Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys
Abstract
:1. Introduction
| Symbol | Description | Dimension |
|---|---|---|
| NEP | Net ecosystem productivity | g O2 m−2·h−1 |
| PARw | Photosynthetic active radiation (underwater) | W·m−2 |
| PARsurf | Photosynthetic active radiation (above water) | W·m−2 |
| cT | Total attenuation (including water) | m−1 |
| aT | Total absorption (including water) | m−1 |
| bT | Total scattering | m−1 |
| ct | Total attenuation (without water absorption) | m−1 |
| cw | Attenuation by pure water | m−1 |
| cp | Attenuation by particles | m−1 |
| at | Total absorption (without water absorption) | m−1 |
| ag | Absorption by the dissolved fraction | m−1 |
| ap | Absorption by particles | m−1 |
| aw | Absorption by pure water | m−1 |
| bp | Particle scattering | m−1 |
| bbp | Particle backscattering | m−1 |
| Ed | Downwelling irradiance | W m−2·nm−1 |
| Chl(m) | Moored chlorophyll-a fluorescence | Counts |
| CDOM(m) | Moored colored dissolve organic matter fluorescence | Counts |
| Turb(m) | Moored turbidity | Counts |
| Chl(FlowT) | Flow-through chlorophyll-a fluorescence | Counts |
| CDOM(FlowT) | Flow-through colored dissolve organic matter fluorescence | Counts |
| Turb(FlowT) | Flow-through turbidity | Counts |
2. Data and Methodology
2.1. Study Area

| Study Site | Date (mm/dd/yy) | Latitude (Deg.) | Longitude (Deg.) | Depth (m) | Water Level Range (m) | Currents Direction (Deg.) | Currents Speed (cm∙s−1) |
|---|---|---|---|---|---|---|---|
| Patch Reef | 05/15/12–05/23/12 | 24.5616 | -81.5557 | 8 | 0.7 | 187 | 3.92 |
| 10/15/12–10/20/12 | 24.5615 | -81.5548 | 6 | 0.8 | 223 | 8.88 | |
| Seagrass | 05/15/12–05/23/12 | 24.6006 | -81.5531 | 4 | -- | -- | -- |
| 10/15/12–10/20/12 | 24.5999 | -81.5544 | 5 | 0.7 | 201 | 4.63 |
2.2. Optical and Hydrographic Data Collection and Processing
2.3. Net Benthic Productivity
2.4. Photosynthetic Active Radiation (PAR)
2.5. Statistical Analyses
3. Results
3.1. Temporal Variations of Hydrographic and Meteorological Parameters

3.2. Moored Optical Observations

3.3. Water Column Optical Observations


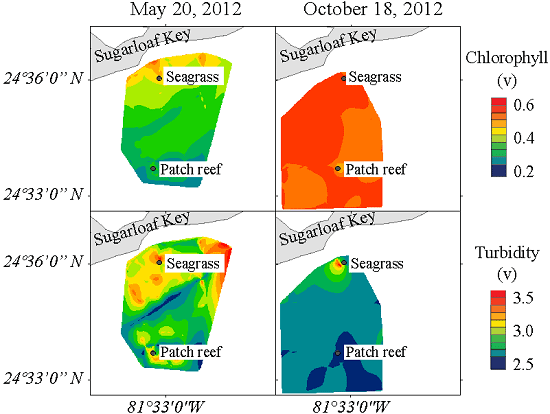
3.4. Spatial Distribution of Hydrographic Parameters at the Surface

3.5. Net Benthic Productivity
| Level | N | Mean | S.D. | df | Z | p | |
|---|---|---|---|---|---|---|---|
| Temporal (PR) | |||||||
| May | 53 | 0.137 | 0.060 | 1 | 2.859 | 0.004 | |
| October | 42 | 0.177 | 0.107 | ||||
| Spatial (Oct) | |||||||
| PR | 42 | 0.177 | 0.107 | 1 | −3.054 | 0.002 | |
| SG | 44 | 0.241 | 0.071 | ||||

| Area/Month | Variables | N | S | p | |||
|---|---|---|---|---|---|---|---|
| Moored Variables | |||||||
| PR (May) | NEPSHARQ | CDOM(m) | 53 | 0.05 | 0.727 | ||
| Chl(m) | 53 | −0.34 | 0.013 * | ||||
| Turb(m) | 53 | −0.18 | 0.204 | ||||
| PARw | 53 | 0.29 | 0.036 * | ||||
| PARsurf | 53 | 0.28 | 0.043 * | ||||
| PR (Oct) | NEPSHARQ | CDOM(m) | 42 | −0.18 | 0.249 | ||
| Chl(m) | 42 | 0.21 | 0.181 | ||||
| Turb(m) | 42 | 0.15 | 0.334 | ||||
| PARw | 42 | 0.21 | 0.191 | ||||
| PARsurf | 42 | 0.42 | 0.005 * | ||||
| SG (Oct) | NEPSHARQ | CDOM(m) | 44 | 0.12 | 0.423 | ||
| Chl(m) | 44 | −0.27 | 0.073 | ||||
| Turb(m) | 44 | −0.15 | 0.335 | ||||
| PARw | 37 | 0.78 | < | 0.001 * | |||
| PARsurf | 37 | 0.71 | < | 0.001 * | |||
| Water Column Variables | |||||||
| PR (May) | NEPSHARQ | aT(406-487) | 5 | >0.80 | > | 0.104 | |
| aT(492-512) | 5 | 0.90 | 0.037 * | ||||
| aT(517-546) | 5 | 1.00 | < | 0.0001 * | |||
