Simultaneous Intake of Euglena Gracilis and Vegetables Synergistically Exerts an Anti-Inflammatory Effect and Attenuates Visceral Fat Accumulation by Affecting Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Diets
2.2. Animals
2.3. Serum and Liver Biochemical Analyses
2.4. Analysis of Gut Microbiota
2.5. Short-Chain Fatty Acids (SCFAs) and Gamma-Aminobutyric Acid (GABA) Analyses
2.6. Histological Analysis
2.7. mRNA Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Parameters
3.2. Biochemical Parameters in Serum and Liver
3.3. Gut Microbiota
3.4. SCFAs and GABA Levels in Feces and Serum
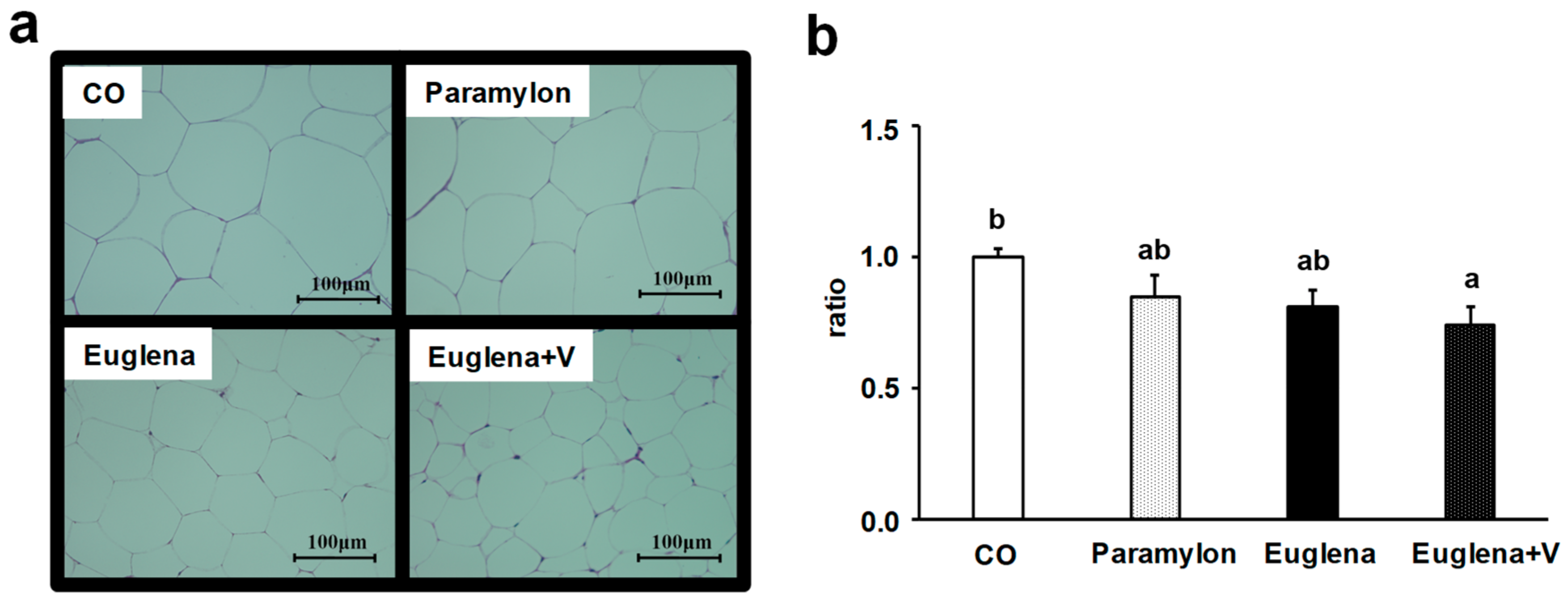
3.5. Histological Analysis of Epididymal Adipose Tissue
3.6. mRNA Levels of Lipid Metabolism-Related Genes in Epididymal Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Founding
Conflicts of Interest
References
- Schwartzbach, S.; Shigeoka, S. Advances in Experimental Medicine and Biology. In Euglena: Biochemistry, Cell and Molecular Biology; Springer: Basel, Switzerland, 2017; ISBN 978-3-319-54910-1. [Google Scholar]
- Marchessault, R.H.; Deslamdes, Y. Fine structure of (1→3)-β-d-glucans: Curdlan and paramylon. Carbohydr. Res. 1979, 75, 231–242. [Google Scholar] [CrossRef]
- Shimada, R.; Fujita, M.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Nakashima, A.; Suzuki, K. Oral administration of green algae, Euglena gracilis, inhibits hyperglycemia in OLETF rats, a model of spontaneous type 2 diabetes. Food Funct. 2016, 7, 4655–4659. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Kato, A.; Hojo, H.; Nozoe, S.; Takeuchi, M.; Ochi, K. Cytokine-Related Immunopotentiating Activities of Paramylon, a β-(1→3)-d-Glucan from Euglena gracilis. J. Pharm. Dyn. 1992, 15, 617–621. [Google Scholar] [CrossRef]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R.; Mitra, S.; Arashida, R.; Asayama, Y.; Yabuta, Y.; Takeuchi, T. Oral Administration of Paramylon, a β-1,3-d-Glucan Isolated from Euglena gracilis Z Inhibits Development of Atopic Dermatitis-Like Skin Lesions in NC/Nga Mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Prosky, L.; Asp, N.G.; Schweizer, T.F.; DeVries, J.W. Furda Determination of insoluble, soluble and total dietary fiber in foods and food products: Interlaboratory study. J. Assoc. Off. Anal. Chem. 1988, 71, 1017–1023. [Google Scholar] [PubMed]
- Papathanasopoulos, A.; Camilleri, M. Dietary fiber supplements: Effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010, 138, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Galisteo, M.; Duarte, J.; Zarzuelo, A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 2008, 19, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F.H. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Kushida, M.; Okouchi, R.; Iwagaki, Y.; Asano, M.; Du, M.X.; Yamamoto, K.; Tsuduki, T. Fermented soybean suppresses visceral fat accumulation in mice. Mol. Nutr. Food Res. 2018, e1701054. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Kushida, M.; Iwagaki, Y.; Asano, M.; Yamamoto, K.; Tomata, Y.; Tsuji, I.; Tsuduki, T. The 1975 type Japanese diet improves lipid metabolic parameters in younger adults: A randomized controlled trial. J. Oleo Sci. 2018, 67, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Guo, X.; Sugawara, S.; Iwagaki, Y.; Yamamoto, K.; Tsuduki, T. Effect of the Japanese diet during pregnancy and lactation or post-weaning on the risk of metabolic syndrome in offspring. Biosci. Biotechnol. Biochem. 2018, 82, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, Y.; Sakamoto, Y.; Sugawara, S.; Mizowaki, Y.; Yamamoto, K.; Sugawara, T.; Kimura, K.; Tsuduki, T. Identification of characteristic components and foodstuffs in healthy Japanese diet and the health effects of a diet with increased use frequency of these foodstuffs. Mol. Nutr. Food Res. 2017, 61, 1700430. [Google Scholar] [CrossRef] [PubMed]
- Iwagaki, Y.; Sugawara, S.; Huruya, Y.; Sato, M.; Wu, Q.; E, S.; Yamamoto, K.; Tsuduki, T. The 1975 Japanese diet has a stress reduction effect in mice: Search for physiological effects using metabolome analysis. Biosci. Biotechnol. Biochem. 2018, 82, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Yamamoto, K.; Hatakeyama, Y.; Tsuduki, T. Effects of fatty acid quality and quantity in the Japanese diet on the suppression of lipid accumulation. J. Oleo Sci. 2016, 65, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; Tokuyama, Y.; Igarashi, M.; Miyazawa, T. Tumor growth suppression by alpha-eleostearic acid, a linolenic acid isomer with a conjugated triene system, via lipid peroxidation. Carcinogenesis 2004, 25, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Fleischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipid 1970, 5, 494–496. [Google Scholar] [CrossRef]
- Shimizu, T.; Mori, K.; Ouchi, K.; Kushida, M.; Tsuduki, T. Effects of Dietary Intake of Japanese Mushrooms on Visceral Fat Accumulation and Gut Microbiota in Mice. Nutrients 2018, 10, 610. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Cao, H. Fast quantification of short chain fatty acids and ketone bodies by liquid chromatography-tandem mass spectrometry after facile derivatization coupled with liquid-liquid extraction. J. Chromatogr. 2018, 1083, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Iwagaki, Y.; Sugawara, S.; Kushida, M.; Okouchi, R.; Yamamoto, K.; Tsuduki, T. Effects of the Japanese diet in combination with exercise on visceral fat accumulation. Nutrition 2018, 57, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Mizowaki, Y.; Sugawara, S.; Yamamoto, K.; Sakamoto, Y.; Iwagaki, Y.; Kawakami, Y.; Igarashi, M.; Tsuduki, T. Comparison of the effects of the 1975 Japanese diet and the modern Mediterranean diet on lipid metabolism in mice. J. Oleo Sci. 2017, 66, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Guo, X.; Sugawara, S.; Iwagaki, Y.; Yamamoto, K.; Konno, A.; Nishiuchi, M.; Tsuduki, T. Influence of Japanese diet consumption during pregnancy and lactation on lipid metabolism in offspring. Nutrition 2018, in press. [Google Scholar] [CrossRef]
