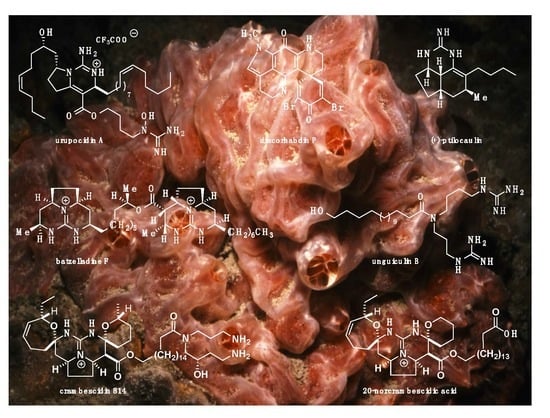
Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas
Abstract
:1. Introduction
2. Chemistry and Biology of Natural Products Isolated from Batzella, Crambe and Monanchora
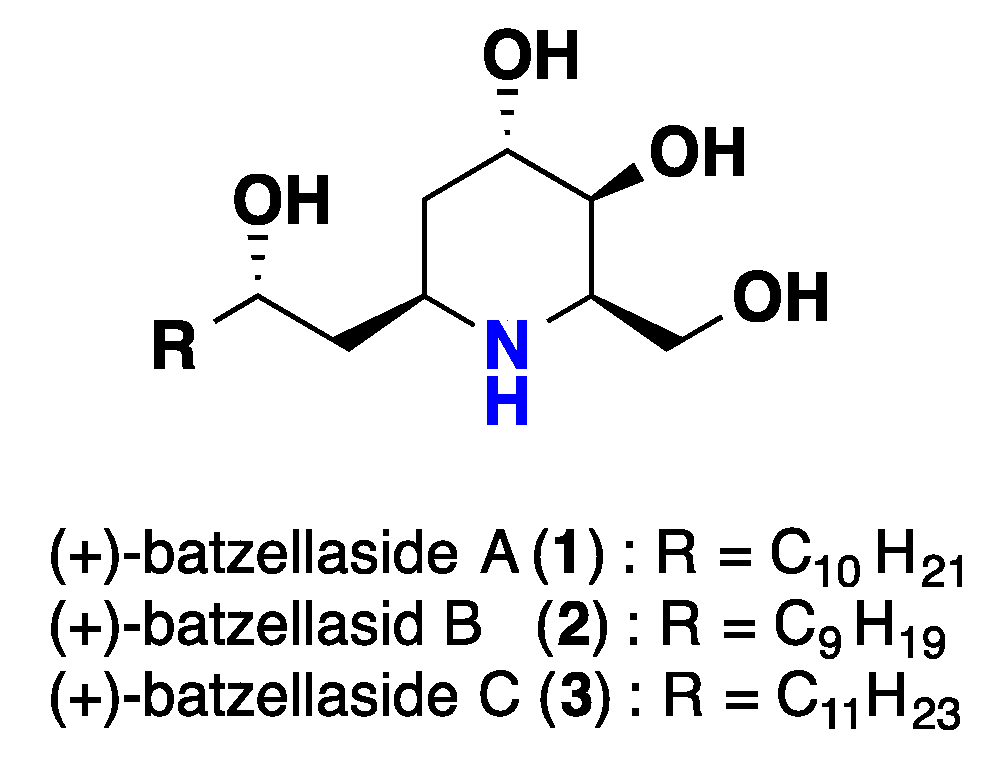
2.1. Piperidine Iminosugars Alkaloids
2.2. Bicyclic Guanidine Alkaloids
2.3. Tricyclic Guanidine Alkaloids Bearing Ptilocaulin
2.4. Tricyclic Pyrroloquinoline Alkaloids
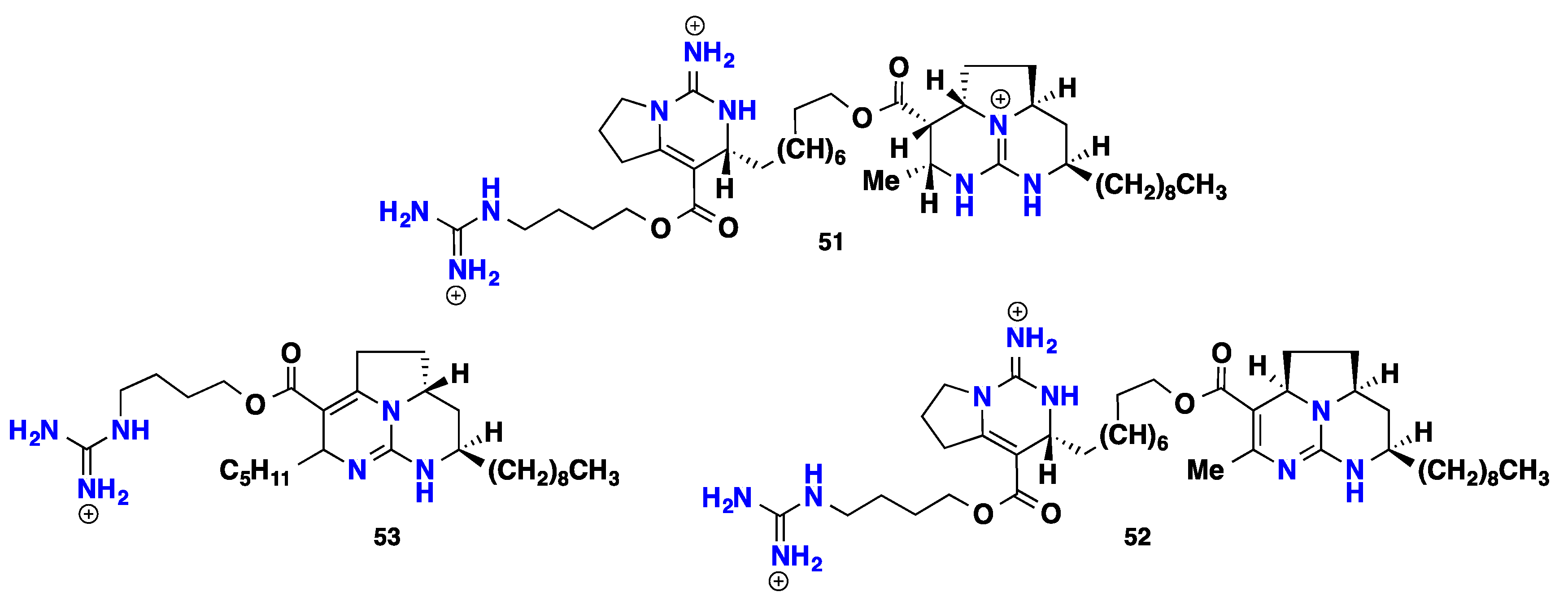
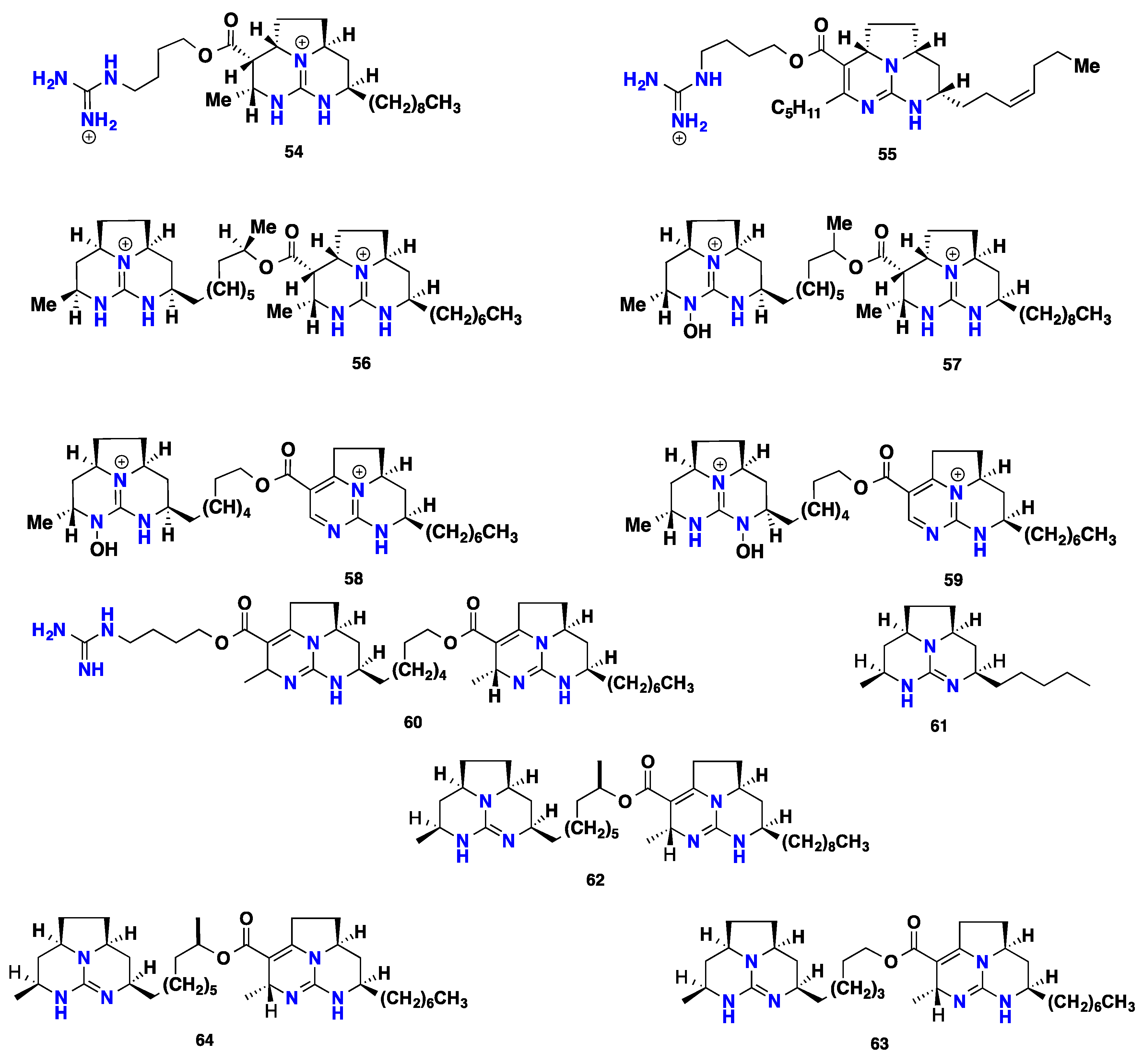
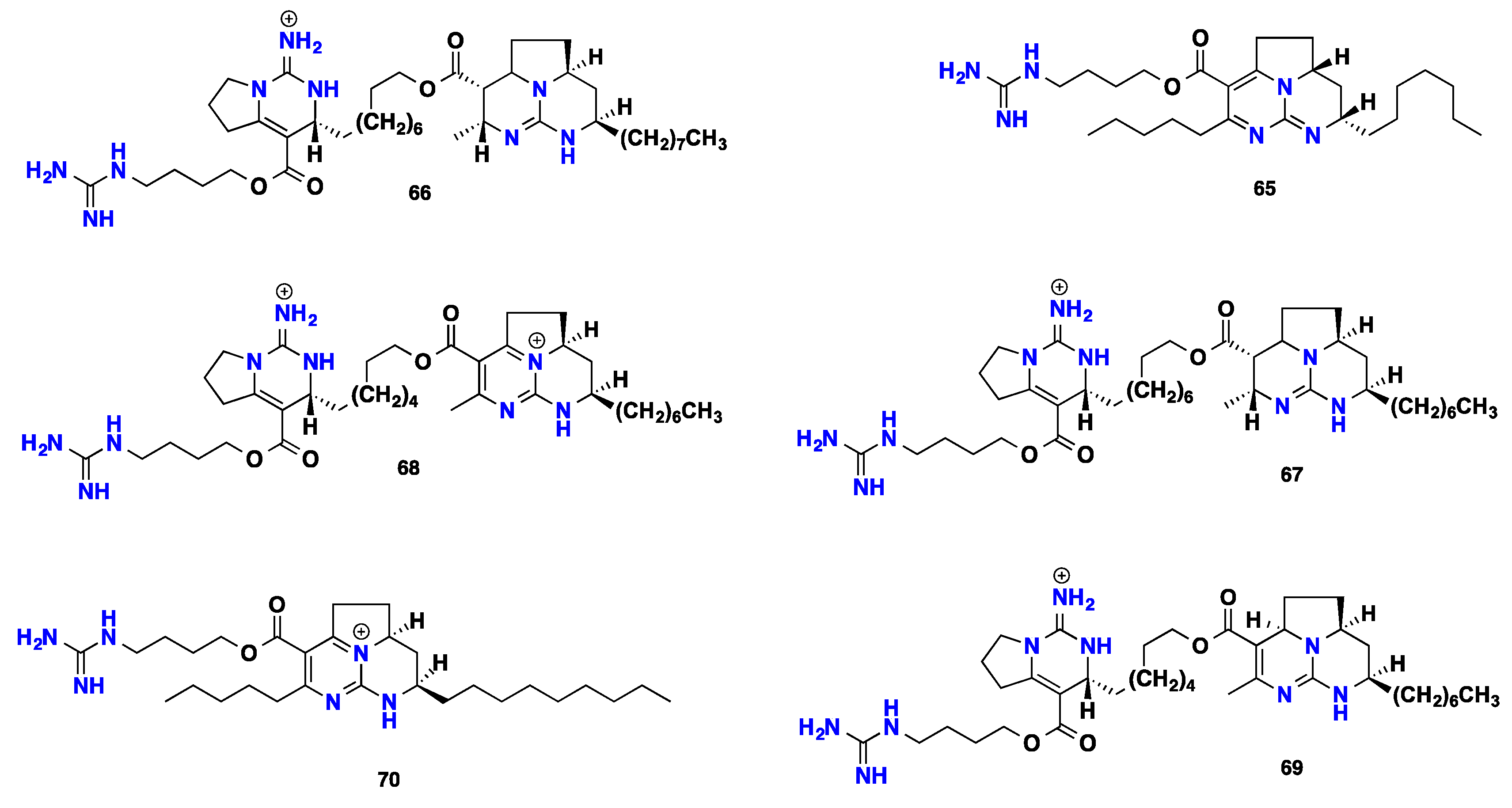
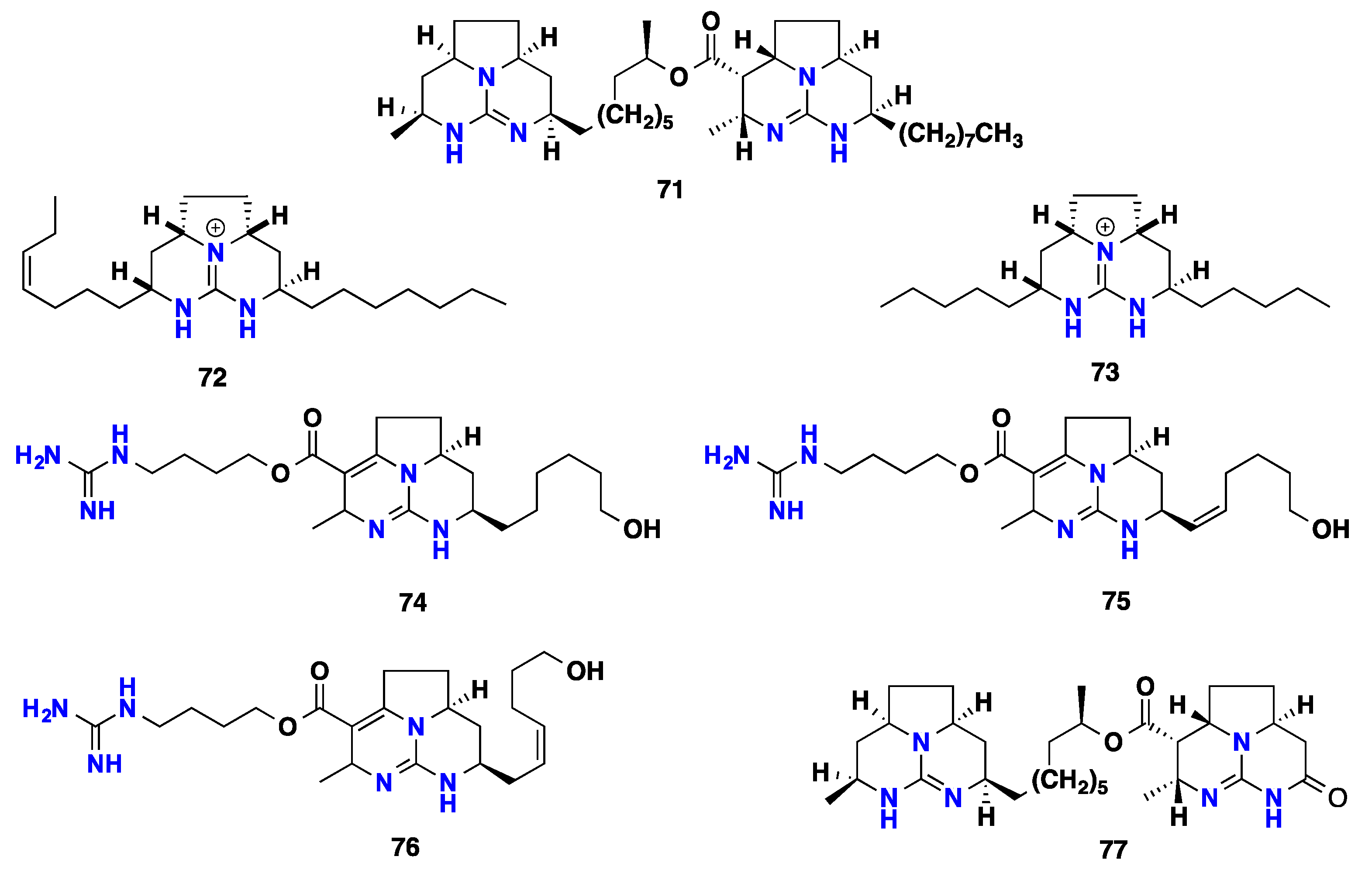
2.5. Polycyclic Alkaloids Bearing Batzelladine
2.6. Pentacyclic Alkaloids Bearing Crambescidin
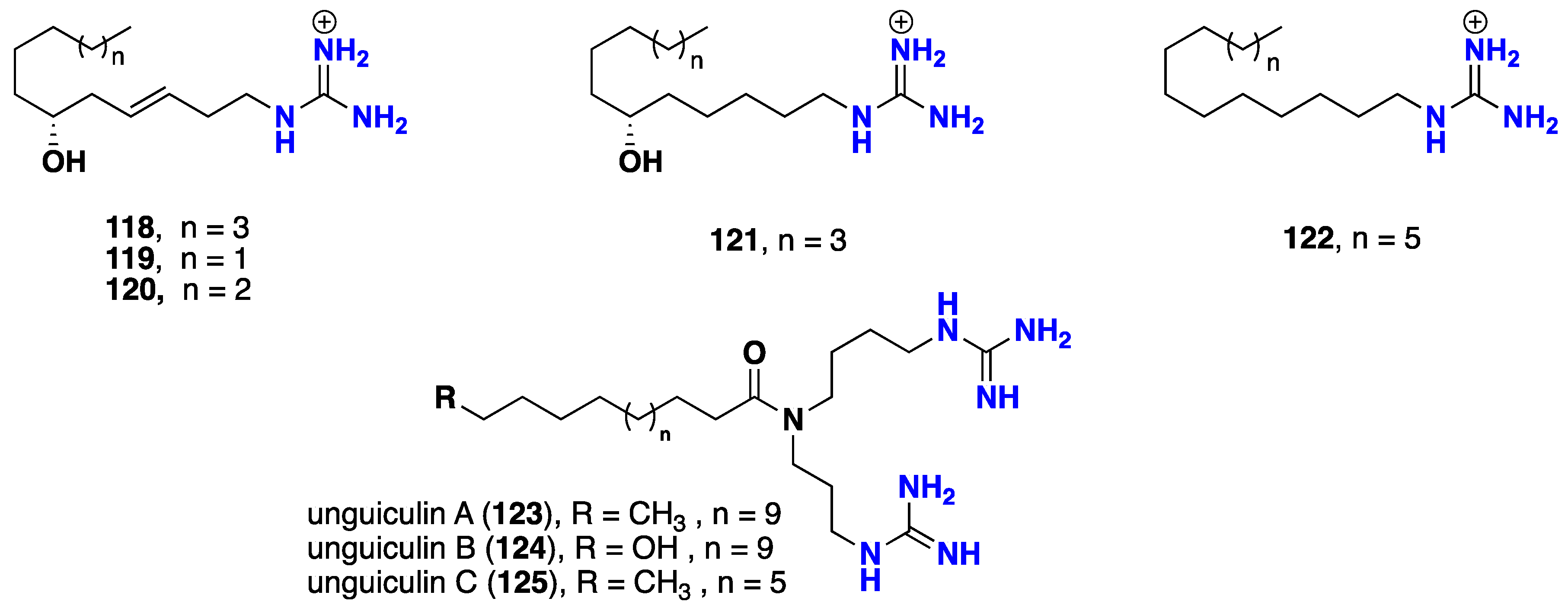
2.7. Acyclic Guanidine Alkaloids
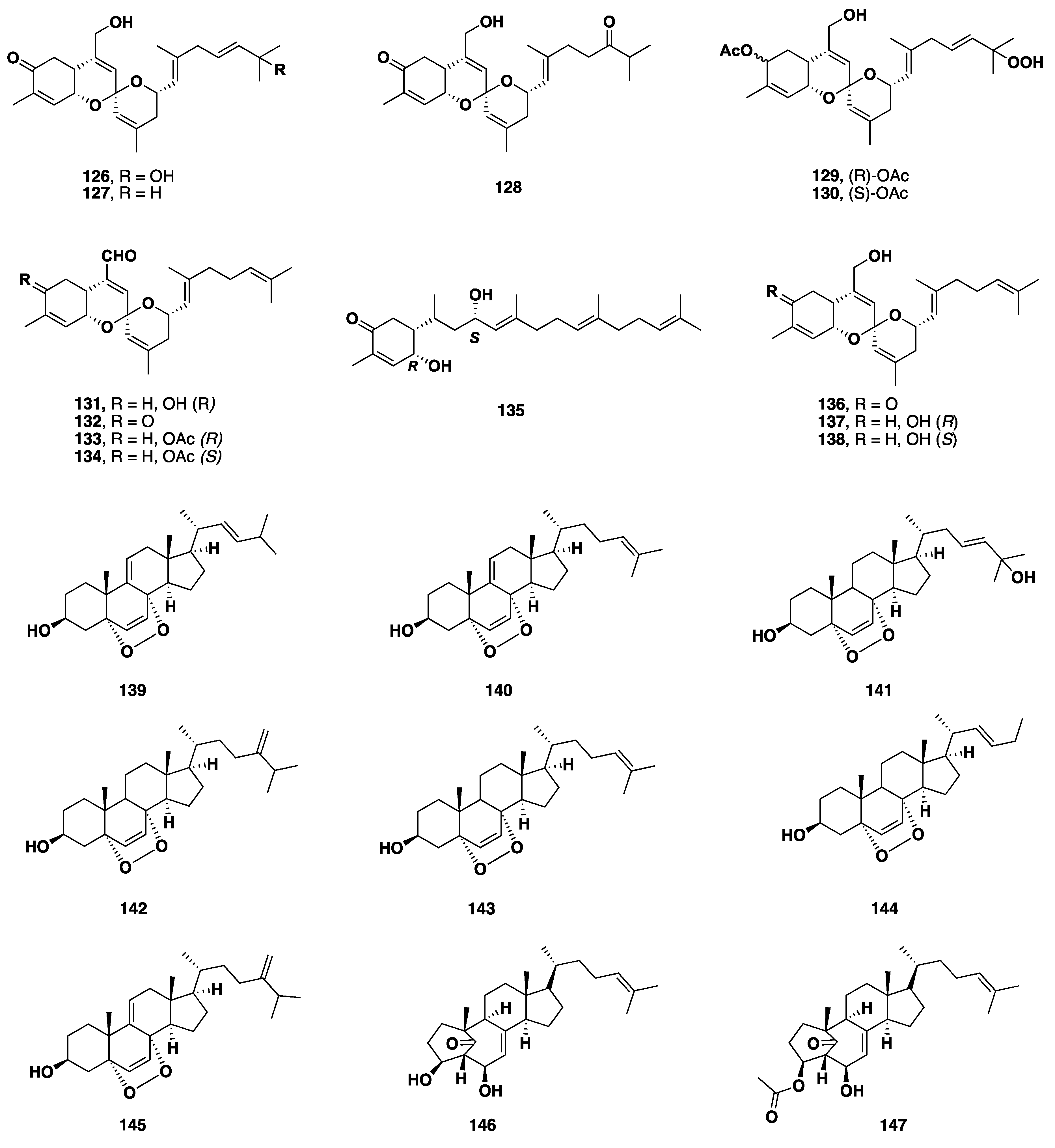
2.8. Terpenoid Compounds
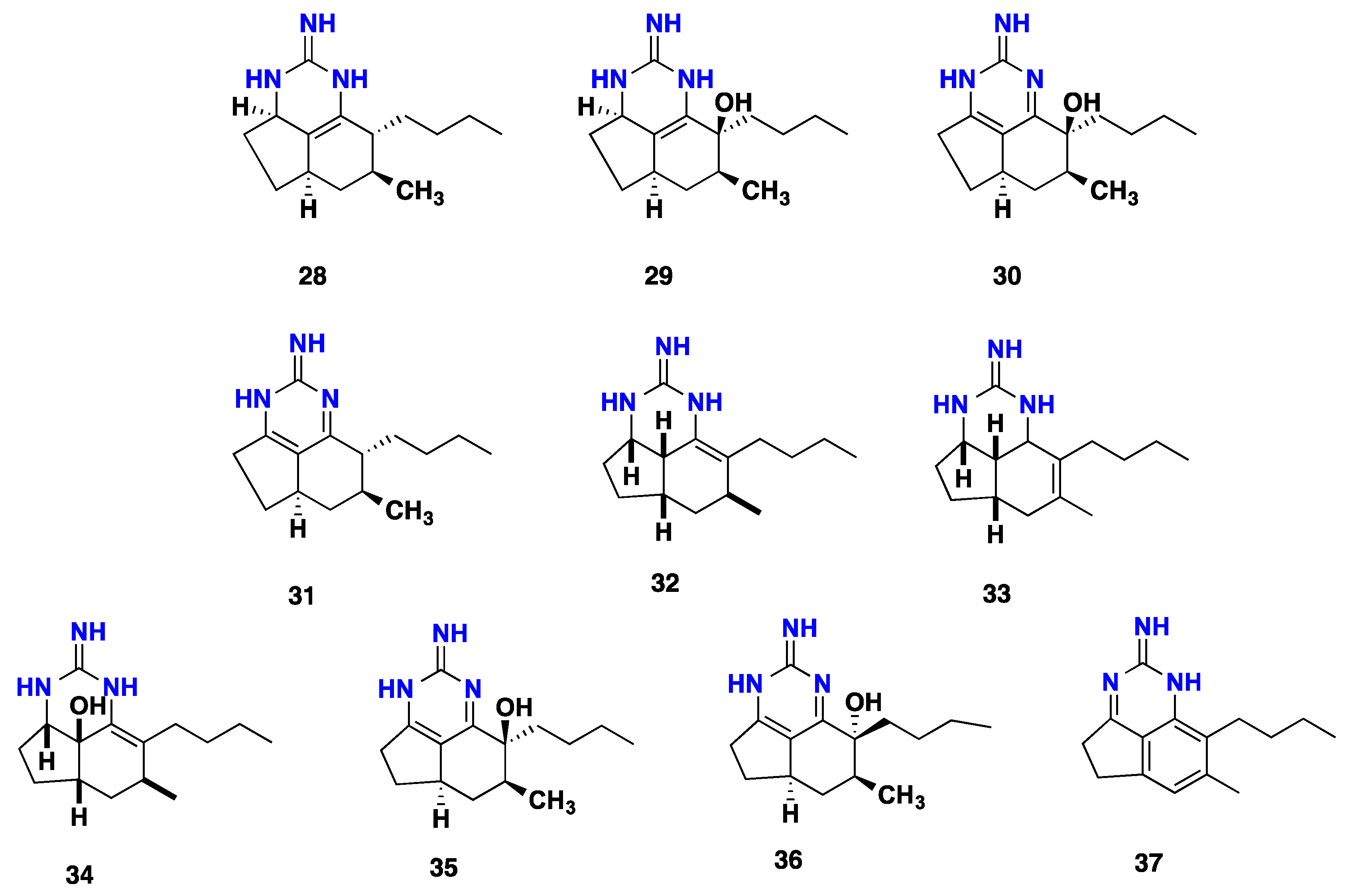
3. Biomimetic Landmarks of Polycyclic Guanidinium Motifs
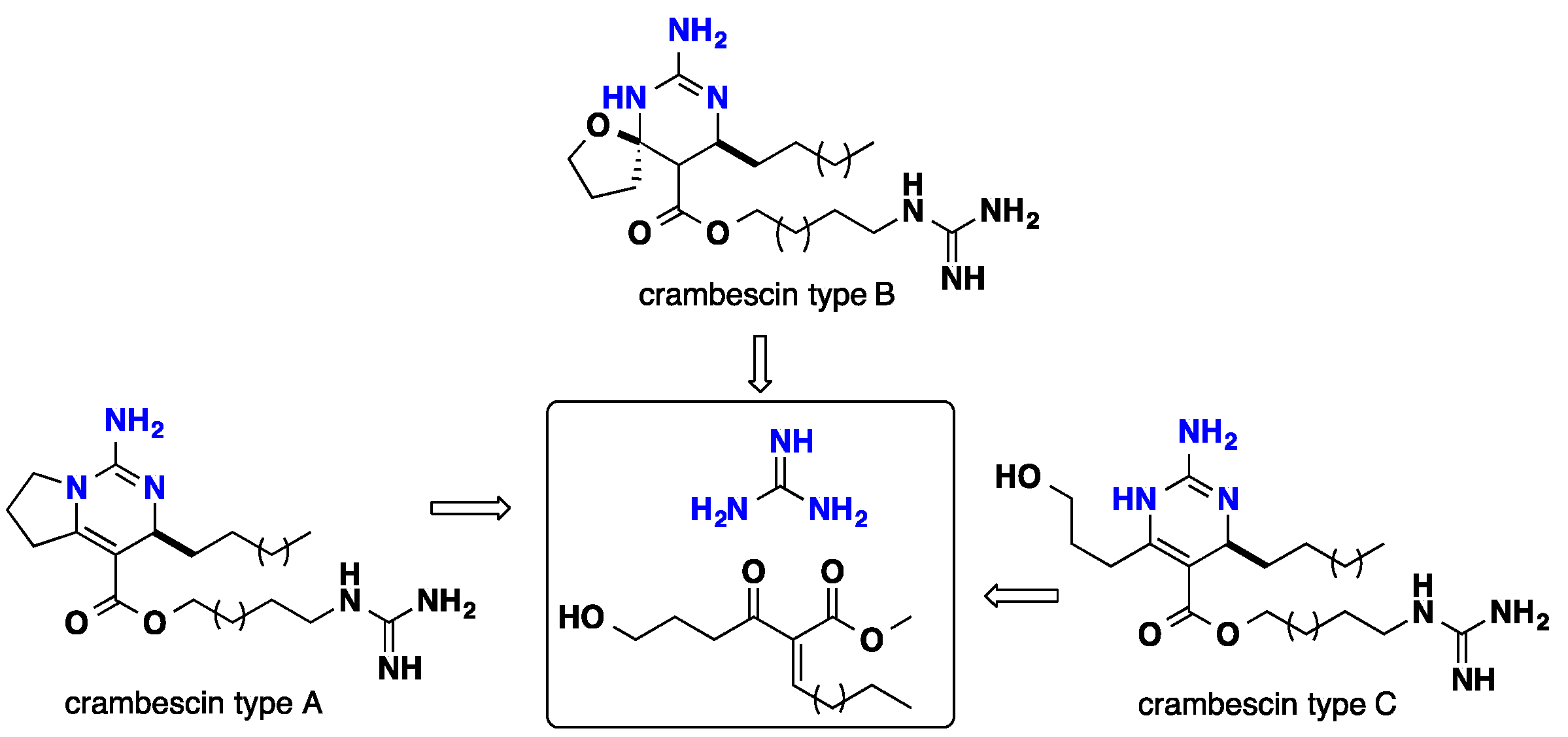
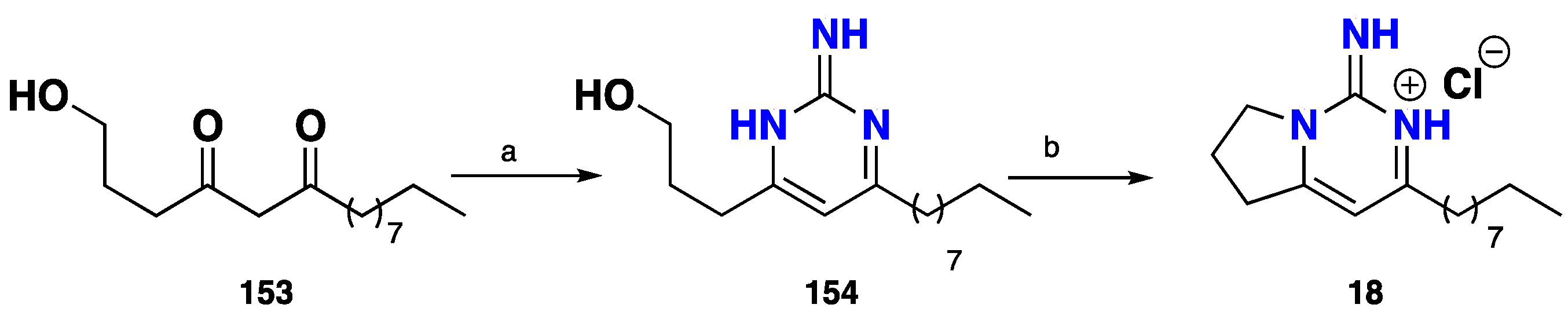
3.1. Bicyclic Compounds Possessing Crambescins Type A, B and C
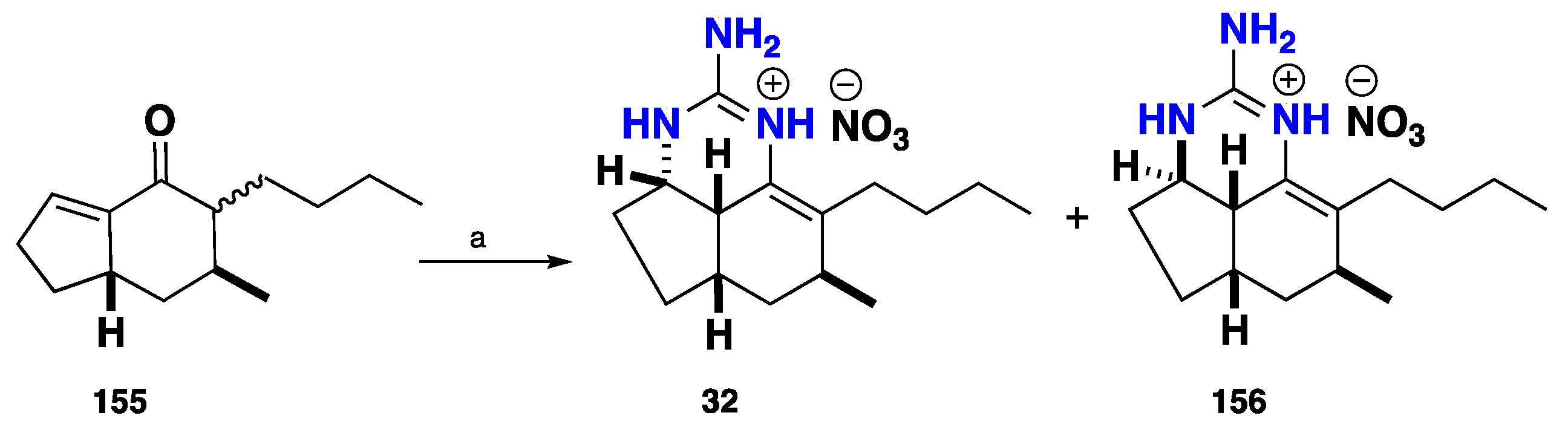
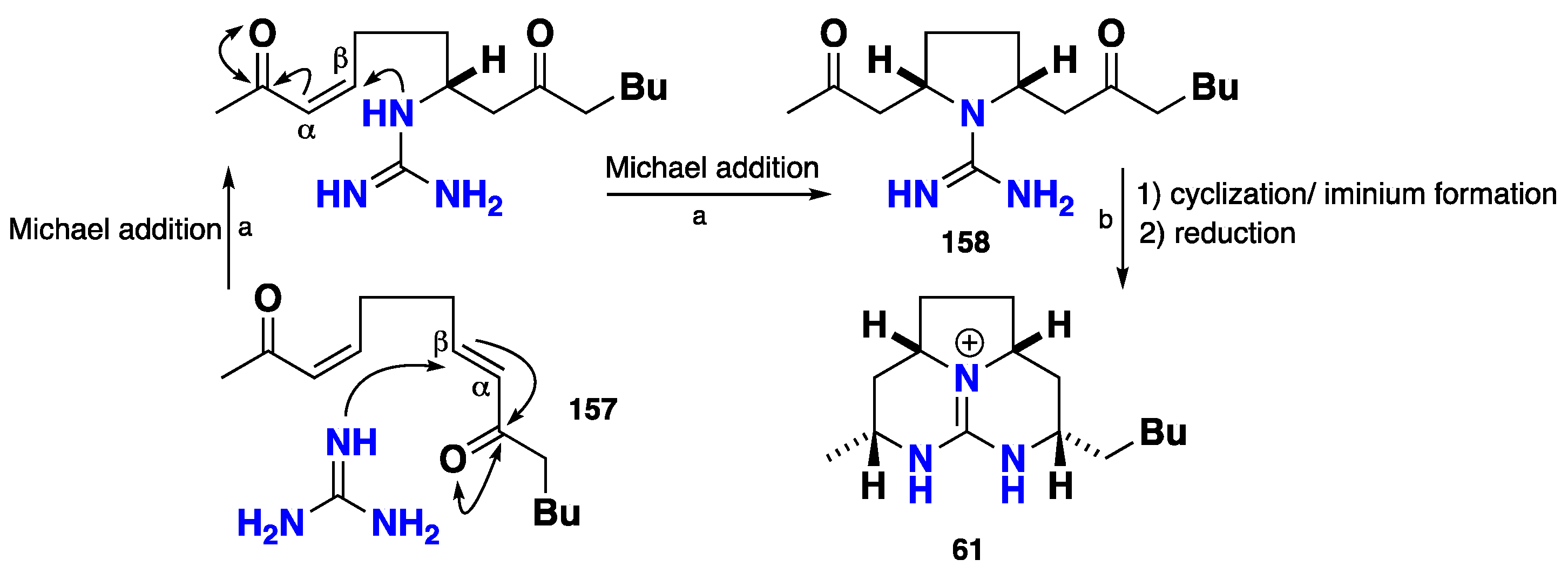
3.2. Tricyclic Possessing Ptilocaulin/Batzelladine
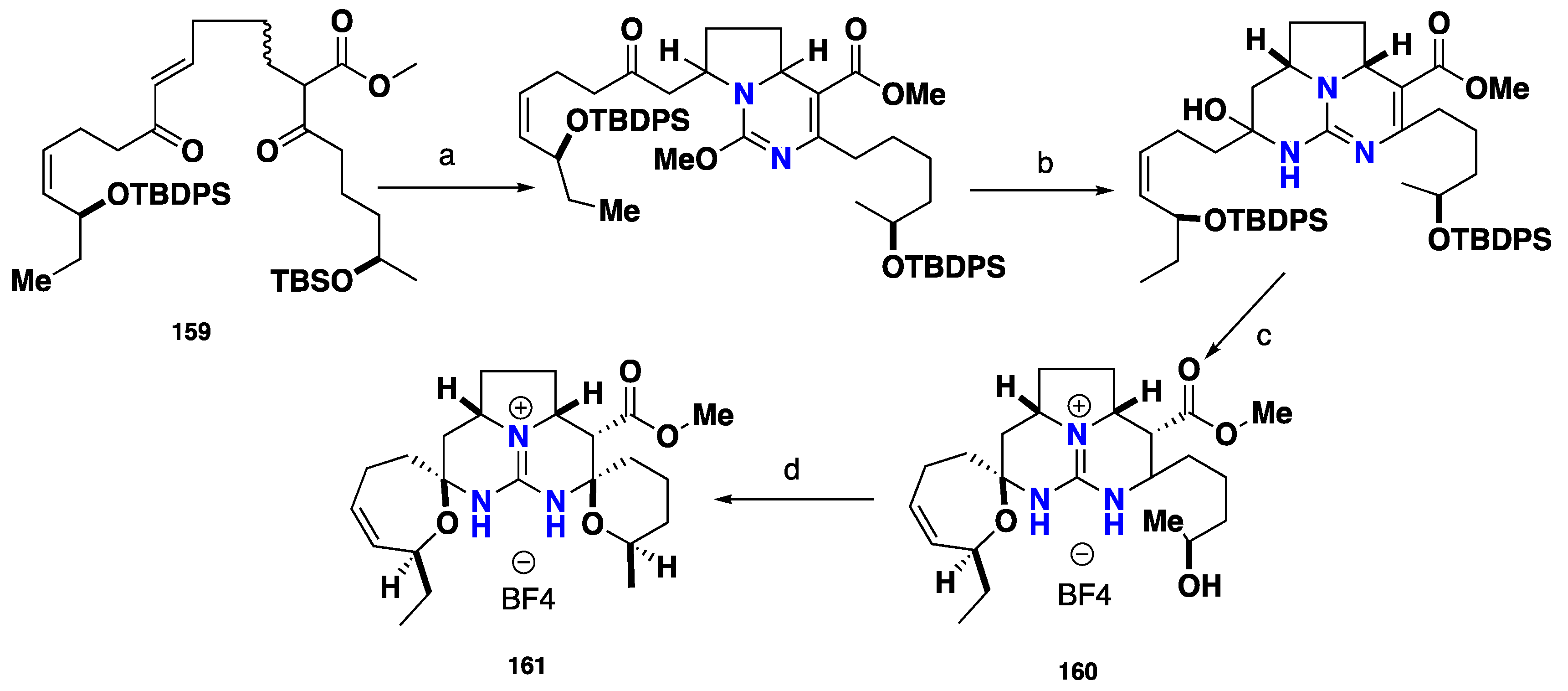
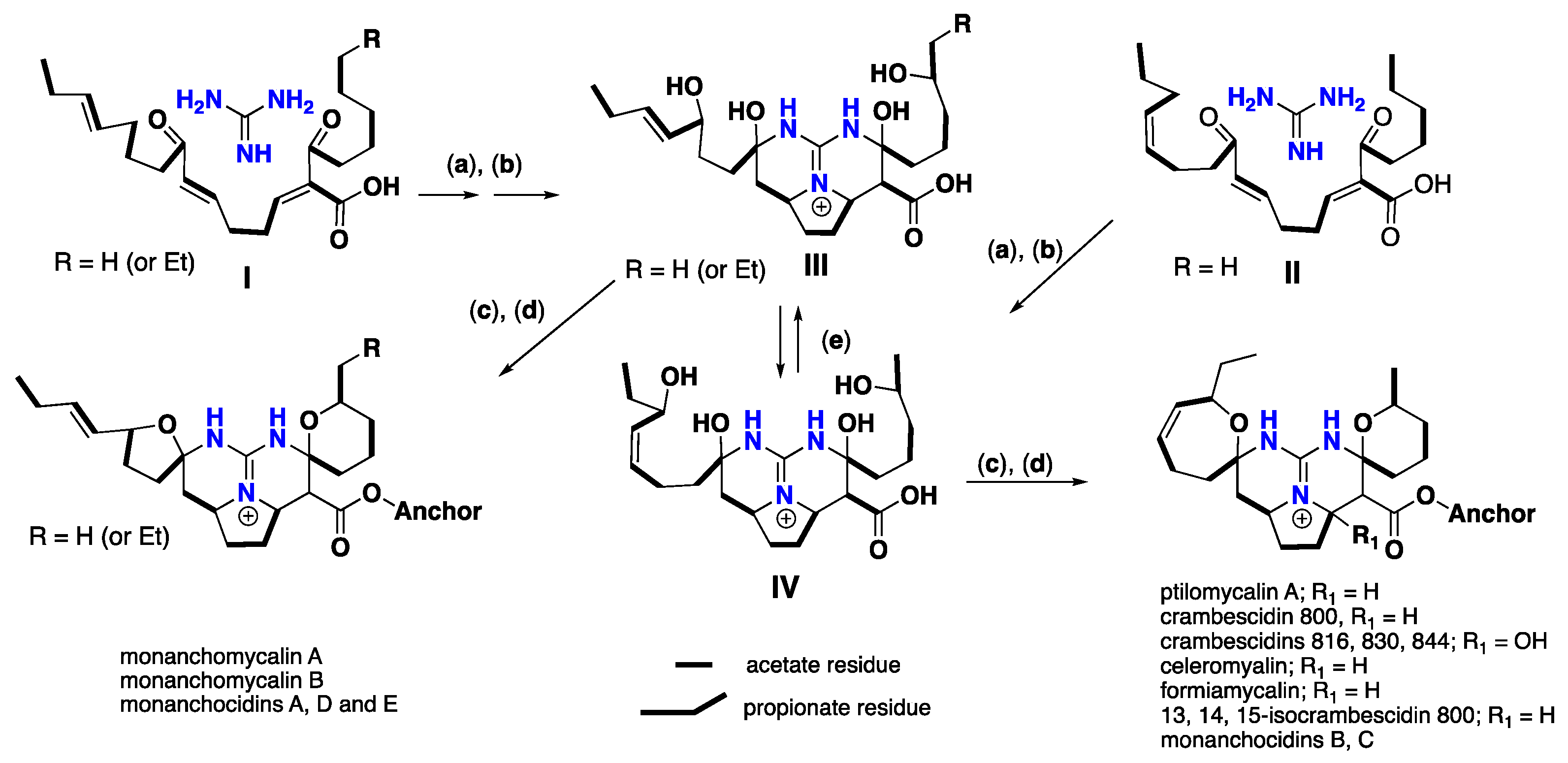
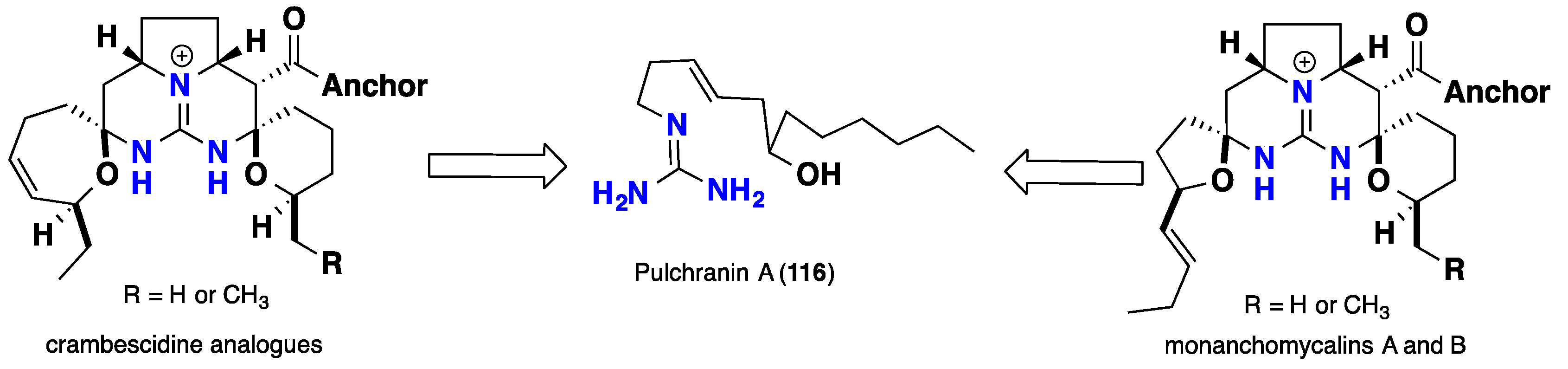
3.3. Pentacyclic Possessing Ptilomycalins, Crambescidins and Monanchomycalins
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADMET | absorption, distribution, metabolism and excretion–toxicity in pharmacokinetics |
| EC50 | Half maximal effective concentration |
