Abstract
Alzheimer’s disease (AD) is the main form of dementia and has a steadily increasing prevalence. As both oxidative stress and metal homeostasis are involved in the pathogenesis of AD, it would be interesting to develop a dual function agent, targeting the two factors. Curcumin, a natural compound isolated from the rhizome of Curcuma longa, is an antioxidant and can also chelate metal ions. Whether the complexes of curcumin with metal ions possess neuroprotective effects has not been evaluated. Therefore, the present study was designed to investigate the protective effects of the complexes of curcumin with Cu(II) or Zn(II) on hydrogen peroxide (H2O2)-induced injury and the underlying molecular mechanisms. The use of rat pheochromocytoma (PC12) cells, a widely used neuronal cell model system, was adopted. It was revealed that curcumin–Cu(II) complexes systems possessed enhanced O2·–-scavenging activities compared to unchelated curcumin. In comparison with unchelated curcumin, the protective effects of curcumin–Cu(II) complexes systems were stronger than curcumin–Zn(II) system. Curcumin–Cu(II) or –Zn(II) complexes systems significantly enhanced the superoxide dismutase, catalase, and glutathione peroxidase activities and attenuated the increase of malondialdehyde levels and caspase-3 and caspase-9 activities, in a dose-dependent manner. The curcumin–Cu(II) complex system with a 2:1 ratio exhibited the most significant effect. Further mechanistic study demonstrated that curcumin–Cu(II) or –Zn(II) complexes systems inhibited cell apoptosis via downregulating the nuclear factor κB (NF-κB) pathway and upregulating Bcl-2/Bax pathway. In summary, the present study found that curcumin–Cu(II) or –Zn(II) complexes systems, especially the former, possess significant neuroprotective effects, which indicates the potential advantage of curcumin as a promising agent against AD and deserves further study.
1. Introduction
As the major form of dementia, Alzheimer’s disease (AD) is a progressive and irreversible neurodegenerative disease, which is related to aging and has a steadily increasing prevalence [1,2,3]. Due to the considerable burden on patients, family and society, screening efficient drugs for AD is a major challenge in drug discovery. Despite much effort being devoted to drug discovery of AD over the past decades, there is no effective therapeutic drug available to treat AD. The pathogenesis of AD remains far from being fully elucidated and oxidative damage to neurons caused by excessive reactive oxygen species (ROS) is widely recognized as an important factor [4,5,6,7]. Antioxidant supplement represents a promising strategy to combat AD. In addition, brain metal dysregulation, including copper (Cu(II)) and zinc (Zn(II)) ions, is intimately involved in the pathogenesis of AD [8,9,10]. The interaction of Cu(II) with both amyloid β (Aβ) peptides and the amyloid precursor protein (APP) has gained much attention in recent years [8,9,10]. Therefore, regulation of metal homeostasis is also considered to be a key therapeutic target. In recent years, many attempts have been made to explore metal chelators as potential agents against AD [11,12,13].
Curcumin is a yellow phenolic compound, isolated from the rhizome of turmeric (Curcuma longa), which has been intensively studied in the past decades owing to its wide spectrum of pharmacological activities, including antioxidant, anticancer, anti-inflammatory, and antimicrobial effects [14,15,16,17]. Curcumin can scavenge ROS and also chelate various metal ions [18,19,20]. Our previous studies found that the complexes of curcumin with metal ions possess superoxide dismutase (SOD)-like activity [21,22,23]. Thus, considering its abilities to scavenge free radicals and bind metal ions, curcumin may act as a promising agent to combat AD, especially in view of its good clinical safety, even at high dosage. In addition, it has been reported that curcumin can inhibit Aβ aggregation and fibril formation, promote the clearance of Aβ plaques, attenuate tau hyperphosphorylation, and inhibit acetylcholinesterase activity [24,25,26]. Rat pheochromocytoma (PC12) is a cell line derived from a pheochromocytoma of the rat adrenal medulla, which possesses the characteristics of neural cells and is widely used as a cell model system for neurodegenerative diseases [27,28,29,30,31]. With the aim of further exploring the neuroprotective effects of the metal ion complexes of curcumin and elucidating underlying mechanisms, we investigated the protective effects of curcumin and curcumin–Cu(II) or –Zn(II) complexes against hydrogen peroxide (H2O2)-induced injury in PC12 cells. Further mechanistic study demonstrated that curcumin–Cu(II) or –Zn(II) complexes inhibited cell apoptosis via downregulating the nuclear factor κB (NF-κB) and upregulating Bcl-2/Bax pathways.
2. Materials and Methods
2.1. Materials
Penicillin-streptomycin and Nutrient Mixture F-12 Ham were purchased from Sigma–Aldrich Shanghai Trading Co. (Shanghai, China). Horse serum (HS) was purchased from Solarbio Biological Technology Co., Ltd. (Beijing, China), fetal bovine serum (FBS) from Gibco (Glen Waverley, Australia). Curcumin, an extract from the turmeric powder of Curcuma longa, comprising the three main components—curcumin, demethoxycurcumin and bidemethoxycurcumin—was purchased from Shanghai Macklin Biological Technology Co., Ltd. (Shanghai, China). Copper chloride dehydrate, zinc chloride, pyrogallol, 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) and 2′,7′-dichlorofluorescindiacetate (DCFH-DA) were purchased from Sigma–Aldrich Shanghai Trading Co. Ltd. (Shanghai, China). H2O2 was purchased from Shuangshuang Chemical Co., Ltd. (Yantai, China). The kits for the Bradford protein assay, malondialdehyde (MDA), SOD, catalase (CAT) and glutathione peroxidase (GSH-Px) activities were gained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit, caspase-3 and caspase-9 activity assay kits were purchased from Bibo Biological Technology Co., Ltd. (Nanjing, China). The enhanced chemiluminescence (ECL) kit, Bcl-2, Bax, p65 and β-actin antibody was purchased from Nanjing Enogene Biotech. Co., Ltd. (Nanjing, China). All other reagents were of analytical grade.
2.2. Superoxide Anion Radical Scavenging Assay
The superoxide anion radical (O2·–)-scavenging activities of curcumin and curcumin–Cu(II) or –Zn(II) complexes were measured with a modified pyrogallol autoxidation method [32]. Briefly, a pyrogallol solution (in 1 M HCl) was thoroughly mixed with Tris-HCl buffer at pH 7.4 and the absorption (A325nm) was recorded every 30 s for 5 min at 37 °C using a microplate reader (EVOLUTION 220, Thermo Fisher Scientific Oy, Vantaa, Finland). The O2·–-scavenging rate was estimated according to the following formula (T = 5 min):
The concentrations of curcumin and curcumin–Cu(II) or –Zn(II) complexes for 50% O2·–-scavenging was defined as the IC50 value.