| aT(551) | 5 | 0.90 | 0.037 * | ||||
| aT(556-591) | 5 | 0.70 | 0.188 | ||||
| aT(595-614) | 5 | 1.00 | < | 0.0001 * | |||
| aT(619-661) | 5 | 0.70 | 0.188 | ||||
| aT(666-699) | 5 | −0.50 | 0.391 | ||||
| ap(402-438) | 5 | 0.90 | 0.037 * | ||||
| ap(443-587) | 5 | 0.70 | 0.188 | ||||
| ap(591) | 5 | 0.90 | 0.037 * | ||||
| ap(595-699) | 5 | <−0.10 | > | 0.624 | |||
| ag(402-699) | 5 | <0.70 | > | 0.104 | |||
| Ed(402-700) | 5 | 0.90 | 0.037 * | ||||
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paulay, G. Diversity and distribution of reef organisms. In Life and Death of Coral Reefs; Birkeland, C., Ed.; Chapman & Hall: New York, NY, USA, 1997; pp. 298–353. [Google Scholar]
- Costanza, R.; D’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Short, F.; Carruthers, T.; Dennison, W.; Waycott, M. Global seagrass distribution and diversity: A bioregional model. J. Exp. Mar. Biol. Ecol. 2007, 350, 3–20. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in Aquatic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994; p. 509. [Google Scholar]
- Morel, A.; Prieur, L. Analysis of variations in ocean color. Limnol. Oceanogr. 1977, 22, 709–722. [Google Scholar] [CrossRef]
- Morel, A. Optical Modeling of the Upper Ocean in Relation to Its Biogenous Matter Content (Case I Waters). J. Geophys. Res. 1988, 93, 10749–10768. [Google Scholar] [CrossRef]
- Coble, P.G.; Hu, C.; Gould, R.W., Jr.; Chang, G.; Wood, A.M. Colored Dissolved Organic in the Coastal Ocean: An Optical Tool for Coastal Zone Environmental Assessment and Management. Oceanography 2004, 17, 50–59. [Google Scholar] [CrossRef]
- Mobley, C.D.; Stramski, D.W.; Bissett, P.; Boss, E. Optical Modeling of Ocean Waters: Is the Case 1-Case 2 Classification Still Useful? Oceanography 2005, 17, 60–67. [Google Scholar] [CrossRef]
- Schofield, O.; Bergmann, T.; Oliver, M.J.; Irwin, A.; Kirkpatrick, G.; Bissett, W.P.; Moline, M.A.; Orrico, C. Inversion of spectral absorption in the optically complex coastal waters of the Mid-Atlantic Bight. J. Geophys. Res. 2004, 109, C12S04. [Google Scholar] [CrossRef]
- Boss, E.; Pegau, W.S.; Zaneveld, J.R.V.; Barnard, A.H. Spatial and temporal variability of absorption by dissolved material at a continental shelf. J. Geophys. Res. 2001, 106, 9499–9507. [Google Scholar] [CrossRef]
- Fortes, M.D. Wetland conservation and management in the Philippines: Where are we now? The Case of Seagrass and Mangrove. In Wetlands Ecosystems in Asia; Wong, H.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 233–262. [Google Scholar]
- Chen, Z.; Hu, C.; Muller-Karger, F.E.; Luther, M.E. Short-term variability of suspended sediment and phytoplankton in Tampa Bay, Florida: Observations from a coastal oceanographic tower and ocean color satellites. Estuar. Coast. Shelf Sci. 2010, 89, 62–72. [Google Scholar] [CrossRef]
- Jones, B.H.; Lee, C.M.; Toro-Farmer, G.; Boss, E.S.; Gregg, M.C.; Villanoy, C.L. Tidally driven exchange in an Archipelago Strait: Biological and optical responses. Oceanography 2011, 24, 142–155. [Google Scholar] [CrossRef]
- Farfán, L.; D’Sa, E.; Liu, K.; Rivera-Monroy, V. Tropical cyclone impacts on coastal regions: The case of the Yucatán and the Baja California Peninsulas, Mexico. Estuaries Coasts 2014, 37, 1388–1402. [Google Scholar] [CrossRef]
- Edinger, E.N.; Jompa, J.; Limmon, G.V.; Widjatmoko, W.; Risk, M.J. Reef degradation and coral biodiversity in indonesia: Effects of land-based pollution, destructive fishing practices and changes over time. Mar. Pollut. Bull. 1998, 36, 617–630. [Google Scholar] [CrossRef]