- Sugawara, S.; Mizowaki, Y.; Iwagaki, Y.; Sakamoto, Y.; Yamamoto, K.; Tsuduki, T. Standardization of the Japanese diet for use in animal experiments. Br. J. Nutr. 2017, 118, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Honma, T.; Hatakeyama, Y.; Jibu, Y.; Kawakami, Y.; Tsuduki, T.; Nakagawa, K.; Miyazawa, T. The Effect of Japanese Foods which Changed with the Age on the Risk of Obesity in Mice. J. Jpn. Soc. Nutr. Food Sci. 2014, 67, 73–85. [Google Scholar] [CrossRef]
- Honma, T.; Kitano, Y.; Kijima, R.; Jibu, Y.; Kawakami, Y.; Tsuduki, T.; Nakagawa, K.; Miyazawa, T. Comparison of the Health Benefits of Different Eras of Japanese Foods: Lipid and Carbohydrate Metabolism Focused Research. J. Jpn. Soc. Food Sci. Technol. 2013, 60, 541–553. [Google Scholar] [CrossRef]
- Yamamoto, K.; E, S.; Hatakeyama, Y.; Sakamoto, Y.; Honma, T.; Jibu, Y.; Kawakami, Y.; Tsuduki, T. The Japanese diet from 1975 delays senescence and prolongs life span in SAMP8 mice. Nutrition 2016, 32, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Panigrahi, D.P.; Patil, S.; Bhutia, S.K. Autophagy in health and disease: A comprehensive review. Biomed. Pharmacother. 2018, 104, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.D.; Wang, H.; Zhang, J.F.; Kung, H.F.; Zhao, Y.N.; Zhang, Y. Proteomic profile of visceral adipose tissues between low-fat diet-fed obesity-resistant and obesity-prone C57BL/6 mice. Mol. Med. Rep. 2010, 3, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Okuno, A.; Tamemoto, H.; Tobe, K.; Ueki, K.; Mori, Y.; Iwamoto, K.; Umesono, K.; Akanuma, Y.; Fujiwara, T.; Horikoshi, H. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J. Clin. Investig. 1998, 101, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.N.; Ables, G.P.; Otlivanchik, O.A.; Schoiswohl, G.; Zechner, R.; Blaner, W.S.; Goldberg, I.J.; Schwabe, R.F.; Chua, S.C., Jr.; Huang, L.S. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids and ameliorates steatosis. J. Biol. Chem. 2008, 283, 13087–13099. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Yde, C.C.; Jensen, H.M.; Morovic, W.; Hibberd, A.A.; Ouwehand, A.C.; Saarinen, M.T.; Forssten, S.D.; Wiebe, L.; Marcussen, J.; et al. Metabolic fate of 13C-labelled polydextrose and impact on the gut microbiome: A triple-phase study in a colon simulator. J. Proteome Res. 2018, 17, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-del-Río, I.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Kimura, I.; Inoue, D.; Maeda, T.; Hara, T.; Ichimura, A.; Miyauchi, S.; Kobayashi, M.; Hirasawa, A.; Tsujimoto, G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl. Acad. Sci. USA 2011, 108, 8030–8035. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829. [Google Scholar] [CrossRef] [PubMed]
