Impact of Circulating Triglycerides Concentration on Atherosclerotic Disease Status in Middle-Aged Saudi Arabian Dwellers
Abstract
:1. Introduction
2. Methods
2.1. Subject Selection
2.2. Study Design
2.2.1. Health Questionnaire and Anthropometry
2.2.2. Blood Test Measurements
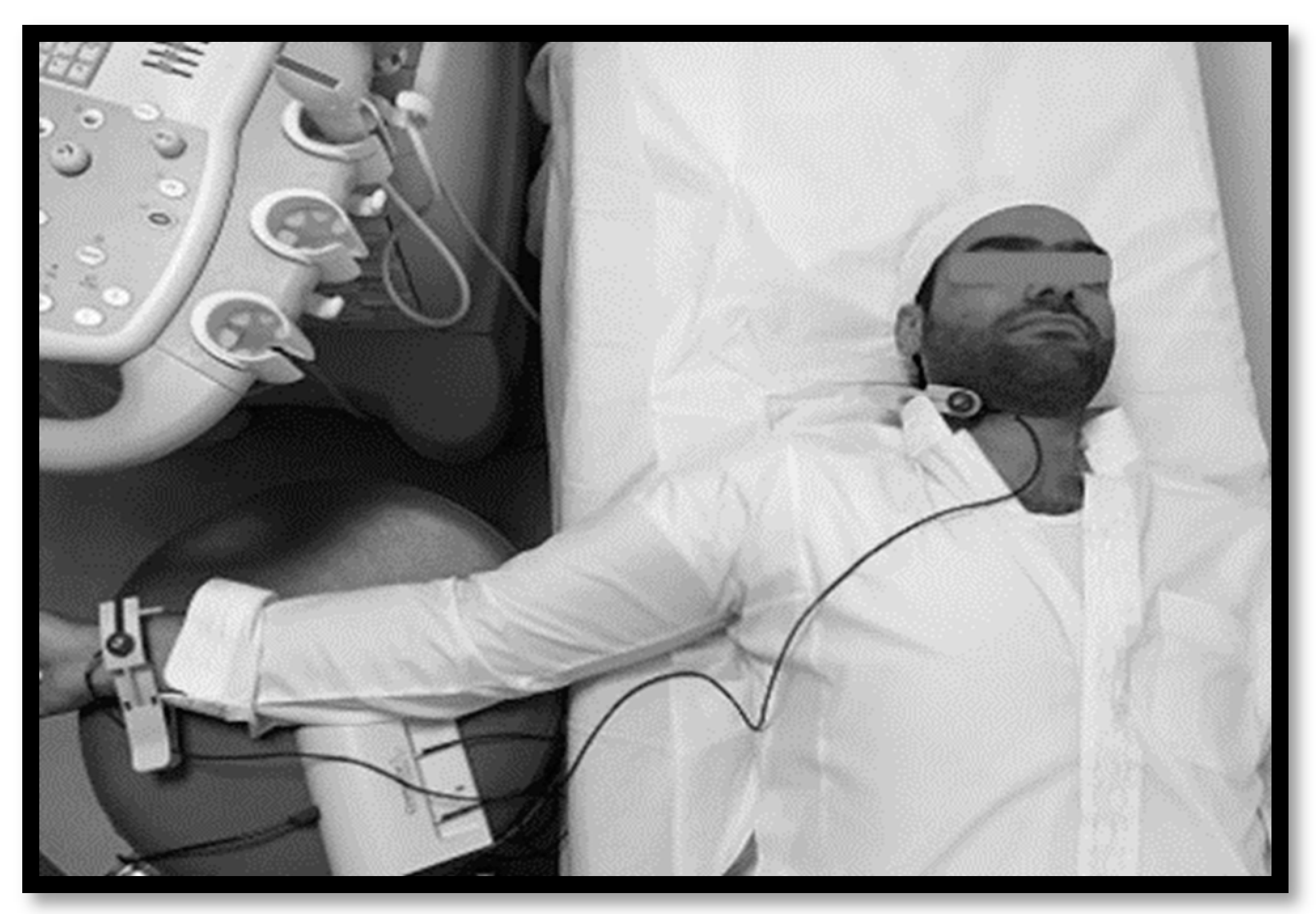
2.2.3. Vascular Structure and Kinetic Measurements
2.3. Statistical Analyses
3. Results
3.1. The Differences in Triglyceride Levels in the Three Clinical Groups
3.2. Triglycerides Associations with Vascular Structure and Kinetic Data, as Well as Endocrine Profile
3.3. Between Clinical Groups Comparison of Slopes
3.4. Further Considerations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BHF. Atherosclerosis. Available online: https://www.bhf.org.uk/heart-health/conditions/atherosclerosis (accessed on 22 July 2018).