- Te, F.T. Turbidity and its effects on corals: A model using the extinction coefficient (k) of photosynthetic active radiance (PAR). In Proceedings of the 8th International Coral Reef Symposium, Panama City, Panama, 24–29 June 1996; Volume 2, pp. 1899–1904.
- Carpenter, R.C. Relationships between primary production and irradiance in coral reef algal communities. Limnol. Oceanogr. 1985, 30, 784–793. [Google Scholar] [CrossRef]
- Barnes, D.J.; Chalker, B.E. Calcification and photosynthesis in reef-building corals and algae. In Ecosystems of the World 25: Coral Reefs; Dubinsky, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 109–131. [Google Scholar]
- Falkowski, P.G.; Jokiel, P.L.; Kinzie, R.A.I. Irradiance and corals. In Ecosystems of the World 25: Coral Reefs; Dubinsky, Z., Ed.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 89–107. [Google Scholar]
- Yates, K.K.; Halley, R.B. Measuring coral reef community metabolism using new benthic chamber technology. Coral Reefs 2003, 22, 247–255. [Google Scholar] [CrossRef]
- Falter, J.L.; Lowe, R.J.; Atkinson, M.J.; Cuet, P. Seasonal coupling and de-coupling of net calcification rates from coral reef metabolism and carbonate chemistry at Ningaloo Reef, Western Australia. J. Geophys. Res. 2012, 117, C05003. [Google Scholar] [CrossRef]
- Venti, A.; Andersson, A.; Langdon, C. Multiple driving factors explain spatial and temporal variability in coral calcification rates on the Bermuda platform. Coral Reefs 2014, 33, 1–19. [Google Scholar]
- Larcombe, P.; Ridd, P.V.; Prytz, A.; Wilson, B. Factors controlling suspended sediment on inner-shelf coral reefs, Townsville, Australia. Coral Reefs 1995, 14, 163–171. [Google Scholar] [CrossRef]
- Boss, E.; Zaneveld, J.R.V. The effect of bottom substrate on inherent optical properties: Evidence of biogeochemical processes. Limnol. Oceanogr. 2003, 48, 346–354. [Google Scholar] [CrossRef]
- Ogston, A.S.; Storlazzi, C.D.; Field, M.E.; Presto, M.K. Sediment resuspension and transport patterns on a fringing reef flat, Molokai, Hawaii. Coral Reefs 2004, 23, 559–569. [Google Scholar]
- Piniak, G.A.; Storlazzi, C.D. Diurnal variability in turbidity and coral fluorescence on a fringing reef flat: Southern Molokai, Hawaii. Estuar. Coast. Shelf Sci. 2008, 77, 56–64. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Calado, R.; Cartaxana, P.; Furtado, J.; Serôdio, J. Photobiology and growth of leather coral Sarcophyton cf. glaucum fragments stocked under low light in a recirculated system. Aquaculture 2013, 414–415, 235–242. [Google Scholar] [CrossRef]
- Rocha, R.J.M.; Pimentel, T.; Serôdio, J.; Rosa, R.; Calado, R. Comparative performance of light emitting plasma (LEP) and light emitting diode (LED) in ex situ aquaculture of scleractinian corals. Aquaculture 2013, 402–403, 38–45. [Google Scholar] [CrossRef]
- Wijgerde, T.; van Melis, A.; Silva, C.I.F.; Leal, M.C.; Vogels, L.; Mutter, C.; Osinga, R. Red light represses the photophysiology of the scleractinian coral Stylophora pistillata. PLoS ONE 2014, 9, e92781. [Google Scholar]
- Blondeau-Patissier, D.; Brando, V.E.; Oubelkheir, K.; Dekker, A.G.; Clementson, L.A.; Daniel, P. Bio-optical variability of the absorption and scattering properties of the Queensland inshore and reef waters, Australia. J. Geophys. Res. 2009, 114, C05003. [Google Scholar] [CrossRef]
