Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, Experimental Design and Ethics
2.2. Body Composition and Dietary Intake
2.3. Cell Isolation and Cell Viability Test
2.4. RNA Extraction and Real-Time PCR
2.5. Enzymatic Determinations
2.6. SDS-Polyacrylamide Gel Electrophoresis and Western Blot Analysis
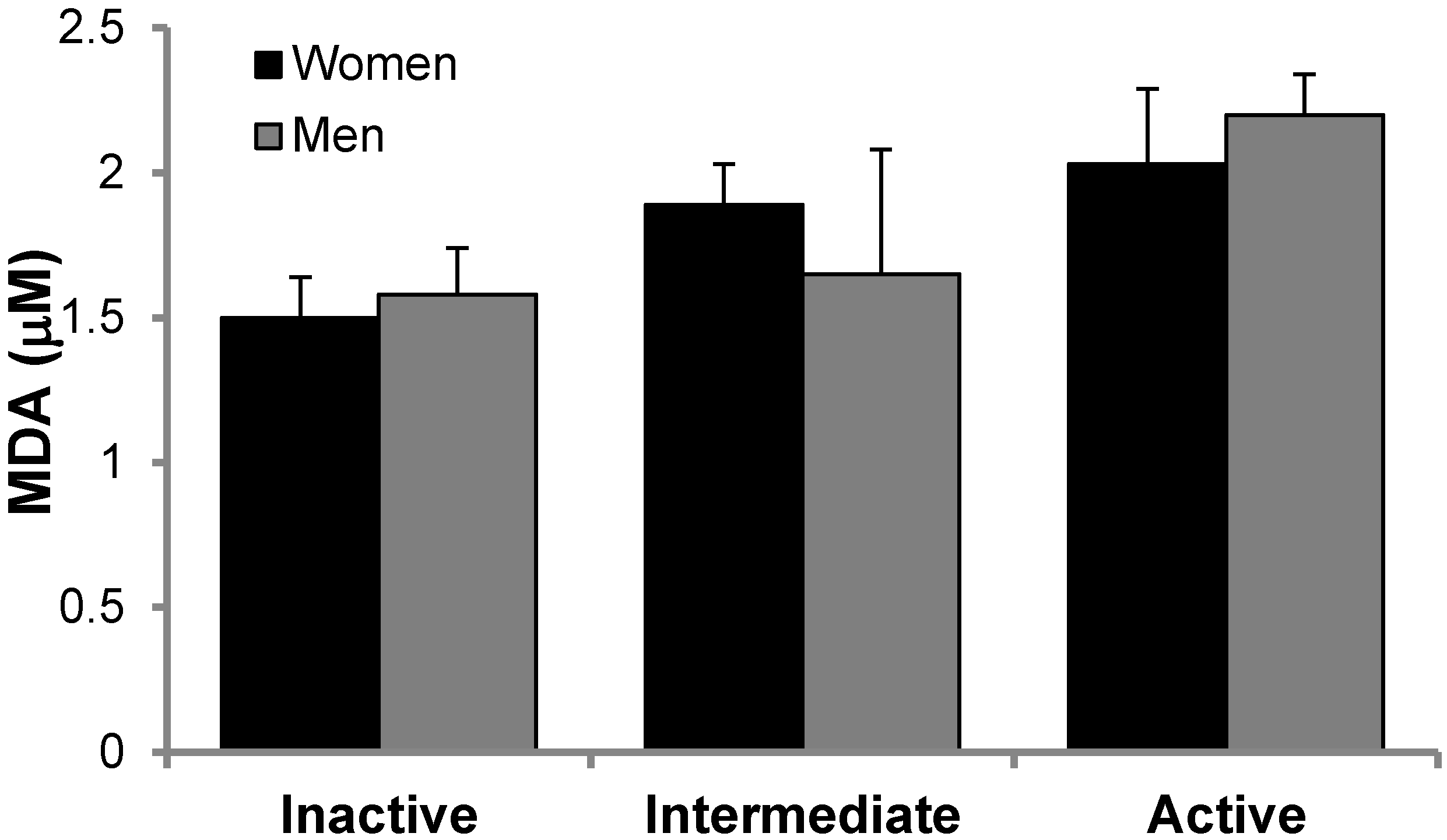
2.7. Malondialdehyde Assay
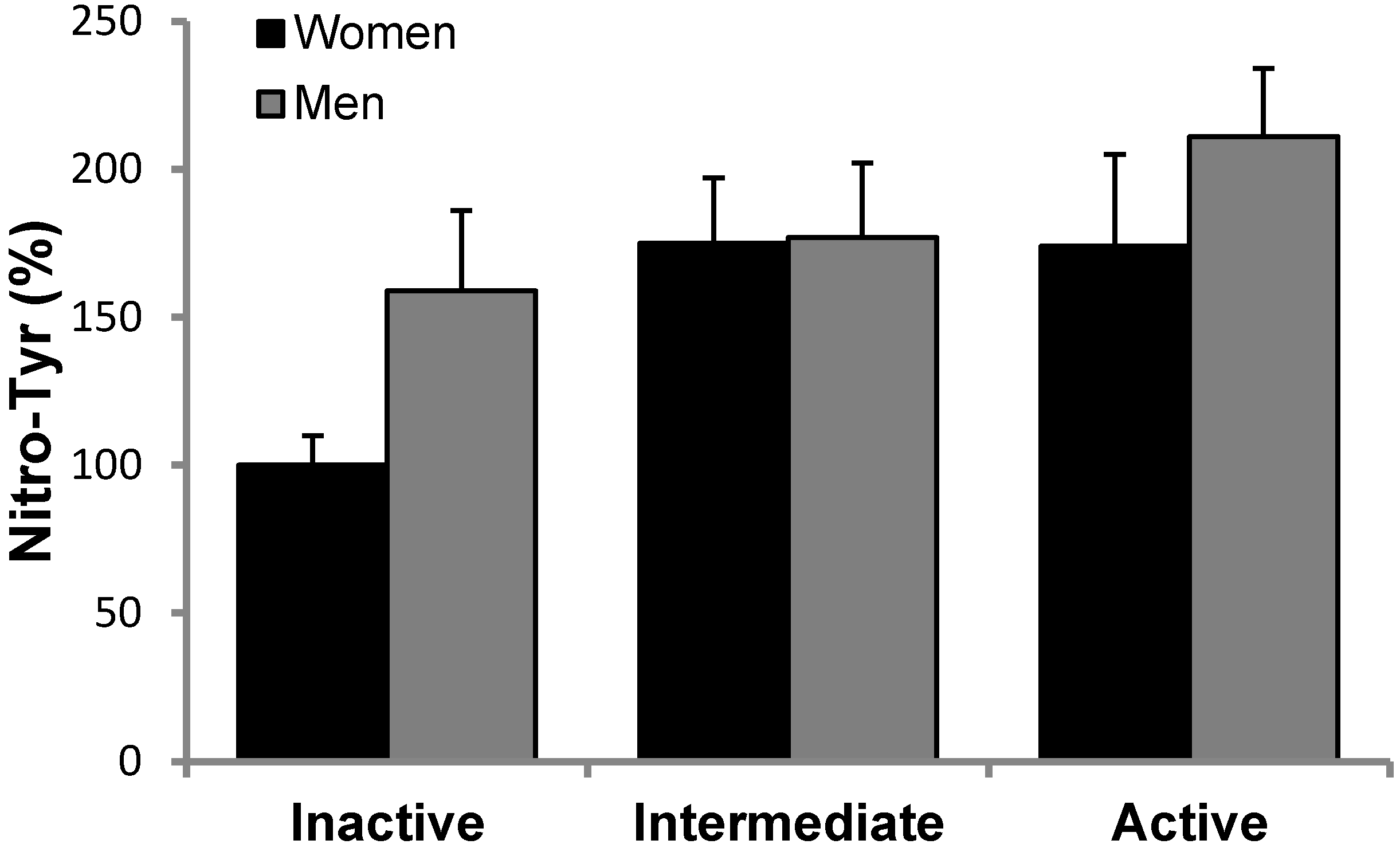
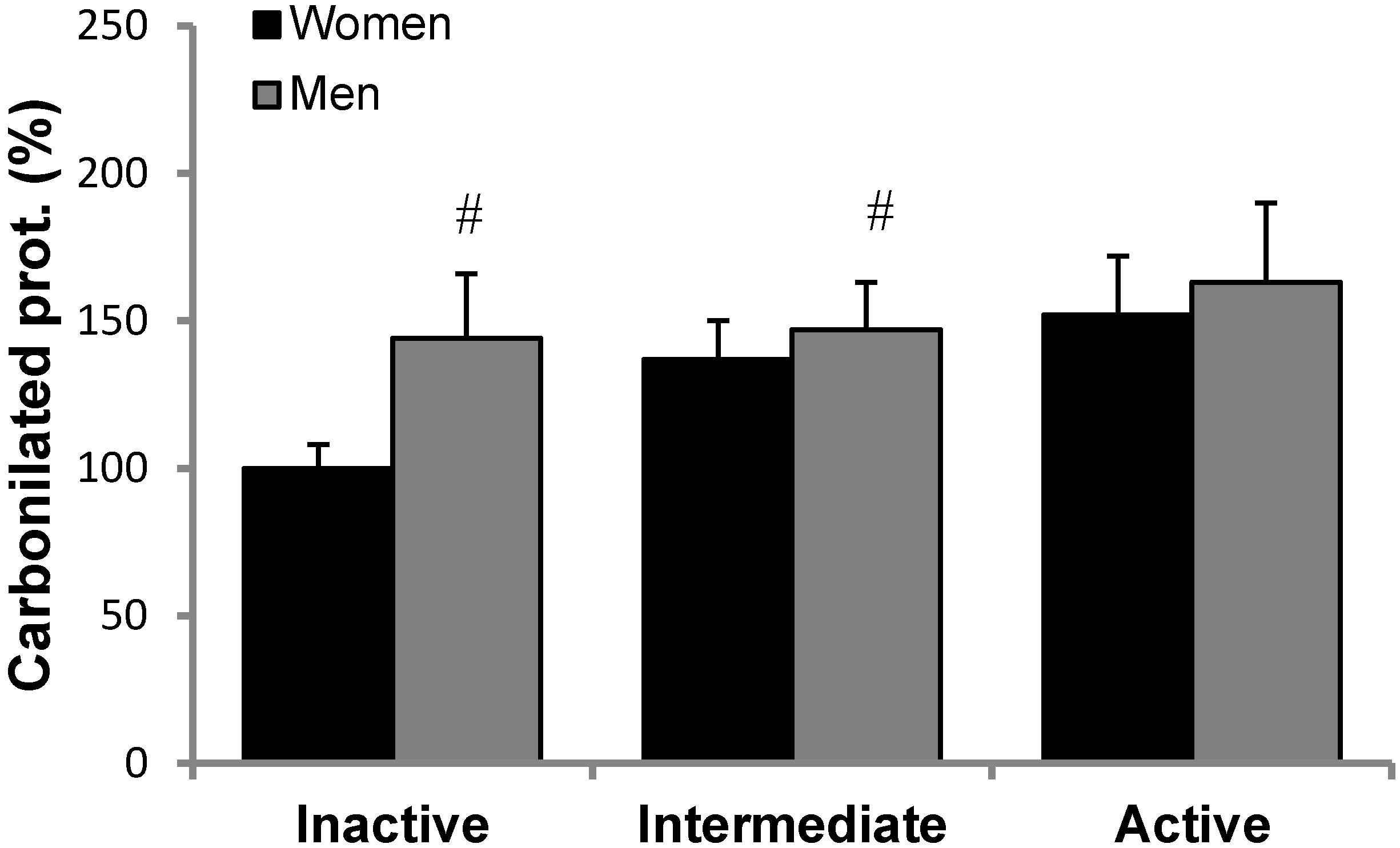
2.8. Protein Carbonyls and Nitrotyrosine Determination
2.9. Statistical Analysis
2.10. Limitations of the Study
3. Results
3.1. Anthropometric Parameters and Dietary Intake
3.2. Antioxidant Protein Levels and Oxidative Stress Markers
3.3. mRNA Relative Expression and Enzymatic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sureda, A.; Del Mar Bibiloni, M.; Julibert, A.; Aparicio-Ugarriza, R.; Palacios-Le Blé, G.; Pons, A.; Gonzalez-Gross, M. Trace element contents in toenails are related to regular physical activity in older adults. PLoS ONE 2017. [Google Scholar] [CrossRef] [PubMed]
- Goloubinoff, P. Mechanisms of protein homeostasis in health, aging and disease. Swiss Med. Wkly. 2016, 146. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Stuckey, M.I.; Petrella, R.J. Prescribing physical activity through primary care: Does activity intensity matter? Phys. Sportsmed. 2014, 42, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Simpkins, J.W.; Ji, X.; Leis, M.; Stambler, I. The Critical Need to Promote Research of Aging and Aging-related Diseases to Improve Health and Longevity of the Elderly Population. Aging Dis. 2015, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, E.; Petrella, R.J. Prescribing physical activity for healthy aging: Longitudinal follow-up and mixed method analysis of a primary care intervention. Phys. Sportsmed. 2014, 42, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Buchner, D.; Piña, I.L.; Balady, G.J.; Williams, M.A.; Marcus, B.H.; Berra, K.; Blair, S.N.; Costa, F.; Franklin, B.; et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Circulation 2003, 107, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Teri, L.; Gibbons, L.E.; Mccurry, S.M.; Logsdon, R.G.; Buchner, D.M.; Barlow, W.E.; Kukull, W.A.; LaCroix, A.Z.; McCormick, W.; Larson, E.B. Exercise plus behavioral management: A randomized controlled trial. JAMA 2015, 290, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Daley, M.J.; Spinks, W.L. Exercise, mobility and aging. Sport Med. 2000, 29, 1–12. [Google Scholar] [CrossRef]