2.3. Cell Culture and Viability Assay
PC12 cells, obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China), were cultured in Nutrient Mixture F-12 Ham medium, supplemented with 10% HS, 5% FBS, 100 U/ml of penicillin and streptomycin at 37 °C under 5% CO2. Before treatment, PC12 cells were seeded in 6 or 96-well plates (1 or 3 × 105 cells/ml) and cultured for 24 h. Cells were pretreated with curcumin and curcumin–Cu(II) or –Zn(II) complexes for 0.5 h, before adding H2O2. Cell viability was assessed by an MTT assay [33]. After 24 h, the cells were incubated with H2O2 (500 µM) for an additional 24 h. Finally, 20 µL MTT was added to each well. After 4 h, 200 µL DMSO was added to each well to dissolve the formazan crystals. The absorbance was measured at 570 nm using a Multiwell microplate reader (MultiskanGo, Thermo Fisher Scientific Oy, Vantaa, Finland). Cell viability was expressed as the percentage of the control group.
2.4. ROS Assay
The intracellular ROS level was quantified using a DCFH-DA assay. At 24 h after seeding, the cells were pretreated with 25 µM curcumin and curcumin–Cu(II) or –Zn(II) complexes for 0.5 h and 500 µM H2O2 was added. After 6 h of incubation, the cells were washed with PBS (0.1 M, pH 7.4) and incubated with 10 µM DCFH-DA for 0.5 h in the dark. After treatment, the cells were washed with serum-free medium three times, and then pictures were taken with a fluorescence microscope (Olympus IX73, Tokyo, Japan), and relative fluorescence intensity was measured with a fluorescent microplate reader (Varioskan Flash, Thermo Fisher Scientific Oy, Vantaa, Finland). The intracellular ROS level in the control group was defined as 100% and the other groups were assessed as a percentage of the control.
2.5. Apoptosis Assay
Cell apoptosis was measured by annexin V-FITC/PI Staining. At 24 h after seeding, the cells were pretreated with curcumin and curcumin–Cu(II) or –Zn(II) systems for 0.5 h and H2O2 was added. After 6 h of incubation, the treated cells were washed with ice-cold PBS (0.1 M, pH 7.4) twice. Cells were resuspended in mixture of 500 µL of 1× binding buffer, 5 µL annexin V-FITC and PI for 10 min in the dark, and then analyzed by a fluorescence microscope (Olympus IX73, Tokyo, Japan).
2.6. MDA and Antioxidant Enzymes Assay
Treatment with curcumin or curcumin–Cu(II) or –Zn(II) complexes on PC12 cells was same as the above assay. After 6 h of incubation, the treated cells were washed twice with ice-cold PBS (0.1 M, pH 7.4) and homogenized. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C, and then supernatant was collected for further experiments. The MDA content was evaluated using the 2-thiobarbituric acid assay [34]. Protein content was measured by the Bradford method [35]. The levels of intracellular antioxidant enzyme, CAT, total SOD and GSH-Px activities were determined according to the manuals of assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.7. Caspase-3 and Caspase-9 Activity
Treatment of PC12 cells with curcumin or curcumin–Cu(II) or–Zn(II) complexes was in accordance with the above assay. After 12 h of incubation, the treated cells were washed twice with ice-cold PBS and homogenized. The homogenate was centrifuged at 12,000 rpm for 15 min at 4 °C and then the supernatant of cell lysates was collected for further experiment. The activity of caspase-3 and caspase-9 were measured using assay kits (Nanjing Bibo Biological Technology, Nanjing, China).
2.8. Western Blotting
The cell lysates were centrifuged at 12,000 rpm for 15 min at 4 °C and then protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes, which were blocked in 5% skim milk powder for 1 h, and then incubated with primary antibodies overnight at 4 °C. On the second day, the membrane was washed by TBST thrice, and then incubated with the secondary antibody for 1.5 h. The membrane was developed in an ECL reagent and visualized by a chemiluminescence detection system (ChemiScope 5300, Clinx, Shanghai, China).
2.9. Statistical Analysis
All independent experiments were conducted in triplicate. The data were shown as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS (16.0) software (IBM Corporation, Armonk, NY, USA). The difference in the different groups was analyzed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons post-hoc test. The significance levels were defined as # p < 0.01, ** p < 0.01 and * p < 0.05.
3. Results
3.1. O2·–-Scavenging Activities
O2·–-scavenging activities of curcumin and curcumin–Cu(II) or –Zn(II) complexes were examined and the results are shown in Table 1. It was found that curcumin–Cu(II) complexes exhibited enhanced O2·–-scavenging activities significantly more than parent curcumin and curcumin–Zn(II) complexes.

Table 1.
Superoxide anion radical-scavenging activities of curcumin and the complexes. Data were expressed as the mean ± standard deviation (SD), n = 3. (* p < 0.05 and ** p < 0.01 versus curcumin).