- Arrazuria, R.; Elguezabal, N.; Juste, R.A.; Derakhshani, H.; Khafipour, E. Mycobacterium avium subspecies paratuberculosis infection modifies gut microbiota under different dietary conditions in a rabbit model. Front. Microbiol. 2016, 7, 446. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.H.; Liang, S.C.; Lu, J.Q.; He, Y.; Luo, Y.M.; You, C.; Xia, G.H.; Li, P.; Zhou, H.W. Effect of intermittent fasting on physiology and gut microbiota in presenium rats. J. South. Med. Univ. 2016, 37, 423–430. [Google Scholar]
- Heilig, H.G.; Zoetendal, E.G.; Vaughan, E.E.; Marteau, P.; Akkermans, A.D.; de Vos, W.M. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 2002, 68, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activity and γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yokozawa, T.; Nakagawa, T.; Sasaki, S. Protective effect of gamma-aminobutyric acid against glycerol-induced acute renal failure in rats. Food Chem. Toxicol. 2004, 42, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Franklin, D.; Lowy, M.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar]
- Bian, X.; Tu, P.; Chi, L.; Gao, B.; Ru, H.; Lu, K. Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions. Food Chem. Toxicol. 2017, 107, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M. Infections caused by Streptococcus pneumoniae: Clinical spectrum, pathogenesis, immunity and treatment. Clin. Infect. Dis. 1992, 14, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; Story, J.A.; MacDonald-Clarke, C.J.; McCabe, L.D.; McCabe, G.P.; Weaver, C.M. Soluble corn fiber increases calcium absorption associated with shifts in the gut microbiome: A randomized dose-response trial in free-living pubertal females. J. Nutr. 2016, 146, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Gibert, M.; Popoff, M.R. Clostridium perfringens: Toxinotype and genotype. Trends Microbiol. 1999, 7, 104–110. [Google Scholar] [CrossRef]



| Control Diet | Paramylon Diet | Euglena Diet | Euglena + Vegetables Diet | |
|---|---|---|---|---|
| Ingredient | (g/100 g diet) | |||
| Casein | 16.3 | 16.3 | 16.1 | 16.1 |
| DL-Methionine | 0.3 | 0.3 | 0.3 | 0.3 |
| Corn Starch | 33.8 | 33.7 | 33.5 | 33.3 |
| Maltodextrin 10 | 8.4 | 8.4 | 8.3 | 8.3 |
| Sucrose | 28.5 | 28.4 | 28.2 | 28.1 |
| Cellulose | 4.2 | 4.2 | 4.2 | 4.1 |
| Corn Oil | 4.4 | 4.4 | 4.4 | 4.3 |
| Mineral Mix (S10001) | 2.9 | 2.9 | 2.9 | 2.9 |
| Calcium Carbonate | 0.3 | 0.3 | 0.3 | 0.3 |
| Vitamin Mix (V10001) | 0.8 | 0.8 | 0.8 | 0.8 |
| Choline Bitartrate | 0.2 | 0.2 | 0.2 | 0.2 |
| Paramylon | - | 0.3 | - | - |
| Euglena | - | - | 1.0 | 1.0 |
| Barley leaf | - | - | - | 0.1 |
| Kale | - | - | - | 0.1 |
| Aghitaba | - | - | - | 0.1 |
| Others | 0.1 | |||
| (g/100 g diet) | ||||
| Total Dietary Fiber | 4.2 | 4.5 | 4.5 | 4.7 |
| (energy %) | ||||
| Protein | 17.1 | 17.1 | 17.3 | 17.5 |
| Carbohydrate | 72.7 | 72.7 | 72.2 | 72.1 |