- Frankel, J.E.; Bean, J.F.; Frontera, W.R. Exercise in the elderly: Research and clinical practice. Clin. Geriatr. Med. 2006, 22, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidant enzyme response to exercise and aging. Med. Sci. Sports Exerc. 1993, 25, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Harmful and beneficial role of ROS. Oxid. Med. Cell Longev. 2016, 2016, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, S.; Awad, M.; Harrington, E.; Sellke, F.; Abid, M. Subcellular reactive oxygen species (ROS) in cardiovascular pathophysiology. Antioxidants 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Ludovico, P.; Burhans, W.C. Reactive oxygen species, ageing and the hormesis police. FEMS Yeast Res. 2014, 14, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A. Coordination of metabolic plasticity in skeletal muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynés, B.; Díaz-Rúa, R.; Cifre, M.; Oliver, P.; Palou, A. Peripheral blood mononuclear cells as a potential source of biomarkers to test the efficacy of weight-loss strategies. Obesity 2015, 23, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Cifre, M.; Díaz-Rúa, R.; Varela-Calviño, R.; Reynés, B.; Pericás-Beltrán, J.; Palou, A.; Oliver, A. Human peripheral blood mononuclear cell in vitro system to test the efficacy of food bioactive compounds: Effects of polyunsaturated fatty acids and their relation with BMI. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox. Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training and acute exercise modulates mitochondrial dynamics in football players’ blood mononuclear cells. Eur. J. Appl. Physiol. 2017, 117, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Cartoni, R.; Leger, B.; Hock, M.B.; Praz, M.; Crettenand, A.; Pich, S.; Ziltener, J.-L.; Luthi, F.; Dériaz, O.; Zorzano, A.; et al. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J. Physiol. 2005, 567, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.G.R.; Lally, J.; Holloway, G.P.; Heigenhauser, G.J.F.; Bonen, A.; Spriet, L.L. