Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice
Abstract
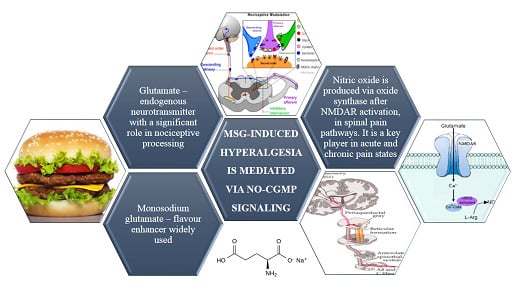
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Formalin-Induced Nociception
2.4. Hot-Plate Latency Assay
2.5. Assay of NO-Synthase (NOS) Activity
2.6. Total Protein Assay
2.7. Statistical Analysis
3. Results
3.1. Formalin-Induced Nociception
3.2. Hot-Plate Latency Assay
3.3. Assay of NO-Synthase (NOS) Activity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kwok, R.H.M. Chinese-Restaurant Syndrome. N. Engl. J. Med. 1968, 278, 796. [Google Scholar] [PubMed]
- Evaluation of Food Additives. Specifications for the identity and purity of food additives and their toxocological evaluation: Some extraction solvents and certain other substances; and a review of the technological efficacy of some antimicrobial agents. World Health Organ. Tech. Rep. Ser. 1971, 462, 1–36.
- Joint FAO/WHO Expert Committee on Food Additives; World Health Organization; Food and Agriculture Organization of the United Nations. Toxicological evaluation of certain food additives with a review of general principles and of specifications. Seventeenth report of the joint FAO-WHO Expert Committee on Food Additives. World Health Organ. Tech. Rep. Ser. 1974, 539, 1–40. [Google Scholar]
- Walker, R.; Lupien, J.R. The safety evaluation of monosodium glutamate. J. Nutr. 2000, 130, 1049S–1052S. [Google Scholar] [PubMed]
- Beyreuther, K.; Biesalski, H.K.; Fernstrom, J.D.; Grimm, P.; Hammes, W.P.; Heinemann, U.; Kempski, O.; Stehle, P.; Steinhart, H.; Walker, R. Consensus meeting: Monosodium glutamate—An update. Eur. J. Clin. Nutr. 2006, 61, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Baad-Hansen, L.; Castrillon, E.; Ghafouri, B.; Stensson, N.; Gerdle, B.; Ernberg, M.; Cairns, B.; Svensson, P.; Svensson Odont, P. Differential effects of repetitive oral administration of monosodium glutamate on interstitial glutamate concentration and muscle pain sensitivity. Nutrition 2015, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30, 10–17. [Google Scholar] [PubMed]
- Hoffman, E.M.; Zhang, Z.; Schechter, R.; Miller, K.E. Glutaminase increases in rat dorsal root ganglion neurons after unilateral adjuvant-induced hind paw inflammation. Biomolecules 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Szekely, J.I.; Torok, K.; Mate, G. The Role of Ionotropic Glutamate Receptors in Nociception with Special Regard to the AMPA Binding Sites. Curr. Pharm. Des. 2002, 8, 887–912. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M. Ionotropic Glutamate Receptors in Spinal Nociceptive Processing. Mol. Neurobiol. 2009, 40, 260–288. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V. Metabotropic glutamate receptors–important modulators of nociception and pain behavior. Pain 2002, 98, 1–8. [Google Scholar] [CrossRef]
- Chiechio, S. Chapter Three-Modulation of Chronic Pain by Metabotropic Glutamate Receptors. Adv. Pharm. 2016, 75, 63–89. [Google Scholar]
- Chen, Z.; Muscoli, C.; Doyle, T.; Bryant, L.; Cuzzocrea, S.; Mollace, V.; Mastroianni, R.; Masini, E.; Salvemini, D. NMDA receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain 2010, 149, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan, V.; Wang, R.; Ulzhöfer, B.; Luo, C.; Bardoni, R.; Bali, K.K.; Agarwal, N.; Tegeder, I.; Hildebrandt, U.; Nagy, G.G.; et al. Peripheral calcium-permeable AMPA receptors regulate chronic inflammatory pain in mice. J. Clin. Investig. 2011, 121, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.D.; Cizkova, D. The role of nitric oxide in nociception. Curr. Rev. Pain 2000, 4, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Toriyabe, M.; Omote, K.; Kawamata, T.; Namiki, A. Contribution of Interaction between Nitric Oxide and Cyclooxygenases to the Production of Prostaglandins in Carrageenan-induced Inflammation. Anesthesiology 2004, 101, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, F.; Skinner, G.O.; Araújo, P.C.; Ferraz, M.M.D.; Tenório, F.; de Almeida, O.M.M.S. Nitric oxide modulates the hyperalgesic response to mechanical noxious stimuli in sleep-deprived rats. BMC Neurosci. 2013, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, K.; Tsuda, M.; Tsutsui, M.; Toyohara, Y.; Tozaki-Saitoh, H.; Shimokawa, H.; Yanagihara, N.; Inoue, K. Reduced spinal microglial activation and neuropathic pain after nerve injury in mice lacking all three nitric oxide synthases. Mol. Pain 2011, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.W.; Sung, B.; Chung, J.M. Nitric oxide mediates behavioral signs of neuropathic pain in an experimental rat model. Neuroreport 1998, 9, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Chacur, M.; Matos, R.J.B.; Alves, A.S.; Rodrigues, A.C.; Gutierrez, V.; Cury, Y.; Britto, L.R.G. Participation of neuronal nitric oxide synthase in experimental neuropathic pain induced by sciatic nerve transection. Brazilian J. Med. Biol. Res. 2010, 43, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Boettger, M.K.; Reif, A.; Schmitt, A.; Üçeyler, N.; Sommer, C. Nitric oxide synthase modulates CFA-induced thermal hyperalgesia through cytokine regulation in mice. Mol. Pain 2010, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Buzescu, A.; Negres, S.; Cǎlin, O.; Chiritǎ, C. Experimental demonstration of hyperalgesia induced by repeated ingestion of dietary monosodium glutamate. Farmacia 2013, 61, 1009–1017. [Google Scholar]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Krohn, R.I. The Colorimetric Detection and Quantitation of Total Protein. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Gunn, A.; Bobeck, E.N.; Weber, C.; Morgan, M.M. The influence of non-nociceptive factors on hot-plate latency in rats. J. Pain 2011, 12, 222–227. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef] [PubMed]