| GI50 | Half maximal growth inhibition |
| gp120 | glycoprotein 120 |
| HIV-1 | Human immunodeficiency virus 1 |
| HSV-1 | Herpes simplex virus 1 |
| IC50 | Half maximal inhibitory concentration |
| MIC | Minimum inhibitory concentration |
References
- Montaser, R.; Luesch, H. Marine natural products: A new wave of drugs. Future Med. Chem. 2011, 3, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical Industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef] [PubMed]
- Rangel, M.; Falkenberg, M. An overview of the marine natural products in clinical trials and on the market. J. Coast. Life Med. 2015, 3, 421–428. [Google Scholar] [CrossRef]
- Shanmugam, A.; Vairamani, S. Biologically active metabolites from sponges and their activities. In Marine Sponges: Chemicobiological and Biomedical Applications; Pallela, R., Ehrlich, H., Eds.; Springer: Berlin, Germany, 2016; pp. 115–142. ISBN 978-81-322-2794-6. [Google Scholar]
- Sfecci, E.; Lacour, T.; Amad, P.; Mehiri, M. Polycyclic guanidine alkaloids from Poecilosclerida marine sponges. Mar. Drugs 2016, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S. Some aspects of guanidine secondary metabolites. Prog. Chem. Nat. Prod. 1995, 119–295. [Google Scholar] [CrossRef]
- Sun, H.H.; Sakemi, S.; Burres, N.; McCarthy, P. Isobatzellines A, B, C and D. cytotoxic and antifungal pyrroloquinoline alkaloids from the marine sponge Batzella sp. J. Org. Chem. 1990, 55, 4964–4966. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Sakai, R.; Rinehart, K.L. Crambescidins: New antiviral and cytotoxic compounds from the sponge Crambe crambe. J. Org. Chem. 1991, 6, 5712–5715. [Google Scholar] [CrossRef]