| Fat | 4.5 | 4.5 | 4.7 | 4.6 |
| Energy (kcal/100 g) | 389 | 388 | 388 | 388 |
| Genbank ID | Gene Name | Primer Sequence (5′ to 3′) | |
|---|---|---|---|
| NM_007988 | Fasn | Forward | CCTGGATAGCATTCCGAACCTG |
| Reverse | TTCACAGCCTGGGGTCATCTTTGC | ||
| NM_008062 | G6pdx | Forward | TGGGTCCACCACTGCCACTTTTG |
| Reverse | ATTGGGCTGCACACGGATGACCA | ||
| NM_001039507 | Hsl | Forward | TTCTCCAAAGCACCTAGCCAA |
| Reverse | TGTGGAAAACTAAGGGCTTGTTG | ||
| M29546 | Me | Forward | GAAAGAGGTGTTTGCCCATGA |
| Reverse | AATTGCAGCAACTCCTATGAGG | ||
| NM_011146 | Pparɤ | Forward | GGAAGACCACTCGCATTCCTT |
| Reverse | TCGCACTTTGGTATTCTTGGAG | ||
| NM_011480 | Srebf1 | Forward | GGAGACATCGCAAACAAGC |
| Reverse | TGAGGTTCCAAAGCAGACTG | ||
| NM_007393 | Actb | Forward | GGCTGTATTCCCCTCCATCG |
| Reverse | CCAGTTGGTAACAATGCCATGT |
| CO | Paramylon | Euglena | Euglena + V | |
|---|---|---|---|---|
| Initial body weight (g) | 22.9 ± 0.3 | 22.9 ± 0.3 | 22.9 ± 0.3 | 22.9 ± 0.3 |
| Final body weight (g) | 29.4 ± 0.6 | 28.5 ± 0.4 | 28.3 ± 0.5 | 28.0 ± 0.9 |
| Food intake (g/day) | 3.01 ± 0.04 | 2.99 ± 0.05 | 3.09 ± 0.06 | 2.96 ± 0.06 |
| Energy intake (kcal/day) | 11.8 ± 0.2 | 11.7 ± 0.2 | 12.1 ± 0.2 | 11.5 ± 0.2 |
| Tissue weight (g/100 g body weight) | ||||
| Brain | 1.57 ± 0.04 | 1.59 ± 0.04 | 1.59 ± 0.03 | 1.58 ± 0.04 |
| Heart | 0.45 ± 0.01 | 0.45 ± 0.01 | 0.46 ± 0.01 | 0.44 ± 0.01 |
| Lung | 0.76 ± 0.05 | 0.87 ± 0.08 | 0.89 ± 0.11 | 0.75 ± 0.05 |
| Liver | 3.31 ± 0.03 | 3.41 ± 0.03 | 3.39 ± 0.06 | 3.37 ± 0.04 |
| Pancreas | 0.68 ± 0.03 | 0.78 ± 0.09 | 0.65 ± 0.03 | 0.73 ± 0.03 |
| Spleen | 0.29 ± 0.02 | 0.28 ± 0.01 | 0.26 ± 0.01 | 0.28 ± 0.01 |
| Kidney | 1.10 ± 0.03 | 1.18 ± 0.03 | 1.14 ± 0.04 | 1.10 ± 0.04 |
| White adipose tissue | ||||
| Mesenteric | 1.29 ± 0.05 | 1.22 ± 0.12 | 0.90 ± 0.12 | 0.91 ± 0.12 |
| Perinephric | 1.38 ± 0.10 a | 1.09 ± 0.16 ab | 0.70 ± 0.07 bc | 0.65 ± 0.08 c |
| Epididymal | 2.61 ± 0.15 a | 2.40 ± 0.26 a | 1.55 ± 0.14 b | 1.51 ± 0.16 b |
| Total | 5.28 ± 0.28 a | 4.70 ± 0.47 a | 3.15 ± 0.30 b | 3.01 ± 0.30 b |
| CO | Paramylon | Euglena | Euglena + V | |
|---|---|---|---|---|
| Serum | ||||
| TG (mmol/L) | 1.38 ± 0.08 | 1.39 ± 0.07 | 1.19 ± 0.07 | 1.23 ± 0.08 |
| TC (mmol/L) | 3.29 ± 0.15 | 3.64 ± 0.12 | 3.28 ± 0.12 | 3.59 ± 0.07 |
| PL (mmol/L) | 73.9 ± 2.7 | 79.9 ± 3.1 | 77.5 ± 2.2 | 82.7 ± 1.5 |
| Glucose (mmol/L) | 5.55 ± 0.27 | 5.61 ± 0.16 | 4.88 ± 0.18 | 5.41 ± 0.27 |
| Insulin (μg/L) | 0.19 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| TBARS (µmol/L) | 7.16 ± 0.33 ab | 7.79 ± 0.38 b | 7.44 ± 0.31 ab | 6.35 ± 0.35 a |
| ALT (IU/L) | 5.03 ± 0.41 | 4.63 ± 0.29 | 4.48 ± 0.37 | 4.09 ± 0.21 |
| AST (IU/L) | 54.6 ± 3.3 | 60.5 ± 4.4 | 46.7 ± 4.6 | 54.9 ± 4.1 |
| Leptin (ng/mL) | 4.27 ± 0.80 | 3.96 ± 0.69 | 3.38 ± 0.83 | 2.24 ± 0.47 |
| Liver | ||||
| TG (μmol/g) | 43.2 ± 7.1 | 41.9 ± 5.4 | 42.9 ± 4.3 | 39.6 ± 4.1 |
| TC (μmol/g) | 8.59 ± 1.17 | 8.17 ± 1.18 | 7.60 ± 1.17 | 7.13 ± 0.44 |
