions for the C3 metabolism winter crop and thus potentially allows for the delineation of crop yield from the fruit biomass. However, the models’ ability to reproduce spatial heterogeneities is limited by the spatial detail of the input parameters, so that the modelled variability, without remote sensing input, stays behind the variability observed in the field.
3.3. Validation of Spatial Dynamics
For the validation of the models capacity of reproducing spatial growth patterns based on the temporally highly resolved processes, the model was set up for the test site in northern Germany at a spatial resolution of 20 m. The spatial resolution thereby meets the information needs of farmers, because the machinery currently used for site-specific applications to the knowledge of the authors operates at spatial units between 24 and 36 m. The required spatial inputs (digital terrain model, soil map) were derived from publicly available data sources, while the meteorological driver variables were acquired from a met-service. The geometric features of the modelled fields as well as the dates of sowing and harvest could be derived from the Farm Management Information Systems (FMIS) of the test farms (
Table 4).
Table 4.
Basic input parameters used for the setup of the PROMET model for the test site in Northern Germany at a spatial resolution of 20 m.
Table 4.
Basic input parameters used for the setup of the PROMET model for the test site in Northern Germany at a spatial resolution of 20 m.
| Data Set | Data Source | Year | Resolution |
|---|
| Soil Map | Harmonized World Soil Database (HWSD, [69]) | 2009 | 30 arcsec |
| Digital Terrain Model | Shuttle Radar Topography Mission (SRTM, [70]) | 2008 | 90 m |
| Farm Mask | Farm Management Information System (FMIS) | 2010/2011 | 20 m |
| Meteorology | met-service | 2009–2011 | 10 stations (1 hour) |
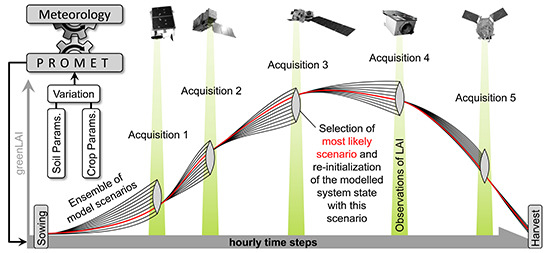
Because the assimilation algorithm relies on multiple observations during the course of one growing season (see
Section 2.3), multi-sensoral inputs should be taken into account to increase the probability of data availability. Spatially explicit maps of photosynthetically active leaf area could be derived from satellite images by applying model inversion techniques based on the complex canopy reflectance model SLC (Soil-Leaf-Canopy) [
61,
62]. A total of five image acquisitions were available for 2010 and seven images for 2011 (
Table 5). All spatial inputs including the greenLAI maps from both sensors were resampled to the common 20 m information unit and geometrically referenced to the model environment.
Table 5.
Earth Observation data available for assimilation into the PROMET model (OZA = Observer Zenith Angle, GSD = Ground Sampling Distance, RE = RapidEye, TM = Landsat 5 TM).
Table 5.
Earth Observation data available for assimilation into the PROMET model (OZA = Observer Zenith Angle, GSD = Ground Sampling Distance, RE = RapidEye, TM = Landsat 5 TM).
| Date 2010 | May 21st | Jun 16th | Jun 29th | Jul 8th | Jul 20th |
|---|
| Sensor | RE | RE | TM | TM | RE |
| OZA [°] | 10.33 | 6.96 | Nadir | Nadir | −12.04 |
| GSD [m] | 5 | 5 | 30 | 30 | 5 |
| Date 2011 | Mar 3rd | Apr 2nd | Apr 18th | May 5th | Jun 2nd | Jun 29th | Jul 27th |
| Sensor | RE | RE | RE | RE | RE | RE | TM |
| OZA [°] | −19.55 | 3.60 | 6.73 | 6.99 | 0.34 | −6.18 | Nadir |
| GSD [m] | 5 | 5 | 5 | 5 | 5 | 5 | 30 |
Taking the models’ good performance with respect to the reproduction of subdivided aboveground biomass accumulation into account (see
Section 3.2), the biomass values modelled for the fruit fraction of the canopy were considered as yield, after they had been reduced by a factor of 0.73 (
Table 1), accounting for dead material such as husks
etc., which contributes to the fruit biomass but is not part of the actual grain yield. The value of this factor is based on [
71], who have observed chaff contents of 27% for a selection of common wheat varieties. Being operated in ensemble mode (see
Section 2.3), the PROMET model was used to integrate the spatial heterogeneity imprinted in the greenLAI signal of the satellite observations into the modelled biomass and yield values. The resulting modelled spatial yield maps show high agreement with the spatial yield measurements for the two investigated seasons with respect to average, variability, standard deviation and spatial patterns (
Table 6).