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010, 588, 4795–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Lira, V.A.; Greene, N.P. Exercise training-induced regulation of mitochondrial quality. Exerc. Sport Sci. Rev. 2012, 40, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Panayiotidis, M.; Collins, A.R. Ex vivo assessment of lymphocyte antioxidant status using the comet assay. Free Radic. Res. 1997, 27, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Bolin, A.P.; Guerra, B.A.; Nascimento, S.J.S.; Otton, R. Changes in lymphocyte oxidant/antioxidant parameters after carbonyl and antioxidant exposure. Int. Immunopharmacol. 2012, 14, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Campoio, T.R.; Oliveira, F.A.; Otton, R. Oxidative stress in human lymphocytes treated with fatty acid mixture: Role of carotenoid astaxanthin. Toxicol. Vitr. 2011, 25, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Ascolani, A.; Balestrieri, E.; Minutolo, A.; Mosti, S.; Spalletta, G.; Bramanti, P.; Mastino, A.; Caltagirone, C.; Macchi, B. Dysregulated NF-κB pathway in peripheral mononuclear cells of Alzheimer’s disease patients. Curr. Alzheimer Res. 2012, 9, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Baglioni, M.; Cecchetti, R.; Cai, J.; Klein, J.B.; Bastiani, P.; Ruggiero, C.; Mecocci, P.; Butterfield, D.A. Lymphocyte mitochondria: Towards identification of peripheral biomarkers in progression of Alzheimer disease. Free Radic. Biol. Med. 2013, 65, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; García, M.; Aguilar, A.; Molina, L.; Covas, M.I.; Marrugat, J. Validation of the minnesota leisure time physical activity questionnaire in spanish women. Med. Sci. Sport Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- Pereira, M.A.; FitzGerald, S.J.; Gregg, E.W.; Joswiak, M.L.; Ryan, W.J.; Suminski, R.R.; Utter, A.C.; Zmuda, J.M. A collection of physical activity questionnaires for health-related research. Med. Sci. Sport Exerc. 1997, 29, S1–S205. [Google Scholar]
- Ainsworth, B.; Hasekll, W.; Leon, A.; Jacobs, D.; Montoye, H.; Sallis, J.; Paffenbarger, R.S., Jr. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sport Exerc. 1992, 25, 71–80. [Google Scholar] [CrossRef]
- Ferrari, M. Intake estimation by means of a 24-hour reminder. Diaeta 2013, 31, 20–25. [Google Scholar]
- Castell, G.S.; Serra-Majem, L.; Ribas-Barba, L. What and how much do we eat? 24-hour dietary recall method. Nutr. Hosp. 2015, 31, 46–48. [Google Scholar]