- Dierssen, H.M.; Zimmerman, R.C.; Burdige, D.J. Optics and remote sensing of Bahamian carbonate sediment whitings and potential relationship to wind-driven Langmuir circulation. Biogeosciences 2009, 6, 487–500. [Google Scholar] [CrossRef]
- McPherson, M.; Hill, V.; Zimmerman, R.; Dierssen, H. The optical properties of Greater Florida Bay: Implications for seagrass abundance. Estuaries Coasts 2011, 34, 1150–1160. [Google Scholar] [CrossRef]
- Schlacher, T.A.; Stark, J.; Fischer, A.B.P. Evaluation of artificial light regimes and substrate types for aquaria propagation of the staghorn coral Acropora solitaryensis. Aquaculture 2007, 269, 278–289. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J.; Apprill, A.; Andréfouët, S. Spectral reflectance of coral. Coral Reefs 2004, 23, 84–95. [Google Scholar] [CrossRef]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Waters; Academic Press: San Diego, CA, USA, 1994; p. 592. [Google Scholar]
- Zaneveld, J.R.V.; Twardowski, M.J.; Barnard, A.; Lewis, M.R. Introduction to radiative transfer. In Remote Sensing of Coastal Aquatic Environments; Miller, R.L., del Castillo, C.E., Mckee, B.A., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2005; pp. 1–20. [Google Scholar]
- Woźniak, B.; Dera, J. Light Absorption in Sea Water; Springer: New York, NY, USA, 2007. [Google Scholar]
- Arnone, R.A.; Wood, M.; Gould, R.W., Jr. The Evolution of Optical Water Mass Classification. Oceanography 2004, 17, 14–15. [Google Scholar] [CrossRef]
- Andréfouët, S.; Mumby, P.; McField, M.; Hu, C.; Muller-Karger, F. Revisiting coral reef connectivity. Coral Reefs 2002, 21, 43–48. [Google Scholar] [CrossRef]
- Hu, C.; Hackett, K.E.; Callahan, M.K.; Andréfouët, S.; Wheaton, J.L.; Porter, J.W.; Muller-Karger, F.E. The 2002 ocean color anomaly in the Florida Bight: A cause of local coral reef decline? Geophys. Res. Lett. 2003, 30, 1151. [Google Scholar] [CrossRef]
- Hu, C.; Muller-Karger, F.E.; Vargo, G.A.; Neely, M.B.; Johns, E. Linkages between coastal runoff and the Florida Keys ecosystem: A study of a dark plume event. Geophys. Res. Lett. 2004, 31, L15307. [Google Scholar] [CrossRef]
- Olascoaga, M.J.; Beron-Vera, F.J.; Brand, L.E.; Kocak, H. Tracing the early development of harmful algal blooms on the West Florida Shelf with the aid of Lagrangian coherent structures. J. Geophys. Res. 2008, 113, C12014. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, J.P.; Hu, C.; English, D.C.; Carder, K.L.; Heil, C.A.; Müller-Karger, F.E. Detection of Karenia brevis blooms on the west Florida shelf using in situ backscattering and fluorescence data. Harmful Algae 2009, 8, 898–909. [Google Scholar] [CrossRef]
- Spalding, M.D.; Ravilious, C.; Green, E.P. World Atlas of Coral Reefs; Universtity of California Press: Berkeley, CA, USA, 2001; p. 416. [Google Scholar]
- Lee, T.N.; Smith, N. Volume transport variability through the Florida Keys tidal channels. Cont. Shelf Res. 2002, 22, 1361–1377. [Google Scholar] [CrossRef]
- Smith, N.P.; Lee, T.N. Volume transport through tidal channels in the Middle Florida Keys. J. Coast. Res. 2003, 19, 254–260. [Google Scholar]
- Smith, N.P. Long-Term gulf-to-Atlantic transport through tidal channels in the Florida Keys. Bull. Mar. Sci. 1994, 54, 602–609. [Google Scholar]
- Sullivan, J.M.; Twardowski, M.S.; Zaneveld, J.R.V.; Moore, C.M.; Barnard, A.H.; Donaghay, P.L.; Rhoades, B. Hyperspectral temperature and salt dependencies of absorption by water and heavy water in the 400–750 nm spectral range. Appl. Opt. 2006, 45, 5294–5309. [Google Scholar] [CrossRef] [PubMed]