- Cury, Y.; Picolo, G.; Gutierrez, V.P.; Ferreira, S.H. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide 2011, 25, 243–254. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.A.; Tambeli, C.H.; Cunha, F.Q.; Ferreira, S.H. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience 2001, 102, 937–944. [Google Scholar] [CrossRef]
- Milano, J.; Oliveira, S.M.; Rossato, M.F.; Sauzem, P.D.; Machado, P.; Beck, P.; Zanatta, N.; Martins, M.A.P.; Mello, C.F.; Rubin, M.A.; et al. Antinociceptive effect of novel trihalomethyl-substituted pyrazoline methyl esters in formalin and hot-plate tests in mice. Eur. J. Pharmacol. 2008, 581, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Zeashan, H.; Amresh, G.; Rao, C.V.; Singh, S. Antinociceptive activity of Amaranthus spinosus in experimental animals. J. Ethnopharmacol. 2009, 122, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Coderre, T.J.; Melzack, R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992, 12, 3665–3670. [Google Scholar] [PubMed]
- Coderre, T.J.; Yashpal, K. Intracellular Messengers Contributing to Persistent Nociception and Hyperalgesia Induced by l-Glutamate and Substance P in the Rat Formalin Pain Model. Eur. J. Neurosci. 1994, 6, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Little, J.W.; Doyle, T.; Salvemini, D. Reactive nitroxidative species and nociceptive processing: Determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids 2012, 42, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Ren, J.; Guo, X.; Yun, K. Morphine Protects Spinal Cord Astrocytes from Glutamate-Induced Apoptosis via Reducing Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2016, 17, 1523. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Martínez, R.; Déciga-Campos, M.; Díaz-Reval, M.I.; González-Trujano, M.E.; López-Muñoz, F.J. Peripheral involvement of the nitric oxide-cGMP pathway in the indomethacin-induced antinociception in rat. Eur. J. Pharmacol. 2004, 503, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Déciga-Campos, M.; López-Muñoz, F.J. Participation of the l-arginine-nitric oxide-cyclic GMP-ATP-sensitive K+ channel cascade in the antinociceptive effect of rofecoxib. Eur. J. Pharmacol. 2004, 484, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hishikawa, K.; Nakaki, T.; Tsuda, M.; Esumi, H.; Ohshima, H.; Suzuki, H.; Saruta, T.; Kato, R. Effect of systemic l-arginine administration on hemodynamics and nitric oxide release in man. Jpn. Heart J. 1992, 33, 41–48. [Google Scholar] [CrossRef] [PubMed]
 ) and in Monosodium glutamate (MSG) treated mice (
) and in Monosodium glutamate (MSG) treated mice (  MSG 150mg/kg;
MSG 150mg/kg;  MSG 300 mg/kg). Values represent means ± standard error of the mean (SEM) of 10 animals.
MSG 300 mg/kg). Values represent means ± standard error of the mean (SEM) of 10 animals.
 ) and in Monosodium glutamate (MSG) treated mice (
) and in Monosodium glutamate (MSG) treated mice (  MSG 150mg/kg;
MSG 150mg/kg;  MSG 300 mg/kg). Values represent means ± standard error of the mean (SEM) of 10 animals.
MSG 300 mg/kg). Values represent means ± standard error of the mean (SEM) of 10 animals.


| Reagents (μL) | Test | Control |
|---|---|---|
| Supernatant | 360 | 360 |
| PBS | 310 | 540 |
| l-Arginine 1 | 60 | - |
| FAD 2 | 60 | - |
| NADPH 3 | 10 | - |
| 37 °C, 60 min | ||
| Cadmium chloride | 0.5 g/mL | 0.5 g/mL |
| 25 °C, 30 min | ||
| Griess reagent | 1000 | 1000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanfirescu, A.; Cristea, A.N.; Nitulescu, G.M.; Velescu, B.S.; Gradinaru, D. Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice. Nutrients 2018, 10, 1. https://doi.org/10.3390/nu10010001
Zanfirescu A, Cristea AN, Nitulescu GM, Velescu BS, Gradinaru D. Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice. Nutrients. 2018; 10(1):1. https://doi.org/10.3390/nu10010001
Chicago/Turabian StyleZanfirescu, Anca, Aurelia Nicoleta Cristea, George Mihai Nitulescu, Bruno Stefan Velescu, and Daniela Gradinaru. 2018. "Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice" Nutrients 10, no. 1: 1. https://doi.org/10.3390/nu10010001
APA StyleZanfirescu, A., Cristea, A. N., Nitulescu, G. M., Velescu, B. S., & Gradinaru, D. (2018). Chronic Monosodium Glutamate Administration Induced Hyperalgesia in Mice. Nutrients, 10(1), 1. https://doi.org/10.3390/nu10010001