- Boyum, A. Separation of white blood cells. Nature 1964, 204, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Alfaro, A.; Ferrer, M.D.; Sureda, A.; Tauler, P.; Marttínez, E.; Bibiloni, M.M.; Micol, V.; Tur, J.A.; Pons, A. Phytoestrogens enhance antioxidant enzymes after swimming exercise and modulate sex hormone plasma levels in female swimmers. Eur. J. Appl. Physiol. 2011, 111, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; Capó, X.; Martorell, M.; Tur, J.A.; Sureda, A.; Pons, A. Training enhances immune cells mitochondrial biosynthesis, fission, fusion, and their antioxidant capabilities synergistically with dietary docosahexaenoic supplementation. Oxid. Med. Cell Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar]
- McCord, J.M. Superoxide dismutase: An enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 214, 6049–6055. [Google Scholar]
- Goldberg, D.M.; Spooner, R.J. Glutathione Reductase. In Methods in Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1984; pp. 258–265. [Google Scholar]
- Flohe, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzym. 1984, 105, 114–120. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 7, 248–254. [Google Scholar] [CrossRef]
- Schatzkin, A.; Kipnis, V.; Carroll, R.J.; Midthune, D.; Subar, A.F.; Bingham, S.; Schoeller, D.A.; Troiano, R.P.; Freedman, L.S. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: Results from the biomarker-based Observing Protein and Energy Nutrition (OPEN) study. Int. J. Epidemiol. 2003, 32, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Freedman, L.S.; Potischman, N.; Kipnis, V.; Midthune, D.; Schatzkin, A.; Thompson, F.E.; Troiano, R.P.; Prentice, R.; Patterson, R.; Carroll, R.; et al. A comparison of two dietary instruments for evaluating the fat-breast cancer relationship. Int. J. Epidemiol. 2006, 35, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.P.; Grune, T.; Speckmann, B. The two faces of reactive oxygen species (ROS) in adipocyte function and dysfunction. Biol. Chem. 2016, 397, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. 2000, 24, 1119–1130. [Google Scholar] [CrossRef]
- Axelsson, J.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. Adipose tissue and its relation to inflammation: The role of adipokines. J. Ren. Nutr. 2005, 15, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Nihi, M.; Manfro, R.; Martins, C. Association between body fat, inflammation and oxidative stress in hemodialysis. J. Bras. 2010, 32, 9–15. [Google Scholar]