- Zaneveld, J.R.V.; Kitchen, J.C.; Moore, C.C. Scattering error correction of reflecting-tube absorption meters. Proc. SPIE 1994, 2258. [Google Scholar] [CrossRef]
- Yates, K.K.; Halley, R.B. CO32− concentration and pCO2 thresholds for calcification and dissolution on the Molokai reef flat, Hawaii. Biogeosciences 2006, 3, 357–369. [Google Scholar] [CrossRef]
- Yates, K.K.; Halley, R.B. Diurnal variation in rates of calcification and carbonate sediment dissolution in Florida Bay. Estuaries Coasts 2006, 29, 24–39. [Google Scholar] [CrossRef]
- Morel, A.; Smith, R.C. Relation between total quanta and total energy for aquatic photosynthesis. Limnol. Oceanogr. 1974, 19, 591–600. [Google Scholar] [CrossRef]
- Cullen, J.J.; Lewis, M.R. Biological processes and optical measurements near the sea surface: Some issues relevant to remote sensing. J. Geophys. Res. 1995, 100, 13255–13266. [Google Scholar] [CrossRef]
- Sackmann, B.S.; Perry, M.J.; Eriksen, C.C. Seaglider observations of variability in daytime fluorescence quenching of chlorophyll-a in Northeastern Pacific coastal waters. Biogeosci. Discuss 2008, 5, 2839–2865. [Google Scholar] [CrossRef]
- Sponaugle, S.; Lee, T.; Kourafalou, V.; Pinkard, D. Florida current frontal eddies and the settlement of coral reef fishes. Limonol. Oceanogr. 2005, 50, 1033–1048. [Google Scholar] [CrossRef]
- Monismith, S.G. Hydrodynamics of Coral Reefs. Annu. Rev. Fluid Mech. 2006, 39, 37–55. [Google Scholar] [CrossRef]
- Boss, E.; Pegau, W.S.; Gardner, W.D.; Zaneveld, J.R.V.; Barnard, A.H.; Twardowski, M.S.; Chang, G.C.; Dickey, T.D. Spectral particulate attenuation and particle size distribution in the bottom boundary layer of a continental shelf. J. Geophys. Res. 2001, 106, 9509–9516. [Google Scholar] [CrossRef]
- Otis, D.B.; Carder, K.L.; English, D.C.; Ivey, J.E. CDOM transport from the Bahamas Banks. Coral Reefs 2004, 23, 152–160. [Google Scholar] [CrossRef]
- Ouillon, S.; Douillet, P.; Lefebvre, J.P.; le Gendre, R.; Jouon, A.; Bonneton, P.; Fernandez, J.M.; Chevillon, C.; Magand, O.; Lefevre, J.; et al. Circulation and suspended sediment transport in a coral reef lagoon: The south-west lagoon of New Caledonia. Mar. Pollut. Bull. 2010, 61, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Leichter, J.J.; Wing, S.R.; Miller, S.L.; Denny, M.W. Pulsed delivery of subthermocline water to Conch Reef (Florida Keys) by internal tidal bores. Limnol. Oceanogr. 1996, 41, 1490–1501. [Google Scholar] [CrossRef]
- Leichter, J.J.; Stewart, H.L.; Miller, S.L. Episodic nutrient transport to Florida coral reefs. Limnol. Oceanogr. 2003, 48, 1394–1407. [Google Scholar] [CrossRef]
- Jouon, A.; Ouillon, S.; Douillet, P.; Lefebvre, J.P.; Fernandez, J.M.; Mari, X.; Froidefond, J.-M. Spatio-temporal variability in suspended particulate matter concentration and the role of aggregation on size distribution in a coral reef lagoon. Mar. Geol. 2008, 256, 36–48. [Google Scholar] [CrossRef]
- Barron, M.G.; Vivian, D.N.; Yee, S.H.; Santavy, D.L. Methods to estimate solar radiation dosimetry in coral reefs using remote sensed, modeled, and in situ data. Environ. Monit. Assess. 2009, 151, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Briceño, H.O.; Boyer, J.N.; Castro, J.; Harlem, P. Biogeochemical classification of South Florida’s estuarine and coastal waters. Mar. Pollut. Bull. 2013, 75, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Fourqurean, J.W.; Willsie, A.; Rose, C.D.; Rutten, L.M. Spatial and temporal pattern in seagrass community composition and productivity in South Florida. Mar. Biol. 2001, 138, 341–354. [Google Scholar] [CrossRef]
