NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling
Abstract
:1. Introduction

2. Results and Discussion
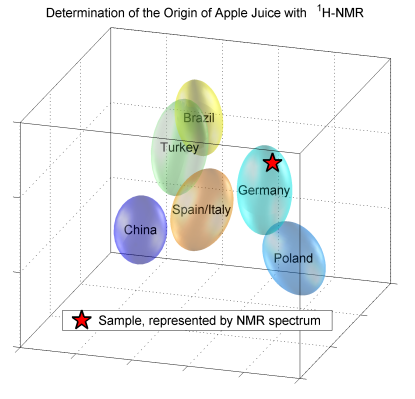
2.1. Quantification (Targeted Analysis)
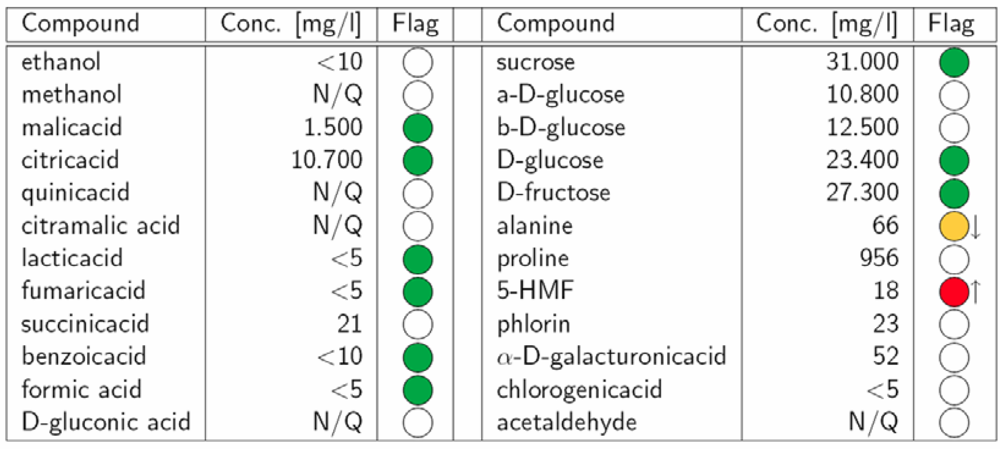 |
2.2. Statistical Analysis (Non-Targeted)
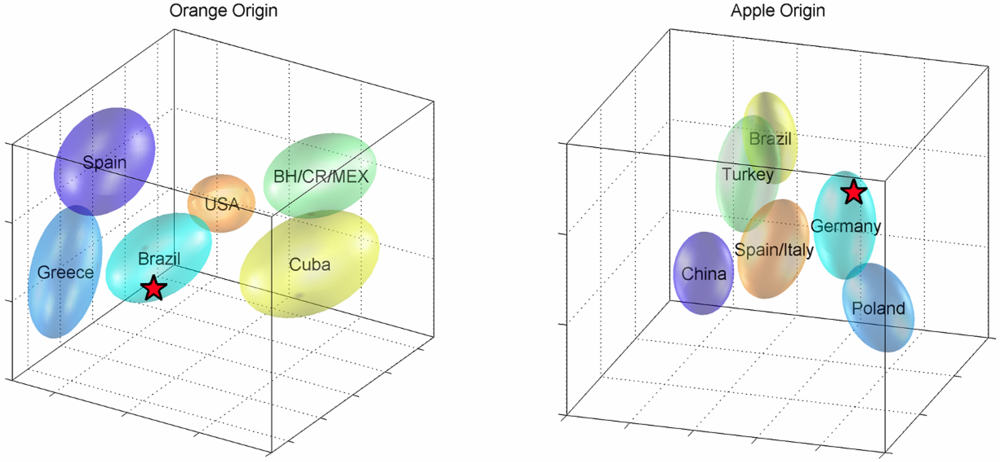

3. Experimental Section
4. Conclusions
References and Notes
- Rinke, P.; Moitrier, S.; Humpfer, E.; Keller, S.; Moertter, M.; Godejohann, M.; Hofmann, G.; Schaefer, H.; Spraul, M. An 1H-NMR-Technique for high throughput screening in quality and authenticity control of fruit juice and fruit raw materials. Fruit Process. 2007, 17, 10–18. [Google Scholar]
- Spraul, M.; Humpfer, E.; Schäfer, H.; Schütz, B.; Mörtter, M.; Rinke, P. NMR-Based Mixture Analysis on the Example of Fruit Juice Quality Control Using Statistics and Quantification. In NMR Spectroscopy in Pharmaceutical Analysis; Holzgrabe, U., Wawer, I., Diehl, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 319–339. [Google Scholar]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. Metabonomics: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar]
- Skordi, E.; Yap, I.; Claus, S.; Martin, F.-P.; Cloarec, O.; Lindberg, J.; Schuppe-Koistinen, I.; Holmes, E.; Nicholson, J.K. Analysis of time-related metabolic fluctuations induced by ethionine in the rat. J. Proteome Res. 2007, 6, 4572–4581. [Google Scholar] [CrossRef] [PubMed]
- Coen, M.; Lenz, E.M.; Nicholson, J.K.; Wilson, I.D.; Pognan, F.; Lindon, J.C. An integrated metabonomic investigation of acetaminophen toxicity in the mouse using NMR spectroscopy. Chem. Res. Toxicol. 2003, 16, 295–303. [Google Scholar]
- Wikoff, W.R.; Gangoitti, J.A.; Barshop, B.A.; Siuzdak, G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin. Chem. 2007, 53, 2169–2176. [Google Scholar]
- Bamforth, F.J.; Dorian, V.; Vallance, H.; Wishart, D.S. Diagnosis of inborn errors of metabolism using 1H NMR spectroscopic analysis of urine. J. Inherit. Metab. Dis. 1999, 22, 297–301. [Google Scholar]