Figure 7 directly compares the measured and modelled yield maps obtained for the season of 2010. Due to reasons of better comparability, the modelled yield map is masked to the pixels, where
in situ measurements were available. Data gaps within the fields are due to failures in the combine harvester yield measuring system. The model is able to produce spatially continuous gap-free yield maps.
Figure 7.
Spatial comparison of measured (
a) and modelled (
b) yield of winter wheat fields from the harvesting season of 2010 in northern Germany. Both maps are calculated for a spatial resolution of 20 × 20 m. The surface area of the displayed winter wheat fields amounts to >180 ha. A quantitative comparison is given in
Figure 8 and
Table 6.
Figure 7.
Spatial comparison of measured (
a) and modelled (
b) yield of winter wheat fields from the harvesting season of 2010 in northern Germany. Both maps are calculated for a spatial resolution of 20 × 20 m. The surface area of the displayed winter wheat fields amounts to >180 ha. A quantitative comparison is given in
Figure 8 and
Table 6.
The visual comparison is supported by a direct correlation of measured and modelled spatial data, which is displayed in
Figure 8 for both respected seasons. Due to the fact that the area considered for the year 2011 is nearly three times larger as the area modelled for 2010, it is not surprising that the overall heterogeneity was higher for 2011 (
Table 6). Although modelled and measured yield maps show a positive correlation for both seasons, a quite high amount of scattering still can be observed. Interpreting the scatterplots in
Figure 8, it has to be taken into account that the data clouds are the result of the comparison of two spatial data sets, so that not only thematic but also geometric distortions may contribute to the overall error.
Figure 8.
X-Y-Plots showing the correlation of modelled
vs. measured yield of all winter wheat pixels for the two consecutive seasons of 2010 (
a) and 2011 (
b). The corresponding statistics are given in
Table 6.
Figure 8.
X-Y-Plots showing the correlation of modelled
vs. measured yield of all winter wheat pixels for the two consecutive seasons of 2010 (
a) and 2011 (
b). The corresponding statistics are given in
Table 6.
Table 6.
Statistics of the validation of winter wheat yield for the two consecutive seasons of 2010 and 2011 corresponding to the X-Y-plots displayed in
Figure 8.
Table 6.
Statistics of the validation of winter wheat yield for the two consecutive seasons of 2010 and 2011 corresponding to the X-Y-plots displayed in Figure 8.
| Indicator | Symbol | 2010 | 2011 |
|---|
| Population: | N [pixels] | 4560 | 13135 |
| Area: | A [ha] | 182.4 | 525.4 |
| Coefficient of Determination: | R² [–] | 0.58 | 0.70 |
| Slope of linear regression: | α [–] | 0.90 | 1.22 |
| Intercept of linear regression: | β [t∙ha-1] | 0.27 | −1.81 |
| Root Mean Square Error: | RMSE [t∙ha−1] | 1.29 | 1.59 |
| Nash−Sutcliffe Coefficient of Model Efficiency: | NSE [–] | 0.50 | 0.67 |
| Average Measured Yield: | Ø Meas. [t∙ha−1] | 8.12 | 7.42 |
| Average Modelled Yield: | Ø Mod. [t∙ha−1] | 7.61 | 7.23 |
| Standard Deviation Measured Yield: | σ Meas. [t∙ha−1] | 1.53 | 1.92 |
| Standard Deviation Modelled Yield: | σ Mod. [t∙ha−1] | 1.81 | 2.79 |
| Minimum Measured Yield: | Min. Meas. [t∙ha−1] | 3.83 | 0.90 |
| Minimum Modelled Yield: | Min. Mod. [t∙ha−1] | 2.39 | 1.11 |
| Maximum Measured Yield: | Max. Meas. [t∙ha−1] | 11.58 | 12.90 |
| Maximum Modelled Yield: | Max. Mod. [t∙ha−1] | 11.74 | 14.58 |
The direct comparison of the statistics of both seasons is difficult, mostly because the locations of fields cultivated with winter wheat differ between the years. Crop rotation in the test area normally replaces winter wheat with rapeseed; in very few cases a crop rotation system with two consecutive seasons of wheat cultivation was applied. Field 33 is one example (
Figure 9 and
Figure 10), where winter wheat was cultivated during both respected seasons. Field 33 thus allows for a direct comparison of the two seasons and enables a view on the model’s capacity of also tracing distinct variations between different growth years.