| PL (μmol/g) | 35.2 ± 0.6 | 35.1 ± 1.0 | 34.7 ± 0.7 | 33.4 ± 1.0 |
| TBARS (nmol/g) | 73.1 ± 2.7 b | 68.1 ± 2.7 ab | 65.6 ± 3.1 ab | 60.2 ± 2.8 a |
| CO | Paramylon | Euglena | Euglena + V | Euglena + V/Euglena | |
|---|---|---|---|---|---|
| Erysipelotrichaceae; Other (%) | 0.00 | 0.01 | 0.01 | 0.04 | 4.01 |
| YS2;f__;g__ (%) | 0.01 | 0.02 | 0.03 | 0.05 | 2.10 |
| Lactobacillus (%) | 2.51 | 2.08 | 2.41 | 4.91 | 2.04 |
| Staphylococcus (%) | 0.02 | 0.18 | 0.09 | 0.03 | 0.28 |
| Jeotgalicoccus (%) | 0.07 | 0.66 | 0.10 | 0.02 | 0.21 |
| Sporosarcina (%) | 0.01 | 0.90 | 0.14 | 0.02 | 0.14 |
| Streptococcus (%) | 0.00 | 0.02 | 0.01 | 0.00 | 0.10 |
| Sutterella (%) | 0.15 | 0.02 | 0.01 | 0.00 | 0.10 |
| Clostridiaceae; Other (%) | 1.01 | 0.01 | 0.00 | 0.00 | 0.01 |
| CO | Paramylon | Euglena | Euglena + V | Gene Function | |
|---|---|---|---|---|---|
| Fasn | 1.00 ± 0.10 | 1.02 ± 0.14 | 0.95 ± 0.06 | 0.82 ± 0.07 | Fatty acid synthesis |
| G6pdx | 1.00 ± 0.14 a | 0.79 ± 0.08 ab | 0.74 ± 0.10 ab | 0.53 ± 0.09 b | |
| Me | 1.00 ± 0.06 ab | 1.27 ± 0.16 a | 0.97 ± 0.06 ab | 0.81 ± 0.07 b | |
| Srebf1 | 1.00 ± 0.11 ab | 1.09 ± 0.11 a | 0.77 ± 0.03 ab | 0.76 ± 0.05 b | |
| Pparɤ | 1.00 ± 0.06 b | 1.06 ± 0.06 b | 1.13 ± 0.08 ab | 1.36 ± 0.08 a | Cell division |
| Hsl | 1.00 ± 0.06 c | 1.09 ± 0.08 bc | 1.40 ± 0.13 ab | 1.50 ± 0.08 a | Lipolysis |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakanoi, Y.; E, S.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K.; et al. Simultaneous Intake of Euglena Gracilis and Vegetables Synergistically Exerts an Anti-Inflammatory Effect and Attenuates Visceral Fat Accumulation by Affecting Gut Microbiota in Mice. Nutrients 2018, 10, 1417. https://doi.org/10.3390/nu10101417
Sakanoi Y, E S, Yamamoto K, Ota T, Seki K, Imai M, Ota R, Asayama Y, Nakashima A, Suzuki K, et al. Simultaneous Intake of Euglena Gracilis and Vegetables Synergistically Exerts an Anti-Inflammatory Effect and Attenuates Visceral Fat Accumulation by Affecting Gut Microbiota in Mice. Nutrients. 2018; 10(10):1417. https://doi.org/10.3390/nu10101417
Chicago/Turabian StyleSakanoi, Yuto, Shuang E, Kazushi Yamamoto, Toshikuni Ota, Kentarou Seki, Mayumi Imai, Ryuki Ota, Yuta Asayama, Ayaka Nakashima, Kengo Suzuki, and et al. 2018. "Simultaneous Intake of Euglena Gracilis and Vegetables Synergistically Exerts an Anti-Inflammatory Effect and Attenuates Visceral Fat Accumulation by Affecting Gut Microbiota in Mice" Nutrients 10, no. 10: 1417. https://doi.org/10.3390/nu10101417
APA StyleSakanoi, Y., E, S., Yamamoto, K., Ota, T., Seki, K., Imai, M., Ota, R., Asayama, Y., Nakashima, A., Suzuki, K., & Tsuduki, T. (2018). Simultaneous Intake of Euglena Gracilis and Vegetables Synergistically Exerts an Anti-Inflammatory Effect and Attenuates Visceral Fat Accumulation by Affecting Gut Microbiota in Mice. Nutrients, 10(10), 1417. https://doi.org/10.3390/nu10101417