- Assfalg, M.; Bertini, I.; Colangiuli, L.C.; Schäfer, H.; Schütz, B.; Spraul, M. Evidence of different metabolic phenotypes in humans. PNAS 2008, 105, 1420–1424. [Google Scholar]
- Lachenmeier, D.; Humpfer, E.; Fang, F.; Schütz, B.; Dvortsak, P.; Sproll, C.; Spraul, M. NMR-Spectroscopy for nontargeted screening and simultaneous quantification of health-relevant compounds in foods: The example of melamine. J. Agric. Food Chem. 2009, 57, 7194–7199. [Google Scholar]
- Kent, J.T.; Bibby, J.M.; Mardia, K.V. Multivariate Analysis; Academic Press Inc.: Maryland Heights, MO, USA, 1980. [Google Scholar]
- Eugene, S.T. Randomization Tests; Marcel Dekker Ltd: New York, NY, USA, 1995. [Google Scholar]
- Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments; Wiley-VCH: Berlin, Germany, 1998. [Google Scholar]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Spraul, M.; Schütz, B.; Rinke, P.; Koswig, S.; Humpfer, E.; Schäfer, H.; Mörtter, M.; Fang, F.; Marx, U.C.; Minoja, A. NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling. Nutrients 2009, 1, 148-155. https://doi.org/10.3390/nu1020148
Spraul M, Schütz B, Rinke P, Koswig S, Humpfer E, Schäfer H, Mörtter M, Fang F, Marx UC, Minoja A. NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling. Nutrients. 2009; 1(2):148-155. https://doi.org/10.3390/nu1020148
Chicago/Turabian StyleSpraul, Manfred, Birk Schütz, Peter Rinke, Susanne Koswig, Eberhard Humpfer, Hartmut Schäfer, Monika Mörtter, Fang Fang, Ute C. Marx, and Anna Minoja. 2009. "NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling" Nutrients 1, no. 2: 148-155. https://doi.org/10.3390/nu1020148
APA StyleSpraul, M., Schütz, B., Rinke, P., Koswig, S., Humpfer, E., Schäfer, H., Mörtter, M., Fang, F., Marx, U. C., & Minoja, A. (2009). NMR-Based Multi Parametric Quality Control of Fruit Juices: SGF Profiling. Nutrients, 1(2), 148-155. https://doi.org/10.3390/nu1020148