- Folsom, A.R.; Pankow, J.S.; Tracy, R.P.; Arnett, D.K.; Peacock, J.M.; Hong, Y.; Djoussé, L.; Eckfeldt, J.H. Association of C-reactive protein with markers of prevalent atherosclerotic disease. Am. J. Cardiol. 2001, 88, 112–117. [Google Scholar] [CrossRef]
- Silva, L.C.R.; de Araújo, A.L.; Fernandes, J.R.; Matias, M.D.S.T.; Silva, P.R.; Duarte, A.J.S. Moderate and intense exercise lifestyles attenuate the effects of aging on telomere length and the survival and composition of T cell subpopulations. Age 2016, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate exercise is an antioxidant: Upregulation of antioxidant genes by training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.D.; Tauler, P.; Sureda, A.; Tur, J.A.; Pons, A. Antioxidant regulatory mechanisms in neutrophils and lymphocytes after intense exercise. J. Sports Sci. 2009, 27, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Ferrando, B.; Brioche, T.; Sanchis-Gomar, F.; Viña, J. Exercise and antioxidant supplements in the elderly. J. Sport Heal. Sci. 2013, 2, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Chung, H.Y.; Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008, 44, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sport Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef]
- Tauler, P.; Gimeno, I.; Aguiló, A.; Guix, M.P.; Pons, A. Regulation of erythrocyte antioxidant enzyme activities in athletes during competition and short-term recovery. Pflugers Arch. Eur. J. Physiol. 1999, 438, 782–787. [Google Scholar] [CrossRef]
- Ji, L.L. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic. Biol. Med. 2008, 44, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Parise, G.; Brose, A.N.; Tarnopolsky, M.A. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp. Gerontol. 2005, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Conley, K.E.; Jubrias, S.A.; Esselman, P.C. Oxidative capacity and ageing in human muscle. J. Physiol. 2000, 526, 203–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidball, J.G.; Villalta, S.A. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillon, N.J.; Bilan, P.J.; Fink, L.N.; Klip, A. Cross-talk between skeletal muscle and immune cells: Muscle-derived mediators and metabolic implications. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E453–E465. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Albany, NY, USA, 2010; Volume 60, ISBN 978-92-4-159-997-9. [Google Scholar]