- Gochfeld, D.J.; El-Sayed, K.A.; Yousaf, M.; Hu, J.F.; Bartyzel, P.; Dunbar, D.C.; Wilkins, S.P.; Zjawiony, J.K.; Schinazi, R.F.; Schlueter, W.S.; et al. Marine natural products as lead anti-HIV agents. Mini Rev. Med. Chem. 2003, 3, 401–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Yang, B.; Lin, X.; Yang, X.; Liu, Y. Marine natural products with anti-HIV activities in the last decade. Curr. Med. Chem. 2013, 20, 953–973. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; López-Alonso, H.; Roel, M.; Vieytes, M.R.; Thomas, O.; Ternon, E.; Vega, F.V.; Botana, L.M. Mechanism of cytotoxic action of crambescidin-816 on human liver-derived tumour cells. Br. J. Pharmacol. 2014, 171, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, K.A. Natural products as antiviral agents. Stud. Nat. Prod. Chem. 2000, 24, 473–572. [Google Scholar]
- Mai, S.H.; Nagulapalli, V.K.; Patil, A.D.; Truneh, A.; Westley, J.W. Marine Compounds as HIV Inhibitors. U.S. Patent Application No. WO9301193 (A1), 21 January 1993. [Google Scholar]
- Patil, A.D.; Kumar, N.V.; Kokke, W.; Bean, M.F.; Freyer, A.J.; Brosse, C.D.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel alkaloids from the sponge Batzella sp. Inhibitors of HIV gp120-Human CD4 Binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar] [CrossRef]
- Nakao, Y.; Fusetani, N. Enzyme inhibitors from marine invertebrates. J. Nat. Prod. 2007, 70, 689–710. [Google Scholar] [CrossRef] [PubMed]