3.2. Cell Viability
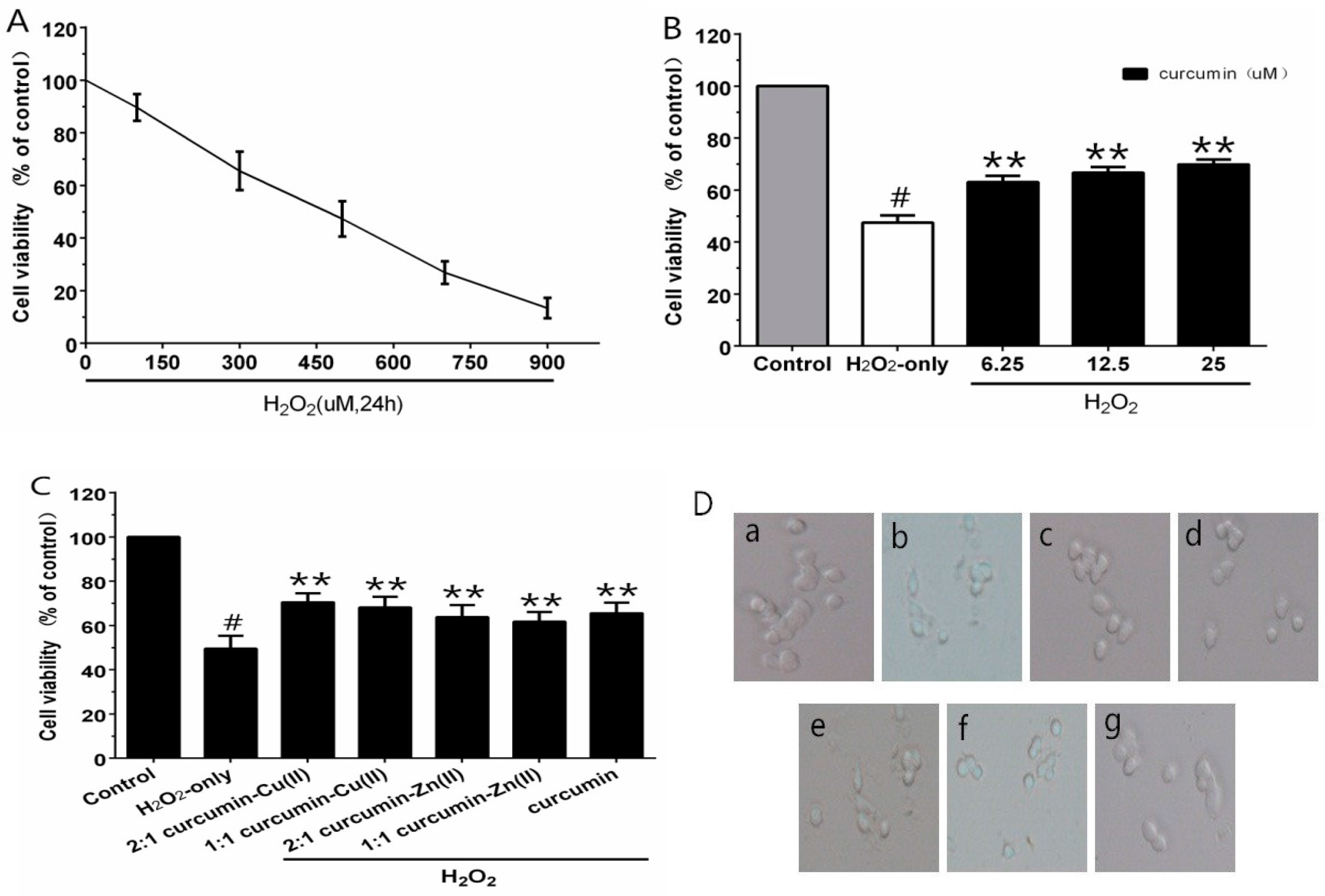
Different concentrations of H2O2 were employed for cytotoxic tests, to build the oxidative injury model of PC12 cells. As estimated by the MTT assay, cell viability was significantly decreased to 47.3% after treatment with 500 µM H2O2 for 24 h (Figure 1A). Thus, 500 μM H2O2 was regarded as the oxidative injury model for the subsequent experiments. When cells were pre-incubated with curcumin (6.25–25 µM) for 0.5 h, as shown in Figure 1B, curcumin (25 µM) showed an efficient neuroprotective effect and the cell viability increased significantly by 22.4% compared with H2O2-treated cells. The cytotoxicity experiment indicated no obvious reduction in cell viability with the treatment of curcumin alone. Therefore, this concentration was used for further experiments. In the complex protection experiment, the curcumin–Cu(II) complex further increased cell viability in comparison with the curcumin group. However, the protective effects of the curcumin–Zn(II) complex was lower than curcumin (Figure 1C). It indicated that the curcumin–Cu(II) complex had relatively stronger protective effects than curcumin or the curcumin–Zn(II) complex, and the 2:1 curcumin–Cu(II) complex exhibited the most significant protective effect.
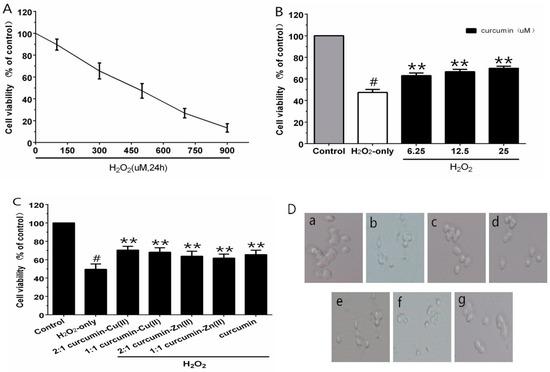
Figure 1.
Curcumin and the complexes systems protected PC12 cells against H2O2-induced damage. Cytotoxic effects of H2O2 (A) on PC12 cells and the protective effects of curcumin (B) and the complexes (C) on H2O2-induced cytotoxicity to PC12 cells and morphological alteration (D). (a) control cells; (b) cells treated with H2O2 only; (c) cells pretreated with the 2:1 curcumin–Cu(II) complex system and co-treated with H2O2; (d) cells pretreated with the 1:1 curcumin–Cu(II) complex system and co-treated with H2O2; (e) cells pretreated with the 2:1 curcumin–Zn(II) complex system and co-treated with H2O2; (f) cells pretreated with the 1:1 curcumin-Zn(II) complexsystem and co-treated with H2O2; (g) cells pretreated with curcumin and co-treated with H2O2. (# p < 0.01 versus control; * p < 0.05 and ** p < 0.01 versus H2O2-treated cells).