- Sallam, N.; Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell Longev. 2016, 2016, 46–54. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer | Temp. of Annealing | |
|---|---|---|---|
| PGC-1α | Fw: | 5′-CACTTACAAGCCAAACCAACAACT-3′ | 60 °C |
| Rv: | 5′-CAATAGTCTTGTTCTCAAATGGGGA-3′ | ||
| COXIV | Fw: | 5′-AGAAGCACTATGTGTACGGCCC-3′ | 63 °C |
| Rv: | 5′-GGTTCACCTTCATGTCCAGCAT-3′ | ||
| MitND5 | Fw: | 5′-CGGCTGAGAGGGCGTAGG-3′ | 63 °C |
| Rv: | 5′-GATGAAACCGATATCCGGCCGA-3′ | ||
| Mtf1 | Fw: | 5′-TGT TTT GGT CGC AAA CTC TG-3′ | 60 °C |
| Rv: | 5′-CTG TCT GCG TAC GTC TTC CA-3′ | ||
| Mtf2 | Fw: | 5′-ATG CAT CCC CAC TTA AGC AC-3′ | 60 °C |
| Rv: | 5′-CCA GAG GGC AGA ACT TTG TC-3′ | ||
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| (n = 66) | (n = 61) | ||||||
| Inactive | Intermediate | Active | Inactive | Intermediate | Active | ||
| (n = 20) | (n = 26) | (n = 20) | (n = 20) | (n = 15) | (n = 26) | ANOVA | |
| Age (years) | 67.2 ± 1.1 | 67.0 ± 0.8 | 68.1 ± 0.9 | 66.0 ± 1.1 | 66.9 ± 1.3 | 64.8 ± 1.1 $ | G |
| Weight (kg) | 68.6 ± 1.1 | 64.8 ± 0.8 | 64.1 ± 0.9 | 83.4 ± 1.1 $ | 80.4 ± 1.3 $ | 77.2 ± 1.1*$ | G, E |
| Height (cm) | 157.1 ± 1.1 | 156.9 ± 0.9 | 155.3 ± 1.1 | 170.5 ± 1.3 $ | 168.6 ± 1.4 $ | 169.4 ± 1.3 $ | G |
| BMI (kg/m2) | 27.8 ± 0.7 | 26.4 ± 0.8 | 26.6 ± 0.9 | 28.6 ± 0.6 | 28.2 ± 0.6 | 26.8 ± 0.6 * | E |
| Body fat (%) | 27.0 ± 1.0 | 23.9 ± 1.0 * | 23.1 ± 1.2 * | 22.9 ± 0.9 $ | 22.7 ± 1.1 | 19.2 ± 1.3 *$# | G, E |
| MET min/week | 2764 ± 183 | 5121 ± 125 * | 8234 ± 437 *# | 2605 ± 236 | 5064 ± 297 * | 9135 ± 567 *# | E |
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| (n = 66) | (n = 61) | ||||||
| Inactive | Intermediate | Active | Inactive | Intermediate | Active | ||
| (n = 20) | (n = 26) | (n = 20) | (n = 20) | (n = 15) | (n = 26) | ANOVA | |
| Energy (Kcal) | 1810.2 ± 108 | 1595.7 ± 77 | 1734.5 ± 107 | 1754.2 ± 158 | 1675.9 ± 106 | 1605.9 ± 68 | |
| Water (mL) | 2057.6 ± 123 | 2023.6 ± 130 | 1891.8 ± 109 | 2053.8 ± 157 | 2025.4 ± 144 | 2134.6 ± 150 | |
| Proteins (%) | 16.9 ± 0.8 | 16.8 ± 0.6 | 17.2 ± 1 | 17.4 ± 1 | 19.3 ± 0.8 | 18.2 ± 0.9 | |
| Carbohydrates (%) | 43.3 ± 2 | 45.2 ± 1 | 44.8 ± 2.1 | 46.0 ± 2 | 44.6 ± 2 | 41.9 ± 2 | |