- Carté, B.K. Marine natural products as a source of novel pharmacological agents. Curr. Opin. Biotechnol. 1993, 4, 275–279. [Google Scholar] [CrossRef]
- Berlinck, R.G.S.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Ferri, S.; Spampinato, S.; Speroni, E. Polycyclic guanidine alkaloids from the marine sponge Crambe crambe and Ca2+ channel blocker activity of crambescidin 816. J. Nat. Prod. 1993, 56, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Rubiolo, J.A.; Ternon, E.; Lopez-Alonso, H.; Thomas, O.; Vega, F.V.; Vieytes, M.R.; Botana, L. Crambescidin-816 Acts as a fungicidal with more potency than crambescidin 800 and 830, Inducing cell cycle arrest, increased cell size and apoptosis in Saccharomyces cerevisiae. Mar. Drugs 2013, 11, 4419–4434. [Google Scholar] [CrossRef] [PubMed]
- Amad, P.; Charroin, C.; Baby, C.; Vacelet, J. Antimicrobial activities of marine sponges from the Mediterranean Sea. Mar. Biol. 1987, 94, 271–275. [Google Scholar] [CrossRef]
- Sun, X.; Sun, S.; Ference, C.; Zhu, W.; Zhou, N.; Zhang, Y.; Zhou, K. A potent antimicrobial compound isolated from Clathria cervicornis. Bioorg. Med. Chem. Lett. 2015, 25, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Batra, S. Thiourea and guanidine derivatives as antimalarial and antimicrobial agents. Curr. Med. Chem. 2013, 13, 2011–2025. [Google Scholar] [CrossRef]