Figure 9.
(
a) Zoom image from
Figure 7, showing spatially measured (
a) and modelled (
b) yield of winter wheat on test field 33 for the harvesting season of 2010, including an X-Y-plot of the correlation of the two spatial datasets (
c) as well as the modelled and EO-measured seasonal course of LAI-development for high (yellow pixel) and low (blue pixel) yielding sections of the field (
d). A quantitative comparison is given in
Table 7.
Figure 9.
(
a) Zoom image from
Figure 7, showing spatially measured (
a) and modelled (
b) yield of winter wheat on test field 33 for the harvesting season of 2010, including an X-Y-plot of the correlation of the two spatial datasets (
c) as well as the modelled and EO-measured seasonal course of LAI-development for high (yellow pixel) and low (blue pixel) yielding sections of the field (
d). A quantitative comparison is given in
Table 7.
Figure 9 and
Figure 10 show a detailed view of the approximately 40 ha large field and compare measured and modelled values for both seasons. With 9.26 t∙ha
−1, the average yield of field 33 in 2010 was significantly higher compared to 2011 where only 6.29 t∙ha
−1 were harvested, while the spatial heterogeneity was higher in 2011 compared to 2010, standard deviation increasing from 1.3 to 1.7 t∙ha
−1. This mostly can be traced to a strong scarcity of rainfall during the summer of 2011, resulting in phases of drought especially during May and June 2011. While during the growth cycle of 2010 about 563 mm of precipitation were registered at the test field, in 2011 only 476 mm of rainfall were measured. Under drought conditions, small-scale variations of soil quality become more distinctly visible in the yield map, resulting in a higher difference between low and high yielding sections of the field. For the more favorable growth conditions of 2010, the model was perfectly able to reproduce the measured yield map. For the more extreme meteorological conditions of 2011, the model performed equally well, but tended to overestimate high yielding field sections more distinctly compared to 2010 (
Table 7). The spatial patterns are nonetheless well reproduced by the model also for 2011, resulting in a high coefficient of determination of 0.82.
Figure 10.
Spatially measured (
a) and modelled (
b) yield of winter wheat on test field 33 for the harvesting season of 2011, including an X-Y-plot of the correlation of the two spatial datasets (
c) as well as the modelled and EO-measured seasonal course of LAI-development for high (yellow pixel) and low (blue pixel) yielding sections of the field (
d). A quantitative comparison is given in
Table 7.
Figure 10.
Spatially measured (
a) and modelled (
b) yield of winter wheat on test field 33 for the harvesting season of 2011, including an X-Y-plot of the correlation of the two spatial datasets (
c) as well as the modelled and EO-measured seasonal course of LAI-development for high (yellow pixel) and low (blue pixel) yielding sections of the field (
d). A quantitative comparison is given in
Table 7.
Table 7.
Validation statistics for field 33 (2010
vs. 2011). Also compare
Figure 9 and
Figure 10.
Table 7.
Validation statistics for field 33 (2010 vs. 2011). Also compare Figure 9 and Figure 10.
| Indicator | | 2010 | 2011 |
|---|
| N | = | 1019 [Pixels] | 1019 [pixels] |
| A | = | 40.76 [ha] | 40.76 [ha] |
| R² | = | 0.67 [–] | 0.82 [–] |
| α | = | 0.89 [–] | 1.41 [–] |
| β | = | 1.39 [t∙ha-1] | −2.3 [t∙ha-1] |
| RMSE | = | 0.92 [t∙ha-1] | 1.38 [t∙ha-1] |
| NSE | = | 0.53 [–] | 0.34 [–] |
| Ø Meas. / Mod. | = | 9.16/9.54 [t∙ha−1] | 6.29/6.59 [t∙ha−1] |
| σ Meas. / Mod. | = | 1.30/1.42 [t∙ha−1] | 1.70/2.66 [t∙ha−1] |
| Min. Meas. / Mod. | = | 4.70/4.33 [t∙ha−1] | 1.50/1.62 [t∙ha−1] |
| Max. Meas. / Mod. | = | 11.21/11.40 [t∙ha−1] | 10.33/13.15 [t∙ha−1] |