| Lipids (%) | 35.7 ± 2 | 33.9 ± 1 | 34.6 ± 2 | 33.5 ± 2 | 33.2 ± 2 | 35.8 ± 1 | |
| Fiber (%) | 3.0 ± 1 | 3.2 ± 0.2 | 2.9 ± 0.3 | 3.5 ± 0.4 | 3.1 ± 0.2 | 3.2 ± 0.3 | |
| Vitamin E (mg/day) | 8.2 ± 0.8 | 7.5 ± 0.7 | 8.3 ± 1 | 8.6 ± 0.9 | 11.4 ± 1 | 8.4 ± 1 | |
| Vitamin C (mg/day) | 130 ± 17 | 140 ± 14 | 171 ± 17 | 131 ± 17 | 134 ± 17 | 137 ± 19 | |
| Selenium (µg/day) | 77.5 ± 11 | 75.7 ± 9 | 87,9 ± 6 | 136 ± 6 $ | 112 ± 11 $ | 124 ± 11 $ | G |
| Zinc (mg/day) | 7.6 ± 0.5 | 7.5 ± 0.6 | 8.1 ± 0.5 | 9.7 ± 0.6 $ | 9.5 ± 0.8 $ | 9.2 ± 0.6 $ | G |
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| (n = 66) | (n = 61) | ||||||
| Inactive | Intermediate | Active | Inactive | Intermediate | Active | ||
| (n = 20) | (n = 26) | (n = 20) | (n = 20) | (n = 15) | (n = 26) | ANOVA | |
| PBMCs (103 cells/mm3) | 2.13 ± 0.13 | 1.90 ± 0.19 | 1.65 ± 0.19 * | 1.99 ± 0.19 | 1.91 ± 0.2 | 1.33 ± 0.2 * | E |
| Lymphocytes (%) | 33.2 ± 1.5 | 33.5 ± 1.3 | 32 ± 1.2 | 34 ± 1.2 | 33 ± 1.6 | 32 ± 1.1 | |
| Monocytes (%) | 6.6 ± 0.4 | 7.3 ± 0.4 | 6.5 ± 0.3 | 7.3 ± 0.4 | 7.6 ± 0.4 | 6.6 ± 0.3 | |
| Inactive | Intermediate | Active | ANOVA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | E | SxE | |||||||||||
| CAT (%) | Women | 100 | ± | 9 | 114 | ± | 13 | 146 | ± | 17 # | 0.001 | 0.013 | 0.601 |
| Men | 132 | ± | 12 | 170 | ± | 17 * | 175 | ± | 17 #$ | ||||
| MnSOD (%) | Women | 100 | ± | 10 | 116 | ± | 13 | 156 | ± | 21 # | 0.420 | 0.045 | 0.900 |
| Men | 125 | ± | 13 | 127 | ± | 20 | 162 | ± | 24 | ||||
| GRd (%) | Women | 100 | ± | 10 | 109 | ± | 10 | 165 | ± | 20 # | 0.013 | 0.000 | 0.759 |
| Men | 123 | ± | 20 | 158 | ± | 21 | 209 | ± | 24 #$ | ||||
| GPx (%) | Women | 100 | ± | 12 | 111 | ± | 14 | 168 | ± | 26 # | 0.994 | 0.002 | 0.720 |
| Men | 108 | ± | 9 | 120 | ± | 17 | 152 | ± | 21 # | ||||
| TrxR1 (%) | Women | 100 | ± | 13 | 135 | ± | 16 | 172 | ± | 24 # | 0.056 | 0.001 | 0.853 |
| Men | 124 | ± | 24 | 177 | ± | 23 * | 196 | ± | 21 # | ||||
| UCP3 (%) | Women | 100 | ± | 10 | 102 | ± | 9 | 118 | ± | 15 | 0.099 | 0.039 | 0.565 |
| Men | 112 | ± | 10 | 109 | ± | 8 | 152 | ± | 19 # | ||||
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| (n = 66) | (n = 61) | ||||||
| mRNA levels | Inactive | Intermediate | Active | Inactive | Intermediate | Active | |
| (%) | (n = 20) | (n = 26) | (n = 20) | (n = 20) | (n = 15) | (n = 26) | ANOVA |