- Shimokawa, J.; Ishiwata, T.; Shirai, K.; Koshino, H.; Tanatani, A.; Nakata, T.; Hashimoto, Y.; Nagasawa, K. Total synthesis of (+)-Batzelladine A.; (−)-Batzelladine D and identification of their target protein. Chem. Eur. J. 2005, 11, 6878–6888. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.G.; Murphy, P.J.; Williams, H.L.; McGown, A.T.; Smith, N.K. Synthetic studies towards ptilomycalin A: Total synthesis of crambescidin 359. Tetrahedron Lett. 2007, 63, 11771–11780. [Google Scholar] [CrossRef]
- Sekine, M.; Iijima, Y.; Iwamoto, O.; Nagasawa, K. Synthesis of (+)-Batzelladine K. Heterocycles 2010, 80, 395–408. [Google Scholar] [CrossRef]
- Wierzejska, J.; Ohshima, M.; Inuzuka, T.; Sengoku, T.; Takahashi, M.; Yoda, H. Total synthesis and absolute stereochemistry of (+)-batzellaside B.; its C8-epimer, a new class of piperidine alkaloids from the sponge Batzella sp. Tetrahedron Lett. 2011, 52, 1173–1175. [Google Scholar] [CrossRef]
- Babij, N.R.; Wolfe, J.P. Asymmetric total synthesis of (+)-Merobatzelladine B. Angew. Chem. Int. Ed. 2012, 51, 4128–4130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; De, S.; Chen, C. Syntheses of cyclic guanidine-containing natural products. Tetrahedron 2015, 71, 1145–1173. [Google Scholar] [CrossRef] [PubMed]
- Parr, B.T.; Economou, C.; Herzon, S.B.A. Concise synthesis of (+)-batzelladine B from simple pyrrole-based starting materials. Nature 2015, 525, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M.; Braekman, J.C.; Faulkner, D.J.; Hajdu, E.; Harper, M.K.; Vacelet, J. The genus Batzella: A chemosystematic problem. Bull. Inst. R. Sci. Nat. Belg. 1996, 66, 89–101. [Google Scholar]
- World Porifera Database. 2017. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=168731 (accessed on 20 March 2017).