3.3. Intracellular ROS Levels
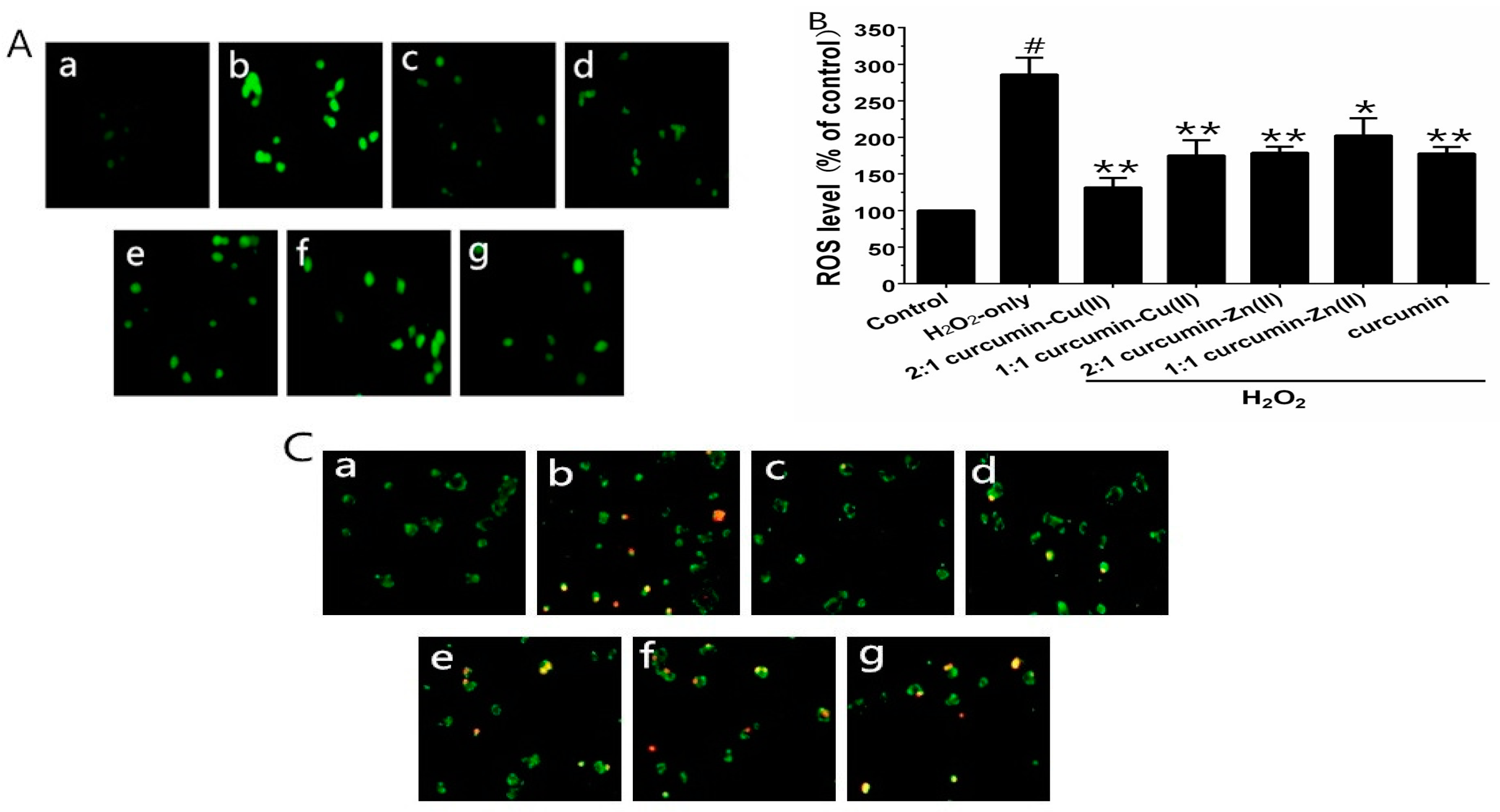
To verify whether curcumin and curcumin–Cu(II) or –Zn(II) complexes systems could prevent H2O2-induced ROS generation, the ROS levels were measured using the fluorescence probe DCFH-DA. Exposure of the cells to 500 µM H2O2 for 6 h significantly increased the intracellular ROS level, in comparison with control group (Figure 2A). PC12 cells pretreated with curcumin and curcumin–Cu(II) or –Zn(II) complexes system significantly reduced ROS levels. The 2:1 curcumin–Cu(II) complex exhibited more significant effects than curcumin and other complexes (Figure 2B). To further verify whether H2O2-induced cell death was via cell apoptosis, AV/PI staining was used to detect apoptotic cells. After treatment with H2O2, the nuclei of most PC12 cells were stained, indicating that cells were apoptotic (Figure 2C). The treatment of curcumin and curcumin–Cu(II) or –Zn(II) complexes systems on H2O2-treated PC12 cells displayed significantly reduced numbers of stained cells (Figure 2C), indicating that both curcumin and curcumin–Cu(II) or –Zn(II) complexes systems could attenuate cell apoptosis induced by H2O2.
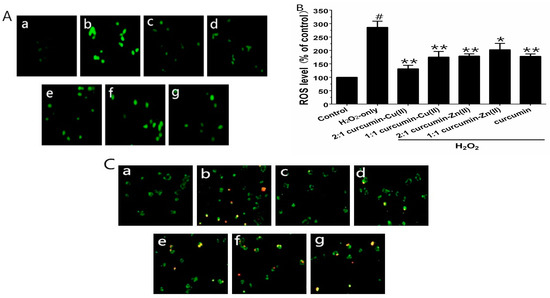
Figure 2.
Inhibitory effects of curcumin and the complexes systems on the H2O2-induced reactive oxygen species (ROS) level and apoptotis in PC12 cells. (A) Representative images of 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) and 2′,7′-dichlorofluorescindiacetate (DCFH-DA) staining in PC12 cells by fluorescent microscopy (×100); (B) Intracellular reactive oxygen species (ROS) level; (C) Representative images of AV-FITC/PI staining under the microscope (×100). (a) control cells; (b) cells treated with H2O2 only; (c) cells pretreated with the 2:1 curcumin–Cu(II) complex system and co-treated with H2O2; (d) cells pretreated with the 1:1 curcumin–Cu(II) complex system and co-treated with H2O2; (e) cells pretreated with the 2:1 curcumin-Zn(II) complex and co-treated with H2O2; (f) cells pretreated with the 1:1 curcumin-Zn(II) complex system and co-treated with H2O2; (g) cells pretreated with curcumin and co-treated with H2O2. (# p < 0.01 versus control; * p < 0.05 and ** p < 0.01 versus H2O2-treated cells).