- Kuffner, I.B.; Hickey, T.D.; Morrison, J.M. Calcification rates of the massive coral Siderastrea siderea and crustose coralline algae along the Florida Keys (USA) outer-reef tract. Coral Reefs 2013, 32, 987–997. [Google Scholar] [CrossRef]
- Zimmerman, R.C. Light and photosynthesis in seagrass meadows. In Seagrasses: Biology, Ecology and Conservation; Larkum, A., Orth, R.J., Duarte, C., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2006; pp. 303–321. [Google Scholar]
- Nakamura, T.; Nakamori, T. Estimation of photosynthesis and calcification rates at a fringing reef by accounting for diurnal variations and the zonation of coral reef communities on reef flat and slope: A case study for the Shiraho reef, Ishigaki Island, southwest Japan. Coral Reefs 2009, 28, 229–250. [Google Scholar] [CrossRef]
- Schutter, M.; van Velthoven, B.; Janse, M.; Osinga, R.; Janssen, M.; Wijffels, R.; Verreth, J. The effect of irradiance on long-term skeletal growth and net photosynthesis in Galaxea fascicularis under four light conditions. J. Exp. Mar. Biol. Ecol. 2008, 367, 75–80. [Google Scholar] [CrossRef]
- Turk, D.; Yates, K.; Vega-Rodriguez, M.; Toro-Farmer, G.; L’Esperance, C.; Melo, N.; Ramsewak, D.; Dowd, M.; Cerdeira Estrada, S.; Muller-Karger, F.; et al. Community metabolism in shallow coral reef and seagrass ecosystems, lower Florida Keys. Mar. Ecol. Prog. Ser. 2015, 538, 35–52. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Barile, P.J.; Matzie, W.R. Anthropogenic nutrient enrichment of seagrass and coral reef communities in the Lower Florida Keys: Discrimination of local versus regional nitrogen sources. J. Exp. Mar. Biol. Ecol. 2004, 308, 23–58. [Google Scholar] [CrossRef]
- Jantzen, C.; Schmidt, G.M.; Wild, C.; Roder, C.; Khokiattiwong, S.; Richter, C. Benthic reef primary production in response to large amplitude internal waves at the Similan Islands (Andaman Sea, Thailand). PLoS ONE 2013, 8, e81834. [Google Scholar]
- Naumann, M.S.; Jantzen, C.; Haas, A.F.; Iglesias-Prieto, R.; Wild, C. Benthic primary production budget of a Caribbean reef lagoon (Puerto Morelos, Mexico). PLoS ONE 2013, 8, e82923. [Google Scholar]
- Mass, T.; Kline, D.I.; Roopin, M.; Veal, C.J.; Cohen, S.; Iluz, D.; Levy, O. The spectral quality of light is a key driver of photosynthesis and photoadaptation in Stylophora pistillata colonies from different depths in the Red Sea. J. Exp. Biol. 2010, 213, 4084–4091. [Google Scholar] [CrossRef] [PubMed]
- Bidigare, R.R.; Ondrusek, M.E.; Morrow, J.H.; Kiefer, D.A. In-Vivo absorption properties of algal pigments. Proc. SPIE 1990, 1302, 290–302. [Google Scholar]
- Aguirre-Gomez, R.; Weeks, A.R.; Boxall, S.R. The identification of phytoplankton pigments from absorption spectra. Int. J. Remote Sens. 2001, 22, 315–338. [Google Scholar] [CrossRef]
- IOCCG. Remote Sensing of Inherent Optical Properties: Fundamentals, Tests of Algorithms, and Applications; Reports of the International Ocean-Colour Coordinating Group, No. 5; Lee, Z.P., Ed.; IOCCG: Dartmouth, NS, Canada, 2006; p. 126. [Google Scholar]