- World Porifera Database. 2017. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=131931 (accessed on 20 March 2017).
- World Porifera Database. 2017. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=169014 (accessed on 20 March 2017).
- Segraves, N.L.; Crews, P.A. Madagascar sponge Batzella sp. as a source of alkylated iminosugars. J. Nat. Prod. 2005, 68, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Braekman, J.C.; Daloze, D.; Bruno, I.; Riccio, R.; Rogeau, D.; Amade, P. Crambines C1 and C2: Two further cytotoxic guanidine alkaloids from the sponge Crambe crambe. J. Nat. Prod. 1992, 55, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Meragelman, K.M.; McKee, T.C.; McMahon, J.B. Monanchorin, a bicyclic alkaloid from the sponge Monanchora ungiculata. J. Nat. Prod. 2004, 67, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Bensemhoun, J.; Bombarda, I.; Aknin, M.; Vacelet, J.; Gaydou, E.M. Ptilomycalin D, a polycyclic guanidine alkaloid from the marine sponge Monanchora dianchora. J. Nat. Prod. 2007, 70, 2033–2035. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Shubina, L.K.; Kuzmich, A.S.; Lee, H.S.; Stonik, V.A. Monanchocidins B–E: Polycyclic guanidine alkaloids with potent antileukemic activities from the sponge Monanchora pulchra. J. Nat. Prod. 2011, 74, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.; Daloze, D.; Braekman, J.C.; Hajdu, E.; van Soest, R.W.M. 8b-hydroxyptilocaulin, a hew guanidine alkaloid from the sponge Monanchora arbuscula. J. Nat. Prod. 1995, 58, 1139–1142. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Rodrigues-Stien, A.; Petek, S.; Demoy-Schnider, M.; Hall, K.; Hooper, J.N.A.; Debitus, C.; Al-Mourabit, A. Cytotoxic guanidine alkaloids from a French Polynesian Monanchora n. sp. sponge. J. Nat. Prod. 2016, 79, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Berlinck, R.G.S.; Braekman, J.C.; Daloze, D.; Hallenga, K.; Ottinger, R. Two new guanidine alkaloids from the Mediterranean sponge Crambe crambe. Tetrahedron Lett. 1990, 31, 6531–6534. [Google Scholar] [CrossRef]
- Bondu, S.; Genta-Jouve, G.; Leirοs, M.; Vale, C.; Guigonis, J.M.; Botana, L.M.; Thomas, O.P. Additional bioactive guanidine alkaloids from the Mediterranean sponge Crambe crambe. RSC Adv. 2012, 2, 2828–2835. [Google Scholar] [CrossRef]
- Chang, L.C.; Whittaker, N.F.; Bewley, C.A. Crambescidin 826 and Dehydrocrambine A: New polycyclic guanidine alkaloids from the marine sponge Monanchora sp. that inhibit HIV-1 Fusion. J. Nat. Prod. 2003, 66, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.C.; Harper, P.M.; Williams, D.E.; Mesquita, J.T.; Pinto, E.G.; Da Costa-Silva, T.A.; Hajdu, E.; Ferreira, A.G.; Santos, R.A.; Murphy, P.J.; et al. Anti-parasitic guanidine and pyrimidine alkaloids from the marine sponge Monanchora arbuscula. J. Nat. Prod. 2015, 78, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Ogurtsova, E.K.; Deisenko, V.A.; Dmitrenok, P.S.; Tabakmakher, K.M.; Guzii, A.G.; Pislyagin, E.A.; Es’kov, A.A.; Kozhemyako, V.B.; Aminin, D.L.; et al. Urupocidin A: A new, inducing iNOS sxpression bicyclic guanidine alkaloid from the marine sponge Monanchora pulchra. Org. Lett. 2014, 16, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Jamison, M.T.; Molinski, T.F. Antipodal crambescin A2 homologues from the marine sponge Pseudaxinella reticulata. Antifungal structure–activity relationships. J. Nat. Prod. 2015, 78, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Offen, P.; Bean, M.F.; Johnson, R.K. Three new tricyclic guanidine alkaloids from the sponge Batzella sp. J. Nat. Prod. 1997, 60, 704–707. [Google Scholar] [CrossRef]
- Harbour, G.C.; Tymiak, A.A.; Rinehart, K.L.; Shaw, P.D.; Hughes, R.G.; Mizsak, S.A.; Coats, J.H.; Zurenko, G.E.; Li, L.H.; Kuentzel, S.L. Ptilocaulin and isoptilocaulin, antimicrobial and cytotoxic Cyclic guanidines from the Caribbean Sponge ptilocaulis aff. P. spiculifer (Lamarck, 1814). J. Am. Chem. Soc. 1981, 103, 5604–5606. [Google Scholar] [CrossRef]
- Kossuga, M.H.; Delira, S.P.; Nascimento, A.M.; Gambardella, M.T.P.; Berlinck, R.G.S.; Torres, Y.R.; Nascimento, G.G.F.; Pimenta, E.F.; Silva, M.; Thiemann, O.H.; et al. Isolation and biological activities of secondary metabolites from the sponges Monanchora aff. arbuscula, Aplysina sp. Petromica ciocalyptoides and Topsentia ophiraphidies, from the ascidian Didemnum ligulum and from the octocoral Carijoa riisei. Quim. Nova 2007, 30, 1194–1202. [Google Scholar] [CrossRef]
- Hua, H.M.; Peng, J.; Fronczek, F.R.; Kelly, M.; Hamann, M.T. Crystallographic and NMR studies of antiinfective tricyclic guanidine alkaloids from the sponge Monanchora unguifera. Bioorg. Med. Chem. 2004, 12, 6461–6464. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.G.; Wilke, D.V.; Jimenez, P.C.; De oliveira, J.R.; Pessoa, O.D.L.; Silveria, E.R.; Viana, F.A.; Pessoa, C.; Maraes, M.O.; Hajdu, E.; et al. Guanidine alkaloids from Monanchora arbuscula: Chemistry and antitumor potential. Chem. Biodivers. 2011, 8, 1433–1445. [Google Scholar] [CrossRef]
- Sakemi, S.; Sun, H.H.; Jefford, C.W.; Bemardinelli, G. Batzellines A, B and C. Novel pyrroloquinoline alkaloids from the sponge Batzella sp. Tetrahedron Lett. 1989, 30, 2517–2520. [Google Scholar] [CrossRef]
- Sun, H.H.; Sakemi, S.I. Pyrroloquinoline Alkaloids, Batzellines and Isobatzellines from Marine Sponge and Methods of Use. U.S. Patent Application No. US5028613 (A), 2 July 1991. [Google Scholar]
- Gunasekera, S.P.; McCarthy, P.J.; Longley, R.E.; Pomponi, S.A.; Wright, A.E.; Lobkovsky, E.; Clardy, J. Discorhabdin P, a new enzyme inhibitor from a deep-water Caribbean sponge of the genus Batzella. J. Nat. Prod. 1999, 62, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Zuleta, I.A.; Longley, R.E.; Wright, A.E.; Pomponi, S.A. Discorhabdins S, T and U, new cytotoxic pyrroloiminoquinones from a deep-water Caribbean sponge of the genus Batzella. J. Nat. Prod. 2003, 66, 1615–1617. [Google Scholar] [CrossRef] [PubMed]
- Gunaskera, S.P.; McCarthy, P.J.; Pomponi, S.A.; Wright, A.E.; Longley, R.E. Discorhabdin Compounds and Methods of Use. U.S. Patent Application No. US6057333 (A), 2 May 2000. [Google Scholar]
- Gunasekera, S.P.; McCarthy, P.J.; Longley, R.E.; Pomponi, S.A.; Wright, A.E. Secobatzellines A and B, two new enzyme inhibitors from a deep-water Caribbean sponge of the genus Batzella. J. Nat. Prod. 1999, 62, 1208–1211. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; McCarthy, P.J.; Longley, R.E.; Pomponi, S.A.; Wright, A.E. Aminoiminoquinone and Aminoquinine Alkaloid Compounds as Capase Inhibitors. U.S. Patent No. WO2000002858 (A1), 20 January 2000. [Google Scholar]
- Guzman, E.A.; Johnson, J.D.; Carrier, M.K.; Meyer, C.I.; Pitts, T.P.; Gunasekera, S.P.; Wright, A.E. Selective cytotoxic activity of the marine-derived batzelladine compounds against pancreatic cancer cell lines. Anti-Cancer Drugs 2009, 20, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Miller, M.; Rooney, F. Clathrins A-C: Metabolites from a southern Australian marine sponge Clathria species. J. Nat. Prod. 2001, 64, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Taylor, P.B.; Carté, B.; Zuber, G.; Johnson, R.K.; Faulkner, D.J. Batzelladines F-I, novel alkaloids from the sponge Batzella sp.: Inducers of p56lck-CD4 dissociation. J. Org. Chem. 1997, 62, 1814–1819. [Google Scholar] [CrossRef]