| COXIV | 1 ± 0.4 | 1.39 ± 0.5 | 5.25 ± 1.9 * | 1.23 ± 0.4 | 1.53 ± 0.8 | 4.37 ± 1.9 * | E |
| PGC1α | 1 ± 0.9 | 1.11 ± 0.7 | 1.91 ± 1.0 | 1.08 ± 0.9 | 1.12 ± 0.4 | 1.19 ± 0.6 | |
| MitND5 | 1 ± 0.4 | 0.96 ± 0.5 | 0.72 ± 0.3 | 1.32 ± 0.5 | 0.57 ± 0.01 | 1.05 ± 0.4 | |
| Mtf1 | 1 ± 0.3 | 0.80 ± 0.2 | 1.55 ± 0.8 | 0.79 ± 0.3 | 1.48 ± 0.7 | 1.38 ± 0.5 | |
| Mtf2 | 1 ± 0.5 | 1.02 ± 0.7 | 1.91 ± 0.8 | 0.83 ± 0.3 | 1.13 ± 0.7 | 1.49 ± 0.6 | |
| Women | Men | ||||||
|---|---|---|---|---|---|---|---|
| (n = 66) | (n = 61) | ||||||
| Inactive | Intermediate | Active | Inactive | Intermediate | Active | ||
| (n = 20) | (n = 26) | (n = 20) | (n = 20) | (n = 15) | (n = 26) | ||
| PBMCs | CAT (K/109 cells) | 53 ± 24 | 44 ± 25 | 32 ± 13 | 38 ± 15 | 36 ± 18 | 71 ± 30 |
| SOD (pkat/109 cells) | 57 ± 16 | 84 ± 27 | 110 ± 31 | 71 ± 39 | 69 ± 27 | 64 ± 16 | |
| GPx (nkat/109 cells) | 102 ± 31 | 77 ± 14 | 89 ± 21 | 80 ± 29 | 95 ± 38 | 54 ± 19 | |
| GRd (nkat/109 cells) | 447 ± 174 | 406 ± 102 | 420 ± 166 | 326 ± 100 | 324 ± 67 | 303 ± 84 | |
| Plasma | CAT (K/L) | 43 ± 20 | 51 ± 15 | 54 ± 17 | 41 ± 17 | 44 ± 14 | 64 ± 14 |
| SOD (pkat/L) | 736 ± 117 | 536 ± 72 | 526 ± 76 | 724 ± 108 | 678 ± 103 | 649 ± 67 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busquets-Cortés, C.; Capó, X.; Bibiloni, M.D.M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People. Nutrients 2018, 10, 1555. https://doi.org/10.3390/nu10101555
Busquets-Cortés C, Capó X, Bibiloni MDM, Martorell M, Ferrer MD, Argelich E, Bouzas C, Carreres S, Tur JA, Pons A, et al. Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People. Nutrients. 2018; 10(10):1555. https://doi.org/10.3390/nu10101555
Chicago/Turabian StyleBusquets-Cortés, Carla, Xavier Capó, Maria Del Mar Bibiloni, Miquel Martorell, Miguel D. Ferrer, Emma Argelich, Cristina Bouzas, Sandra Carreres, Josep A. Tur, Antoni Pons, and et al. 2018. "Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People" Nutrients 10, no. 10: 1555. https://doi.org/10.3390/nu10101555
APA StyleBusquets-Cortés, C., Capó, X., Bibiloni, M. D. M., Martorell, M., Ferrer, M. D., Argelich, E., Bouzas, C., Carreres, S., Tur, J. A., Pons, A., & Sureda, A. (2018). Peripheral Blood Mononuclear Cells Antioxidant Adaptations to Regular Physical Activity in Elderly People. Nutrients, 10(10), 1555. https://doi.org/10.3390/nu10101555