3.4. MDA Levels and Antioxidant Enzyme Activities
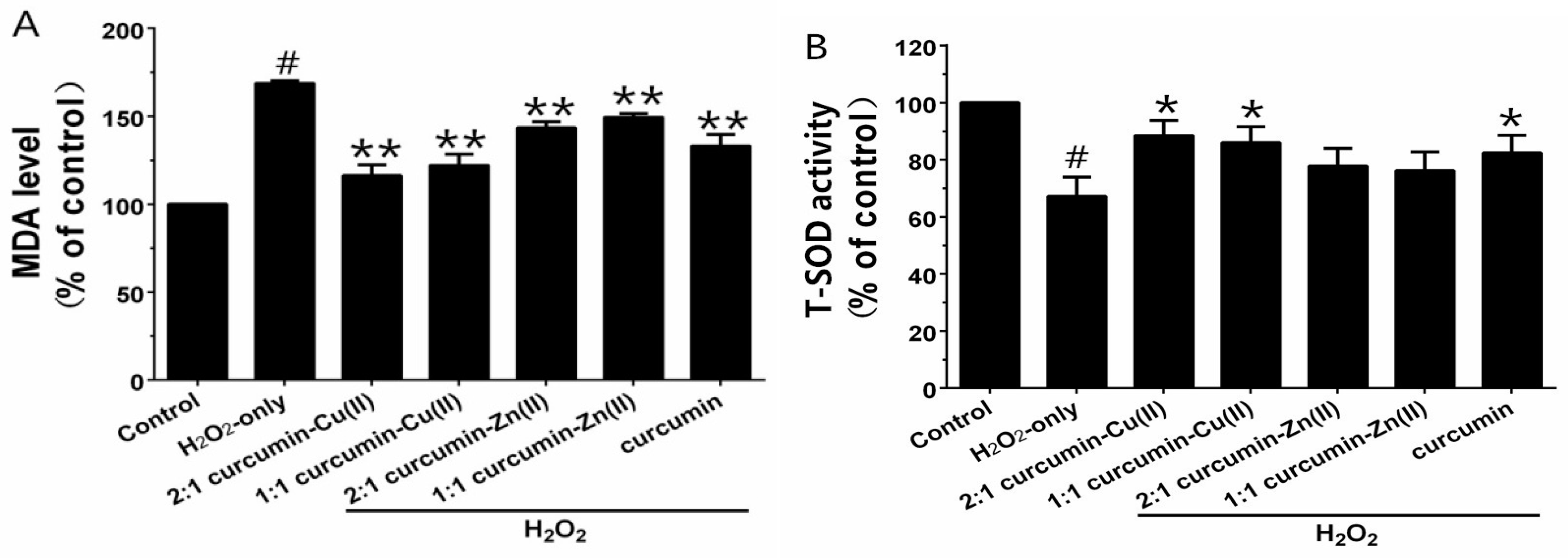
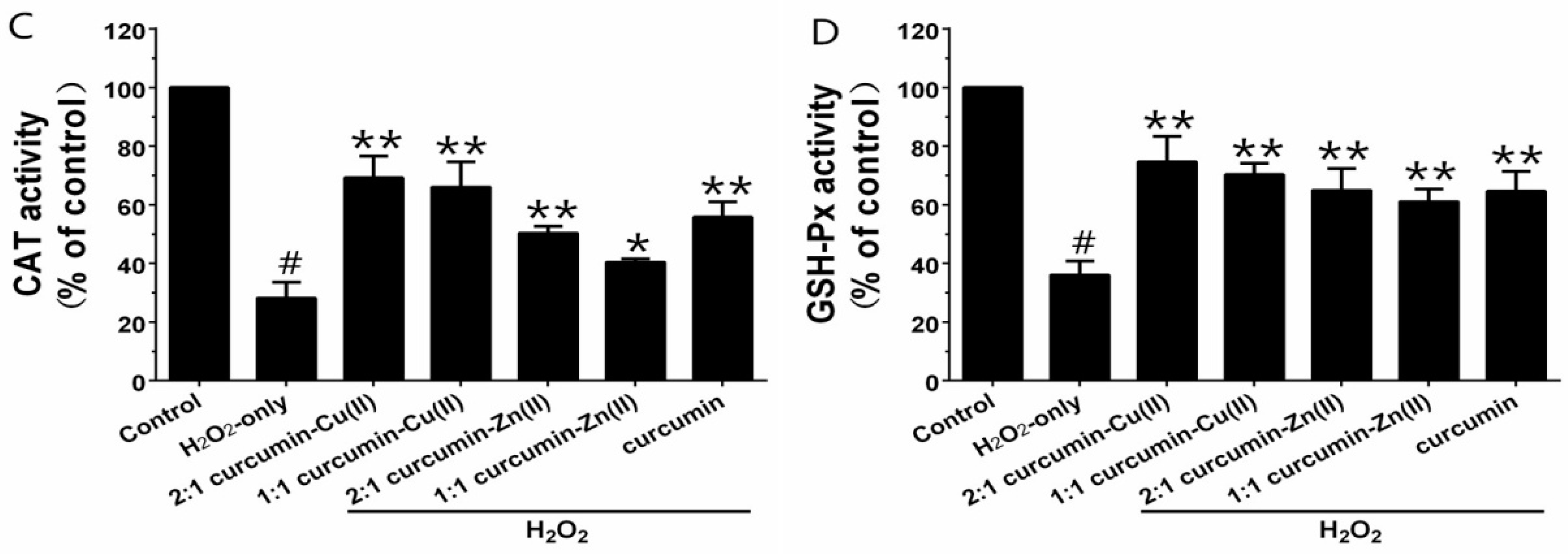
Exposure of PC12 cells to H2O2 caused an increase in the intracellular MDA level, and pretreatment with curcumin and curcumin–Cu(II) or –Zn(II) complexes systems obviously attenuated this increase (Figure 3A). Additionally, exposure of PC12 cells to H2O2 caused a decrease in the activities of SOD, CAT and GSH-Px (Figure 3B-D). Pretreatment with curcumin and curcumin–Cu(II) or –Zn(II) complexes systems significantly attenuated the changes in CAT, SOD and GSH-Px activities induced by H2O2, and the protective effects of the curcumin–Cu(II) complex system were stronger than curcumin or the curcumin–Zn(II) complex system.
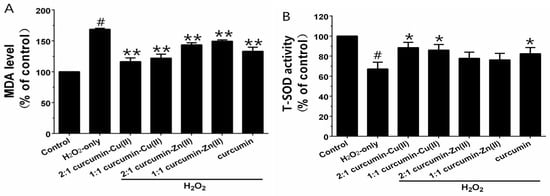
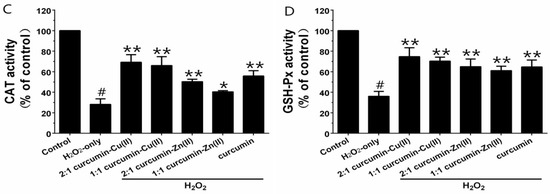
Figure 3.
Effects of curcumin and the complexes on the malondialdehyde (MDA) level (A) and the activities of total superoxide dismutase (SOD) (B); catalase (CAT) (C) and glutathione peroxidase (GSH-Px) (D) in PC12 cells. PC12 cells were pretreated with curcumin and the complexes for 0.5 h before being exposed to 500 µM H2O2 for 6 h. (# p < 0.01 versus control; * p < 0.05 and ** p < 0.01 versus H2O2-treated cells).