- Gallimore, W.A.; Kelly, M.; Scheuer, P.J. Alkaloids from the sponge Monanchora unguifera. J. Nat. Prod. 2005, 68, 1420–1423. [Google Scholar] [CrossRef] [PubMed]
- Braekman, J.C.; Daloze, D.; Tavares, R.; Hajdu, E.; van Soest, R.W.M. Novel polycyclic guanidine alkaloids from two marine sponges of the genus Monanchora. J. Nat. Prod. 2000, 63, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.M.; Peng, J.; Dunber, D.C.; Schinazi, R.F.; Andrews, A.G.C.; Cuevas, C.; Fernandez, L.F.G.; Kelly, M.; Hamann, M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron 2007, 63, 11179–11188. [Google Scholar] [CrossRef]
- Laville, R.; Thomas, O.P.; Berrue, F.; Marquez, D.; Vacelet, J.; Amade, P. Bioactive guanidine alkaloids from two Caribbean marine sponges. J. Nat. Prod. 2009, 72, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Kohon, L.; Porto, P.; Binachi, B.; santos, M.; Berlinck, R.G.S.; Arns, C. Nor-batzelladine L from the sponge Monanchora sp. displays antiviral activity against Herpes Simplex virus type 1. Planta Med. 2012, 78, CL27. [Google Scholar] [CrossRef]
- Pessoa, C.; dos Santos, M.F.C.; Berlinck, R.G.S.; Ferreira, P.M.P.; Cavalcanti, B.C. Cytotoxic batzelladine L from the Brazilian marine sponge Monanchora arbuscula. Planta Med. 2013, 79, PK6. [Google Scholar] [CrossRef]
- Takishima, S.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; Yamada, H.; Omura, S.; Kobayashi, K.; Van Soest, R.W.M.; Matsunaga, S. Merobatzelladines A and B, anti-infective tricyclic guanidines from a marine sponge Monanchora sp. Org. Lett. 2009, 11, 2655–2658. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.F.; Mesquita, J.T.; Pinto, E.G.; Thais, A.; Costa-Silva, T.A.; Borborema, S.E.T.; Junior, A.J.G.; Neves, B.J.; Andrade, C.H.; Al Shuhaib, Z.; et al. Analogues of marine guanidine alkaloids are in vitro effective against Trypanosoma cruzi and selectively eliminate Leishmania (L.) infantum Intracellular amastigotes. J. Nat. Prod. 2016, 79, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Ray, S.; Cohen, F.; Collins, S.K.; Overman, L.E. Inhibition of HIV-1 envelope-mediated fusion by synthetic batzelladine analogues. J. Nat. Prod. 2004, 67, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, A.; Sato, K.; Aron, Z.D.; Cohen, F.; Harris, A.; McDougall, B.R.; Robinson, W.E.; Overman, L.E.; Weiss, G.A. Guanidine alkaloid analogues as inhibitors of HIV-1 Nef interactions with p53, actin and p56lck. Proc. Natl. Acad. Sci. USA 2004, 101, 14079–14084. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brahmbhatt, K.G.; Khan, S.I.; Jacob, M.; Tekwani, B.L.; Sabde, S.; Mitra, D.; Singh, I.; Khan, I.A.; Bhutani, K.K. Synthesis and biological evaluation of tricyclic guanidine analogues of batzelladine K for antimalarial, antileishmanial, antibacterial, antifungal and anti-HIV activities. Chem. Biol. Drug Des. 2013, 81, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.L.; Black, G.P.; Browne, P.; Hizi, A.; Jaffar, M.; Leyland, J.P.; Martin, C.; Oz-Gleenberg, I.; Murphy, P.J.; Roberts, T.D.; et al. Synthesis and biological activity of analogues of batzelladine F. Tetrahedron 2013, 69, 3061–3066. [Google Scholar] [CrossRef]
- Tempone, A.G.; Martins, L.F.; Pinto, E.G.; Mesquita, J.T.; Bennett, E.L.; Black, G.P.; Murphy, P.J. Synthetic marine guanidines are effective antileishmanial compounds by altering the plasma membrane permeability. Planta Med. 2014, 80, P1L154. [Google Scholar] [CrossRef]
- Kashman, Y.; Hirsh, S.; McConnell, O.J.; Ohtani, I.; Kusumi, T.; Kakisawa, H. Ptilomycalin A: A novel polycyclic guanidine alkaloid of marine origin. J. Am. Chem. Soc. 1989, 111, 8925–8926. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Jares-Erijman, E.A. Crambescidins: New Antiviral and Cytotoxic Compounds from the Sponge Crambe crambe. U.S. Patent Application No. 5756734 (A), 26 May 1998. [Google Scholar]
- Martin, V.; Vale, C.; Bondu, S.; Thomas, O.P.; Vieytes, M.R.; Botana, L.M. Differential effects of crambescins and crambescidin 816 in voltage-gated sodium, potassium and calcium channels in neurons. Chem. Res. Toxicol. 2013, 26, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Ternon, E.; Zarate, L.; Chenesseau, S.; Croué, J.; Dumollard, R.; Marcelino, T.; Suzuki, M.T.; Thomas, O.P. Spherulization as a process for the exudation of chemical cues by the encrusting sponge Cramb crambe. Sci. Rep. 2016, 6, 29474. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.G.; Juncal, B.; Silva, S.B.L.; Thomas, O.P.; Vàzquez, V.M.; Alfonso, A.; Vieytes, M.R.; Vale, C.; Botana, L.M. The marine guanidine alkaloid crambescidin 816 induces calcium influx and cytotoxicity in primary cultures of cortical neurons through glutamate receptors. ACS Chem. Neurosci. 2017, 8, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Jares-Erijman, E.A.; Ingrum, A.L.; Carney, J.R.; Rinehart, K.L.; Sakai, R. Polycyclic guanidine-containing compounds from the Mediterranean sponge Crambe crambe: The structure of 13,14,15-isocrambescidin 800 and the absolute stereochemistry of the pentacyclic guanidine moieties of the crambescidins. J. Org. Chem. 1993, 58, 4805–4808. [Google Scholar] [CrossRef]
- Heys, L.; Moore, C.G.; Murphy, P.J. The guanidine metabolites of Ptilocaulis spiculifer and related compounds; isolation and synthesis. Chem. Soc. Rev. 2000, 29, 57–67. [Google Scholar] [CrossRef]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015, 115, 9655–9705. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.G.; Sun, F.; Rinehart, K.L. Crambescidin Compounds. U.S. Patent Application No. WO1998046575 (A1), 22 October 1998. [Google Scholar]
- Rinehart, K.L.; Shi, J.G.; Sun, F. Crambescidin Compounds. U.S. Patent Application No. US006028077 (A), 22 February 2000. [Google Scholar]
- Palagiano, E.; De Marino, S.; Minale, L.; Riccio, R.; Zollo, F.; Iorizzi, M.; Carre, J.; Debitus, C.; Lucarain, L.; Provost, J. Ptilomycalin A, crambescidin 800 and related new highly cytotoxic guanidine alkaloids from the starfishes Fromia monilis and Celerina heffernani. Tetrahedron 1995, 51, 3675–3682. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Saludes, J.P.; Molinski, T.F. Ptilomycalin A inhibits laccase and melanization in Cryptococcus neoformans. Bioorg. Med. Chem. 2011, 19, 6654–6657. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Kong, D.; Matsui, K.; Kobayashi, M. Erythroid differentiation in K562 chronic myelogenous cells induced by crambescidin 800, a pentacyclic guanidine alkaloid. Anticancer Res. 2004, 24, 2325–2330. [Google Scholar] [PubMed]
- Dyshlovoy, S.A.; Venz, S.; Hauschild, J.; Tabakmakher, K.M.; Otte, K.; Madanchi, R.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; et al. Anti-migratory activity of marine alkaloid monanchocidin A, proteomics-based discovery and confirmation. Proteomics 2016, 16, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Hauschild, J.; Amann, K.; Tabakmakher, K.M.; Venz, S.; Walther, R.; Guzii, A.G.; Makarieva, T.N.; Shubina, L.K.; Fedorov, S.N.; et al. Marine alkaloid monanchocidin A overcomes drug resistance by induction of autophagy and lysosomal membrane permeabilization. Oncotarget 2015, 6, 17328–17341. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmakher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenko, P.S.; Kuzmich, A.S.; Lee, H.S.; Stonik, V.A. Monanchomycalins A and B, unusual guanidine alkaloids from the sponge Monanchora pulchra. Tetrahedron Lett. 2012, 53, 4228–4231. [Google Scholar] [CrossRef]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.S.; Lee, Y.J.; Kozlov, S. Marine cyclic guanidine alkaloids monanchomycalin B and urupocidin A act as inhibitors of TRPV1, TRPV2 and TRPV3 but not TRPA1 receptors. Mar. Drugs 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Tabakmakher, K.M.; Denisenko, V.A.; Guzii, A.G.; Dmitrenko, P.S.; Dyshlovoy, S.A.; Lee, H.S.; Makarieva, T.N. Monanchomycalin C, a new pentacyclic guanidine alkaloid from the Far-Eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2013, 8, 1399–1402. [Google Scholar] [PubMed]