- Dupouy, C.; Neveux, J.; Ouillon, S.; Frouin, R.; Murakami, H.; Hochard, S.; Dirberg, G. Inherent optical properties and satellite retrieval of chlorophyll concentration in the lagoon and open ocean waters of New Caledonia. Mar. Pollut. Bull. 2010, 61, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cannizzaro, J.; Carder, K.L.; Muller-Karger, F.E.; Hardy, R. Remote detection of Trichodesmium blooms in optically complex coastal waters: Examples with MODIS full-spectral data. Remote Sens. Environ. 2010, 114, 2048–2058. [Google Scholar] [CrossRef]
- Volpe, V.; Silvestri, S.; Marani, M. Remote sensing retrieval of suspended sediment concentration in shallow waters. Remote Sens. Environ. 2011, 115, 44–54. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Andrefouet, S.; Tyler, M.R. Sea surface correction of high spatial resolution ikonos images to improve bottom mapping in near-shore environments. IEEE Trans. Geosci. Remote Sens. 2003, 41, 1724–1729. [Google Scholar] [CrossRef]
- Purkis, S.J.; Pasterkamp, R. Integrating in situ reef-top reflectance spectra with Landsat TM imagery to aid shallow-tropical benthic habitat mapping. Coral Reefs 2004, 23, 5–20. [Google Scholar] [CrossRef]
- Palandro, D.A.; Andréfouët, S.; Hu, C.; Hallock, P.; Muller-Karger, F.E.; Dustan, P.; Callahan, M.K.; Kranenburg, C.; Beaver, C.R. Quantification of two decades of shallow-water coral reef habitat decline in the Florida Keys National Marine Sanctuary using Landsat data (1984–2002). Remote Sens. Environ. 2008, 112, 3388–3399. [Google Scholar] [CrossRef]
- Hochberg, E.; Atkinson, M. Coral reef benthic productivity based on optical absorptance and light-use efficiency. Coral Reefs 2008, 27, 49–59. [Google Scholar] [CrossRef]
- Lee, Z.; Carder, K.L.; Mobley, C.D.; Steward, R.G.; Patch, J.S. Hyperspectral Remote Sensing for Shallow Waters. 2. Deriving Bottom Depths and Water Properties by Optimization. Appl. Opt. 1999, 38, 3831–3843. [Google Scholar] [CrossRef] [PubMed]
- Botha, E.J.; Brando, V.E.; Anstee, J.M.; Dekker, A.G.; Sagar, S. Increased spectral resolution enhances coral detection under varying water conditions. Remote Sens. Environ. 2013, 131, 247–261. [Google Scholar] [CrossRef]
- Devred, E.; Turpie, K.R.; Moses, W.; Klemas, V.V.; Moisan, T.; Babin, M.; Toro-Farmer, G.; Forget, M.-H.; Jo, Y.-H. Future retrievals of water column bio-optical properties using the Hyperspectral Infrared Imager (HyspIRI). Remote Sens. 2013, 5, 6812–6837. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toro-Farmer, G.; Muller-Karger, F.E.; Vega-Rodríguez, M.; Melo, N.; Yates, K.; Cerdeira-Estrada, S.; Herwitz, S.R. Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys. Remote Sens. 2016, 8, 86. https://doi.org/10.3390/rs8020086
Toro-Farmer G, Muller-Karger FE, Vega-Rodríguez M, Melo N, Yates K, Cerdeira-Estrada S, Herwitz SR. Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys. Remote Sensing. 2016; 8(2):86. https://doi.org/10.3390/rs8020086
Chicago/Turabian StyleToro-Farmer, Gerardo, Frank E. Muller-Karger, Maria Vega-Rodríguez, Nelson Melo, Kimberly Yates, Sergio Cerdeira-Estrada, and Stanley R. Herwitz. 2016. "Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys" Remote Sensing 8, no. 2: 86. https://doi.org/10.3390/rs8020086
APA StyleToro-Farmer, G., Muller-Karger, F. E., Vega-Rodríguez, M., Melo, N., Yates, K., Cerdeira-Estrada, S., & Herwitz, S. R. (2016). Characterization of Available Light for Seagrass and Patch Reef Productivity in Sugarloaf Key, Lower Florida Keys. Remote Sensing, 8(2), 86. https://doi.org/10.3390/rs8020086