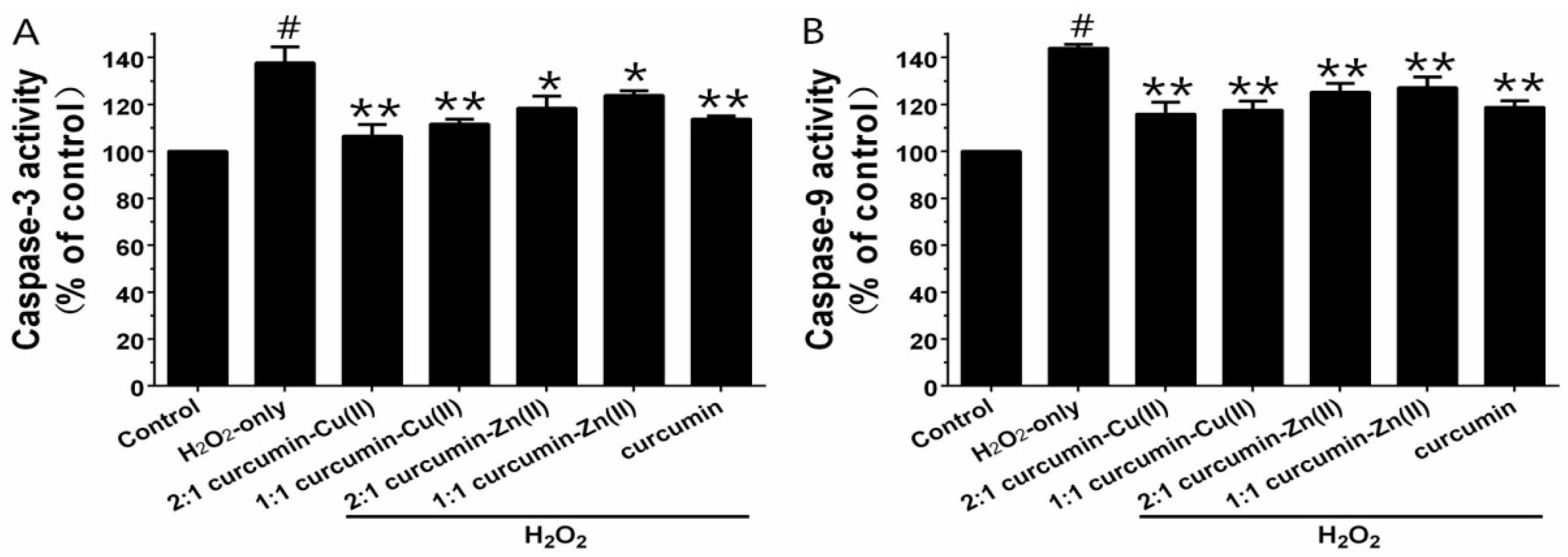
3.5. Caspase-3 and Caspase-9 Activities
Caspase-3 and -9 are the key executive proteins related to apoptosis. As shown in Figure 4, H2O2 obviously increased caspase-3 and -9 activities and pretreatment with curcumin and curcumin–Cu(II) or –Zn(II) complexes attenuated the increases. The effect of the 2:1 curcumin–Cu(II) complex was the most significant—it decreased caspase-3 activity from 137.63 ± 6.8% to 106.37 ± 5.05% and caspase-9 activity from 143.93 ± 1.56% to 115.83 ± 5.08%.
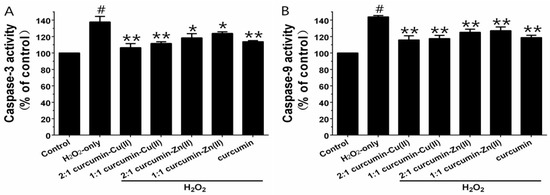
Figure 4.
Effects of curcumin and the complexes systems on caspase-3 (A) and -9 (B) activity in H2O2-induced PC12 cells. Cells were pretreated with curcumin and the complexes systems for 0.5 h before being exposed to 500 µM H2O2 for 6 h. Caspase-3 and -9 activities were determined using a commercial kit, in accordance with the instructions of the manufacturer. (# p < 0.01 versus control; * p < 0.05 and ** p < 0.01 versus H2O2-treated cells).
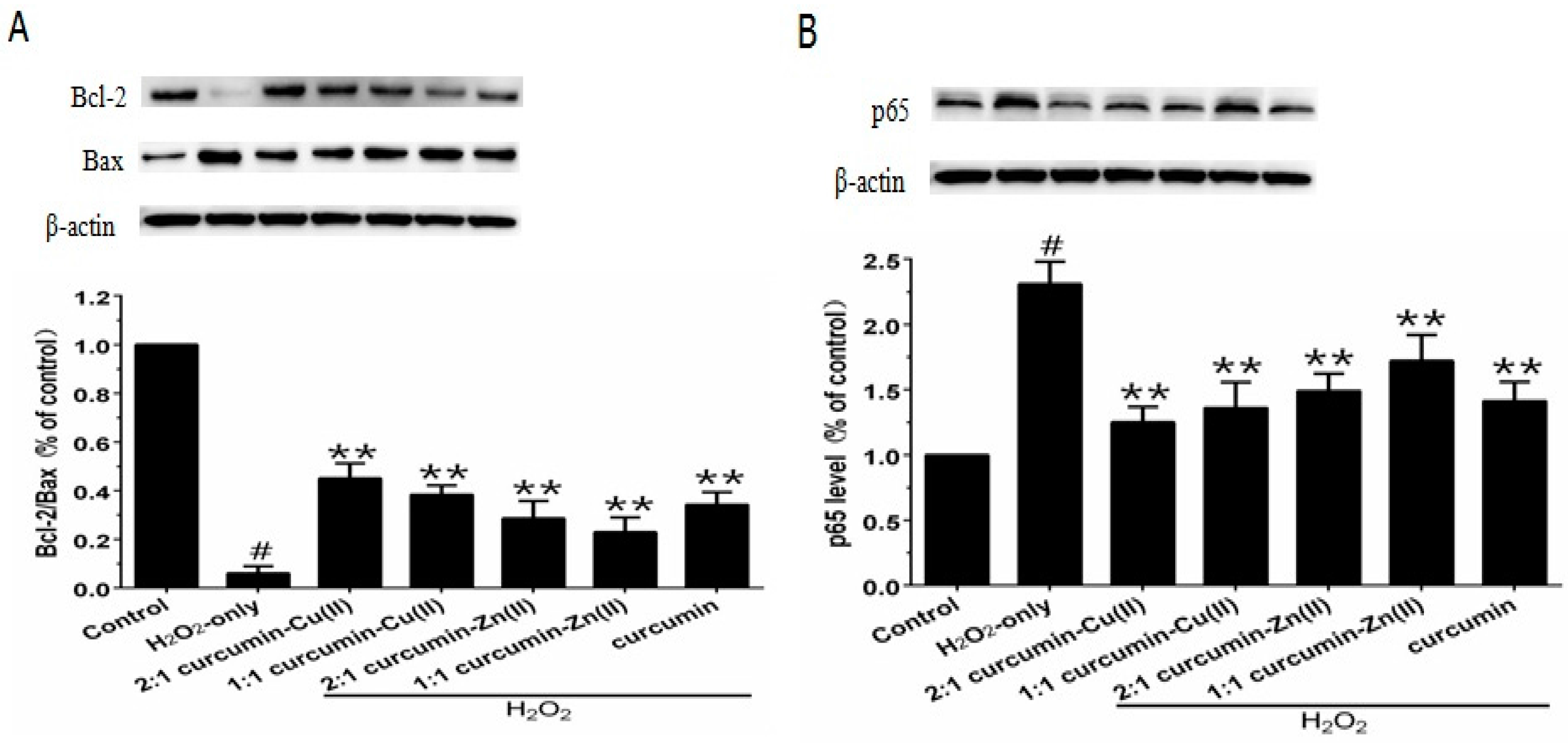
3.6. Bcl-2/Bax Ratio and NF-κB p65 Levels
The Bcl-2/Bax ratio is an important factor in most mitochondria apoptotic proceedings. As shown in Figure 5A, exposing PC12 cells to H2O2 caused a significant decrease in the Bcl-2/Bax ratio, which was reversed by pretreatment with curcumin and curcumin–Cu(II) or –Zn(II) complexes systems. NF-κB is one of the major anti-apoptotic factors induced by ROS [29]. NF-κB is also regulated by various apoptotic stimuli or inhibitors. As shown in Figure 5B, Western blot results showed that p65 expression was increased in the presence of H2O2, while pretreatment with curcumin and curcumin–Cu(II) or –Zn(II) complexes systems significantly decreased the expression of p65 induced by H2O2. The effect of the 2:1 curcumin–Cu(II) complex system was stronger than curcumin or the other complexes.