- Tabakmakher, K.M.; Makarieva, T.N.; Denisenko, V.A.; Guzii, A.G.; Dmitrenko, P.S.; Kuzmich, A.S.; Stonik, V.A. Normonanchocidins A, B and D, new pentacyclic guanidine alkaloids from the Far-Eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2015, 10, 913–916. [Google Scholar] [PubMed]
- Tabakmakher, K.M.; Makarieva, T.N.; Shubina, L.K.; Denisenko, V.A.; Popov, R.S.; Kuzmich, A.S.; Lee, H.S.; Stonik, V.A. Monanchoxymycalins A and B, new hybrid pentacyclic guanidine alkaloids from the Far-Eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2016, 11, 1817–1820. [Google Scholar]
- Campos, P.E.; Queiroz, E.F.; Marcourt, L.; Wolfender, J.M.; Sanchez, A.S.; Illien, B.; Al-Mourabit, A.; Gauvin-Bialecki, A. Isolation and identification of new secondary metabolites from the marine sponge Monanchora unguiculata. Planta Med. 2016, 81, P580. [Google Scholar] [CrossRef]
- Campos, P.E.; Wolfender, J.M.; Queiroz, E.F.; Marcourt, L.; Al-Mourabit, A.; Frederich, M.; Bordignon, A.; De Voogd, N.; Illien, B.; Gauvin-Bialecki, A. Unguiculin A and ptilomycalins E-H, antimalarial guanidine alkaloids from the marine sponge Monanchora unguiculata. J. Nat. Prod. 2017, 80, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmaher, K.M.; Denisenko, V.A.; Dmitrenok, P.S.; Ogurtsova, E.K.; Antonov, A.S.; et al. Pulchranin A, isolated from the Far-Eastern marine sponge, Monanchora pulchra: The first marine non-peptide inhibitor of TRPV-1 channels. Tetrahedron Lett. 2013, 54, 1247–1250. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Ogurtsova, E.K.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Lee, H.S.; et al. Pulchranins B and C, new acyclic guanidine alkaloids From the Far-Eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2013, 8, 1229–1232. [Google Scholar] [PubMed]
- Ogurtsova, E.K.; Makarieva, T.N.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Denisenko, V.A.; Dmitrenok, P.S.; Lee, Y.I.; Grishin, E.V.; Stonik, V.A. New derivatives of natural acyclic guanidine alkaloids with TRPV receptor-regulating properties. Nat. Prod. Commun. 2015, 10, 1171–1173. [Google Scholar] [PubMed]
- Dyshlovoy, S.A.; Tabakmakher, K.M.; Hauschild, J.; Shchekateva, R.K.; Otte, K.; Guzii, A.G.; Makarieva, T.N.; Kudryashova, E.K.; Fedorov, S.N.; Shubina, L.K.; et al. Guanidine alkaloids from the marine sponge Monanchora pulchra show cytotoxic properties and prevent EGF-Induced neoplastic transformation in vitro. Mar. Drugs 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A. Isolation of Bioactive Marine Natural Products and Bio-Inspired Synthesis of Fused Guanidinic Tricyclic Analogues. Unpublished Ph.D. Thesis, University of Paris-Saclay, Paris, France, May 2016. [Google Scholar]
- El-Demerdash, A.; Moriou, C.; Martin, M.T.; Petek, S.; Debitus, C.; Al-Mourabit, A. Unguiculins A-C: Cytotoxic bis-guanidine alkaloids from the French Polynesian sponge, Monanchora n. sp. Nat. Prod. Res. 2017, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kapustina, I.I.; Tabakmakher, K.M.; Makar’eva, T.N. Sterols from the toxin containing Far-Eastern sponge Monanchora pulchra. Chem. Nat. Compd. 2012, 47, 1025–1027. [Google Scholar] [CrossRef]
- Wang, W.; Mun, B.; Lee, Y.; Reddy, M.V.; Park, Y.; Lee, J.; Kim, H.; Hahn, D.; Chin, J.; Ekins, M.; et al. Bioactive sesterterpenoids from a Korean sponge Monanchora sp. J. Nat. Prod. 2013, 76, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Mun, B.; Wang, W.; Kim, H.; Hahn, D.; Tang, I.; Won, D.H.; Kim, E.H.; Lee, J.; Han, C.; Kim, H.; et al. Cytotoxic 5a,8a-epidioxy sterols from the marine sponge Monanchora sp. Arch. Pharm. Res. 2015, 38, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lee, T.G.; Patil, R.S.; Mun, B.; Yang, I.; Kim, H.; Hahn, D.; Won, D.H.; Lee, J.; Lee, Y.; et al. Monanchosterols A and B, bioactive bicyclo [4.3.1] steroids from a Korean sponge Monanchora sp. J. Nat. Prod. 2015, 78, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Shi, Z.J. Biomimetic synthesis of the bicyclic guanidine moieties of crambines A and B. J. Org. Chem. 1992, 57, 2526–2528. [Google Scholar] [CrossRef]
- Snider, B.B.; Faith, W.C. The total synthesis of (±)-ptilocaulin. Tetrahedron Lett. 1983, 24, 861–864. [Google Scholar] [CrossRef]
- Snider, B.B.; Faith, W.C. Total synthesis of (+)- and (−)-ptilocaulin. J. Am. Chem. Soc. 1984, 106, 1443–1445. [Google Scholar] [CrossRef]
- Yu, M.; Pochapsky, S.; Snider, B.B. Synthesis of 7-Epineoptilocaulin, mirabilin B and isoptilocaulin. A unified biosynthetic proposal for the ptilocaulin and batzelladine alkaloids. Synthesis and structure revision of netamines E and G. J. Org. Chem. 2008, 73, 9065–9074. [Google Scholar] [CrossRef] [PubMed]
- Black, G.P.; Murphy, P.J.; Walshe, N.D.A.; Hibbs, D.E.; Hursthouse, M.B.; Malik, K.M.A. A short synthetic route to the tricyclic guanidinium core of the batzelladine alkaloids. Tetrahedron Lett. 1996, 37, 6943–6946. [Google Scholar] [CrossRef]
- Ahmed, N.; Brahmbhatt, K.G.; Singh, I.P.; Bhutani, K.K. Total synthesis of (±) batzelladine K: A biomimetic approach. Synthesis 2010, 15, 2567–2570. [Google Scholar] [CrossRef]
- Snider, B.B.; Shi, Z. Biomimetic synthesis of the pentacyclic nucleus of ptilomycalin A. J. Am. Chem. Soc. 1994, 116, 549–557. [Google Scholar] [CrossRef]
- Moore, C.G.; Murphy, P.J.; Williams, H.L.; McGown, A.T.; Smith, N.K. A synthesis of crambescidin 359. Tetrahedron Lett. 2003, 44, 251–254. [Google Scholar] [CrossRef]
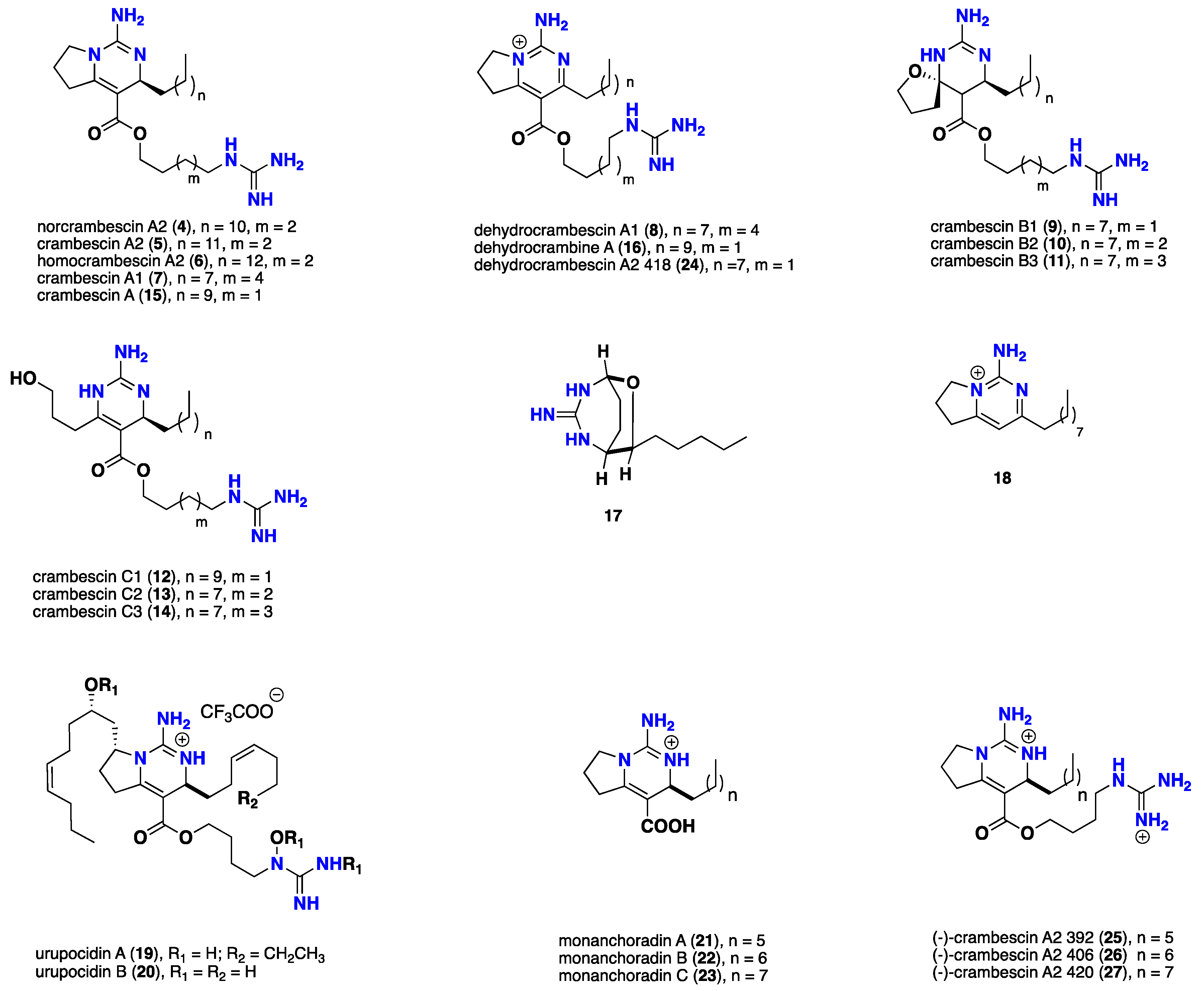


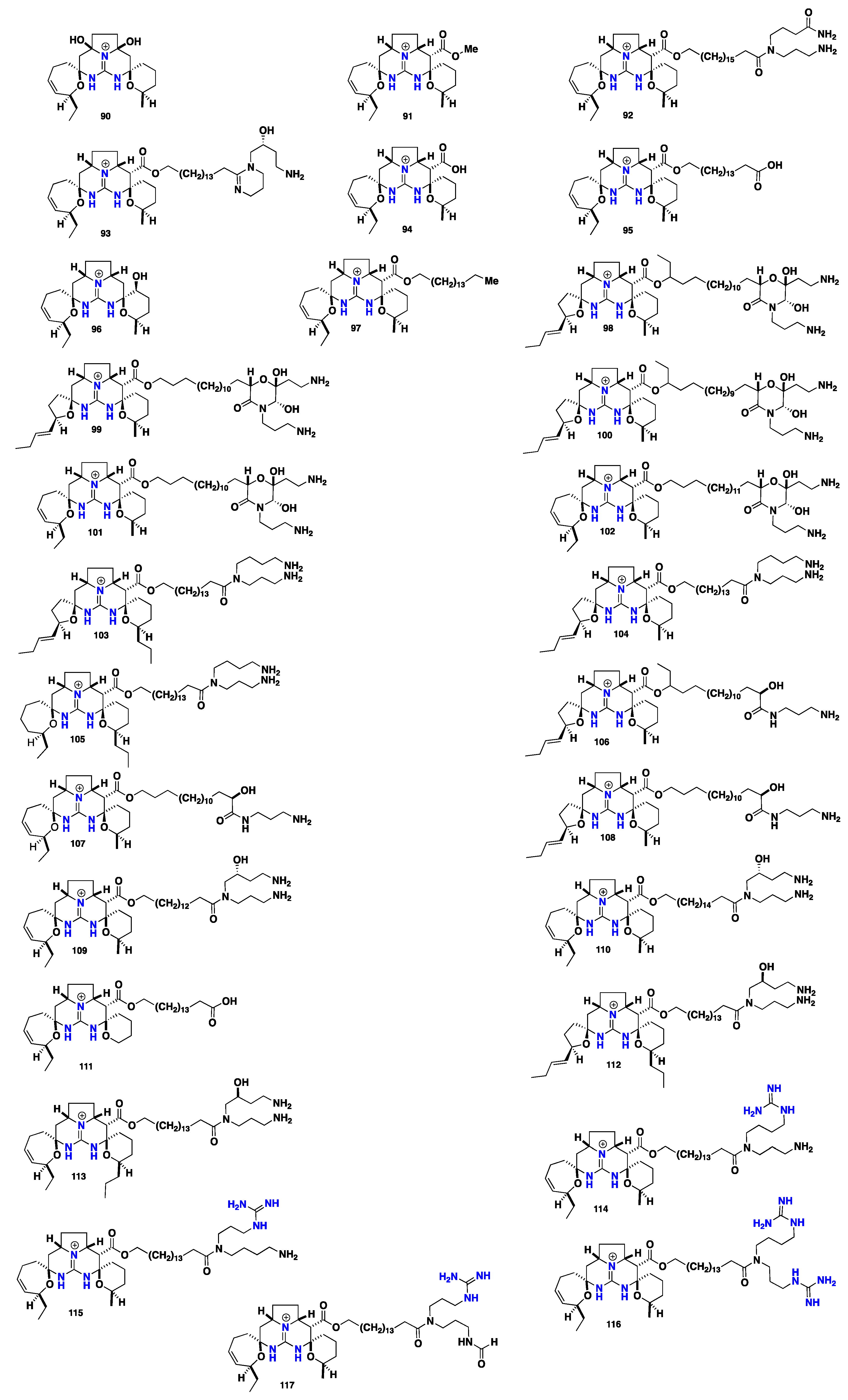


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Demerdash, A.; Atanasov, A.G.; Bishayee, A.; Abdel-Mogib, M.; Hooper, J.N.A.; Al-Mourabit, A. Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas. Nutrients 2018, 10, 33. https://doi.org/10.3390/nu10010033
El-Demerdash A, Atanasov AG, Bishayee A, Abdel-Mogib M, Hooper JNA, Al-Mourabit A. Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas. Nutrients. 2018; 10(1):33. https://doi.org/10.3390/nu10010033
Chicago/Turabian StyleEl-Demerdash, Amr, Atanas G. Atanasov, Anupam Bishayee, Mamdouh Abdel-Mogib, John N. A. Hooper, and Ali Al-Mourabit. 2018. "Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas" Nutrients 10, no. 1: 33. https://doi.org/10.3390/nu10010033
APA StyleEl-Demerdash, A., Atanasov, A. G., Bishayee, A., Abdel-Mogib, M., Hooper, J. N. A., & Al-Mourabit, A. (2018). Batzella, Crambe and Monanchora: Highly Prolific Marine Sponge Genera Yielding Compounds with Potential Applications for Cancer and Other Therapeutic Areas. Nutrients, 10(1), 33. https://doi.org/10.3390/nu10010033