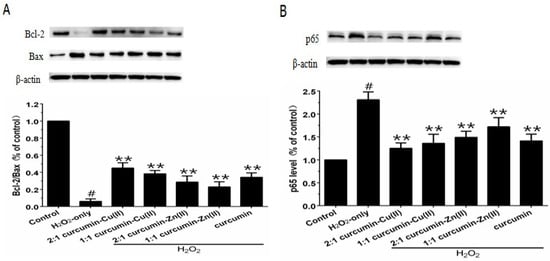
Figure 5.
Effects of curcumin and the complexes system on the ratios of Bcl-2/Bax and p65 levels in PC12 cells. The PC12 cells were pretreated with curcumin and the complexes for 0.5 h and then exposed to 500 μM H2O2 for 12 h. (A) The expression of Bcl-2/Bax, β-actin was used for normalization and verification of protein loading, Bcl-2 and Bax in control, H2O2, curcumin and the complexes systems treatments and β-actin representations of the ratios of Bcl-2/Bax; (B) Following the same treatment, the p65 levels were identified. Data are shown as mean ± SD (n = 3). (# p < 0.01 versus control; ** p < 0.01 versus H2O2-treated cells).
4. Discussion
Screening multi-function drugs from natural products to treat AD has gained much interest in recent years [26,36,37]. Curcumin is an intensively investigated natural polyphenol with antioxidant activity, which can scavenge various ROS and reactive nitrogen species (RNS), and inhibit lipid peroxidation [19]. Curcumin has been found to be a metal ion chelator and can bind various metal ions. It is interesting to explore whether metal ion complexes of curcumin possess neuroprotective effects, which will endow curcumin another advantage against AD. The present study revealed that curcumin can inhibit H2O2-induced injury in neuronal PC12 cells, which is consistent with previous studies [29,38,39]. The effective concentration of curcumin employed in the present study was lower than that in the study of Siddiqui et al., which may arise from the different concentrations of employed H2O2 and a dose-dependent vulnerability of PC12 cells against H2O2 exposure [39]. Both curcumin–Cu(II) and –Zn(II) complexes were found to possess neuroprotective effects on PC12 cells, and the protective effects of the curcumin–Cu(II) complexes were relatively stronger than parent curcumin. The significant neuroprotective effects of curcumin–Cu(II) complexes may arise from the SOD-like activities of curcumin–Cu(II) complexes [21,22,23]. Curcumin–Cu(II) and –Zn(II) complexes significantly increased the activities of antioxidant enzymes, including SOD, CAT and GSH, and attenuated the H2O2-induced increase in MDA levels and caspase-3 and caspase-9 activities in PC12 cells. Previous studies have reported that many genes are involved in cell apoptosis, such as Bcl-2 and Bax genes [40]. In our study, pretreatment with curcumin and curcumin–Cu(II) or -Zn(II) complexes upregulated the expression of anti-apoptotic protein Bcl-2, and down-regulated the expression of pro-apoptotic protein Bax, indicating that curcumin and curcumin-Cu(II) or -Zn(II) complexes systems inhibited cell apoptosis, via upregulating the Bcl-2/Bax pathway. NF-κB acts as an important anti-apoptotic factor and it was demonstrated that the link between oxidative stress and NF-κB mainly arises from the inhibition of NF-κB activation by many antioxidants [41,42]. Our study indicated that curcumin and curcumin–Cu(II) or –Zn(II) complexes inhibit cell apoptosis via downregulating the NF-κB pathway, which is consistent with previous studies [28,43]. However, curcumin was reported to have an extremely poor bioavailability, which makes its pharmacology difficult to be fully elucidated and also hampers its clinical applications. In view of its low stability [44,45], our previous studies proposed that the bioactive degradation products of curcumin should contribute to in its diverse pharmacological effects [25,46,47]. The degradation products retain the main functional groups of curucmin and exhibit similar biological activities and mechanisms as parent curcumin to combat AD, including antioxidants, Aβ fibril formation-, and enzyme-inhibiting activities [25,46]. Additionally, many strategies have been used to improve the bioavailability of curcumin [48]. For instance, it was found that the application of adjuvants, like piperine, can significantly improve the bioavailability of curcumin [49].
5. Conclusions
To summarize, the present findings suggest that metal complexes of curcumin, especially curcumin–Cu(II), exhibit strong neuroprotective effects on neuronal PC12 cells. In view of the antioxidant and metal ion-chelating activities of curcumin, the neuroprotective effect of its metal complexes indicates the great advantages of curcumin as a promising anti-AD agent. In addition, it has been demonstrated that curcumin could inhibit Aβ aggregation and ameliorate Aβ-induced toxicity [25,50,51,52], and inhibit acetylcholinesterase activity [25,53]. Thus, the potentials of curcumin as a natural agent to combat AD deserve more preclinical and clinical studies.
Acknowledgments
This work was supported by the Shandong Provincial Science Foundation for Distinguished Young Scholars (Grant No. JQ201508) and in part by National Natural Science Foundation of China (Grant No. 31370745) and the National Science and Technology Major Projects of New Drugs (Grant No. 2015ZX09102015).
Author Contributions
L.S. and H.-F.J. conceived and designed the experiments; F.-S.Y. and J.-L.S. performed the experiments; F.-S.Y., W.-H.X., H.-F.J. and L.S. analyzed the data; F.-S.Y., L.S. and H.-F.J. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Burns, A.; Iliffe, S. Alzheimer’s disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef] [PubMed]
- Brookmeyer, R.; Johnson, E.; Ziegler-Graham, K.; Arrighi, H.M. Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement. 2007, 3, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Zhu, X.; Perry, G.; Smith, M.A. Oxidative stress in diabetes and Alzheimer’s disease. J. Alzheimers Dis. 2009, 16, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, P.; Cunha, R.A.; Oliveira, C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr. Pharm. Des. 2010, 16, 2766–2778. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Eskici, G.; Axelsen, P.H. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry 2012, 51, 6289–6311. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The Role of Copper in Neurodegenerative Disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Liu, Y.; Nguyen, M.; Meunier, B. Regulation of copper and iron homeostasis by metal chelators: A possible chemotherapy for Alzheimer’s disease. Acc. Chem. Res. 2015, 48, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Kador, P.F. Orally Bioavailable Metal Chelators and Radical Scavengers: Multifunctional Antioxidants for the Coadjutant Treatment of Neurodegenerative Diseases. J. Med. Chem. 2015, 58, 8796–8805. [Google Scholar] [CrossRef] [PubMed]
- Crouch, P.J.; Barnham, K.J. Therapeutic redistribution of metal ions to treat Alzheimer’s disease. Acc. Chem. Res. 2012, 45, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Theoretical study on physicochemical properties of curcumin. Spectrochim. Acta Mol. Biomol. Spectrosc. 2007, 67, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [PubMed]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, H.Y.; Ji, H.F. A Theoretical study on Cu(II)-chelating properties of curcumin and its implications for curcumin as a multipotent agent to combat Alzheimer’s disease. J. Mol. Struct. Theochem. 2005, 757, 199–202. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Kunwar, A.; Kadam, R.M.; Shen, L.; Dutta, S.; Padhye, S.; Satpati, A.K.; Zhang, H.Y.; Indira Priyadarsini, K. Comparative study of copper(II)-curcumin complexes as superoxide dismutase mimics and free radical scavengers. Eur. J. Med. Chem. 2007, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Liu, C.C.; An, C.Y.; Ji, H.F. How does curcumin work with poor bioavailability? Clues from experimental and theoretical studies. Sci. Rep. 2016, 6, 20872. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Zhang, H.Y. Multipotent natural agents to combat Alzheimer’s disease. Functional spectrum and structural features. Acta Pharmacol. Sin. 2008, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Westerink, R.H.S.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Tusi, S.K.; Ansari, N.; Amini, M.; Amirabad, A.D.; Shafiee, A.; Khodagholi, F. Attenuation of NF-κB and activation of Nrf2 signaling by 1,2,4-triazine derivatives, protects neuron-like PC12 cells against apoptosis. Apoptosis 2010, 15, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.S.; Cho, E.K.; Kwon, B.Y.; Phark, S.; Hwang, K.W.; Sul, D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008, 46, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.W.; Lee, H.; Yoo, H.S.; Yun, Y.P.; Oh, K.W.; Ha, T.Y.; Hong, J.T. Inhibitory effect of green tea extract on beta-amyloid-induced PC12 cell death by inhibition of the activation of NF-kappaB and ERK/p38 MAP kinase pathway through antioxidant mechanisms. Brain Res. Mol. Brain Res. 2005, 140, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hoi, C.P.; Ho, Y.P.; Baum, L.; Chow, A.H. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis, on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010, 24, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J. Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 2015, 59, 745–756. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ji, H.F.; Li, X.J.; Zhang, H.Y. Natural products and drug discovery. Can thousands of years of ancient medical knowledge lead us to new and powerful drug combinations in the fight against cancer and dementia? EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Frustaci, A.; Del Bufalo, A.; Fini, M.; Cesario, A. Multitarget drugs of plants origin acting on Alzheimer’s disease. Curr. Med. Chem. 2013, 20, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.Y.; Kim, J.K. Curcuminoids from Curcuma longa L. (Zingiberaceae) that protect PC12 rat pheochromocytoma and normal human umbilical vein endothelial cells from betaA(1-42) insult. Neurosci. Lett. 2001, 303, 57–61. [Google Scholar] [CrossRef]
- Siddiqui, M.A.; Kashyap, M.P.; Kumar, V.; Tripathi, V.K.; Khanna, V.K.; Yadav, S.; Pant, A.B. Differential protection of pre-, co- and post-treatment of curcumin against hydrogen peroxide in PC12 cells. Hum. Exp. Toxicol. 2011, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.S.; Lee, S.D.; Kuo, W.W. Anti-apoptotic and pro-survival effect of protocatechuic acid on hypertensive hearts. Chem. Biol. Interact. 2014, 209, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, D.L.; Galea, E.; Reis, D.J. Suppression of glial nitric oxide synthase induction by heat shock: Effects on proteolytic degradation of IkappaB-alpha. Nitric Oxide 1997, 1, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Surh, Y.J. Beta-amyloid-induced apoptosis is associated with cyclooxygenase-2 up-regulation via the mitogen-activated protein kinase-NF-kappaB signaling pathway. Free Radic. Biol. Med. 2005, 38, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Lin, J.K.; Pan, M.H.; Lin-Shiau, S.Y. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 2000, 13, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Can improving bioavailability improve the bioactivity of curcumin? Trends Pharmacol. Sci. 2014, 35, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Thapa, A.; Jett, S.D.; Chi, E.Y. Curcumin Attenuates Amyloid-β Aggregate Toxicity and Modulates Amyloid-β Aggregation Pathway. ACS Chem. Neurosci. 2016, 7, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, D.; Taguchi, H.; Yamamoto, A.; Shirai, N.; Hirao, K.; Tooyama, I. Curcuminoid binds to amyloid-b1-42 oligomer and fibril. J. Alzheimers Dis. 2011, 24, 33–42. [Google Scholar] [PubMed]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Gilani, A.H. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol. Biochem. Behav. 2009, 91, 554–559. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).