- Libby, P. Atherosclerosis: Disease biology affecting the coronary vasculature. Am. J. Cardiol. 2006, 98, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.; Bojara, W.; Schunk, K. Early diagnosis and treatment of coronary heart disease in symptomatic subjects with advanced vascular atherosclerosis of the carotid artery (type III and IV b findings using ultrasound). Cardiol. Res. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Lertratanakul, A.; Bernatsky, S.; Hanly, J.G.; Isenberg, D.; Rahman, A.; Merrill, J.; Wallace, D.J.; Ginzler, E.; Khamashta, M.; Bruce, I.; et al. 25-hydroxyvitamin D and cardiovascular disease in patients with systemic lupus erythematosus: Data from a large international inception cohort. Arthritis Care Res. 2014, 66, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.T.; Estwick, T.; Zavodni, A.; Huang, C.Y.; Kwan, A.C.; Soule, B.P.; Long Priel, D.A.; Remaley, A.T.; Rudman Spergel, A.K.; Turkbey, E.B.; et al. Assessment of atherosclerosis in chronic granulomatous disease. Circulation 2014, 130, 2031–2039. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- George, S.J.; Johnson, J. Atherosclerosis: Molecular and Cellular Mechanisms; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M. Inflammation and atherosclerosis: Role of c-reactive protein in risk assessment. Am. J. Med. 2004, 116, 9–16. [Google Scholar] [CrossRef] [PubMed]
- URMC. Atherosclerosis. In Health Encyclopedia; University of Rochester Medical Center: New York, NY, USA, 2018. [Google Scholar]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEniery, C.M.; Wallace, S.; Mackenzie, I.S.; McDonnell, B.; Yasmin; Newby, D.E.; Cockcroft, J.R.; Wilkinson, I.B. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension 2006, 48, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Caplan, L.R.; Wong, K.S.L. Intracranial Atherosclerosis; Wiley-Blackwell: Chichester, UK; Hoboken, NJ, USA, 2008. [Google Scholar]
- Polak, J.F.; Szklo, M.; Kronmal, R.A.; Burke, G.L.; Shea, S.; Zavodni, A.E.; O’Leary, D.H. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: The multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000087. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Abbas, H.Y. Ultrasound evaluation of carotid artery intima-media thickness in patients with risk factors for cardiovascular disease. Int. J. Diagn. Imaging 2017, 4, 16. [Google Scholar] [CrossRef]
- Arora, T.; Rehan, H.S. A perspective on role of calcium and vitamin d in cardiovascular outcomes and lipid profile. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hajj, A.; Chedid, R.; Chouery, E.; Megarbane, A.; Gannage-Yared, M.H. Relationship between vitamin D receptor gene polymorphisms, cardiovascular risk factors and adiponectin in a healthy young population. Pharmacogenomics 2016, 17, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Khoo, C.; Furtado, J.; Sacks, F.M. Apolipoprotein C-III and the metabolic basis for hypertriglyceridemia and the dense low-density lipoprotein phenotype. Circulation 2010, 121, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.V. High-density lipoprotein and transport of cholesterol and triglyceride in blood. J. Clin. Lipidol. 2007, 1, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Talayero, B.G.; Sacks, F.M. The role of triglycerides in atherosclerosis. Curr. Cardiol. Rep. 2011, 13, 544. [Google Scholar] [CrossRef] [PubMed]
- Berdanier, C.D.; Zempleni, J. Advanced Nutrition: Macronutrients, Micronutrients, and Metabolism; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Lilley, J.S.; Linton, M.F.; Kelley, J.C.; Graham, T.B.; Fazio, S.; Tavori, H. A case of severe acquired hypertriglyceridemia in a 7-year-old girl. J. Clin. Lipidol. 2017, 11, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Ntai, K.; Kadoglou, N.; Papadakis, I.; Kornelakis, M.; Tritakis, V.; Varoudi, M.; Papadima, T.; Triantafyllidi, H.; Parissis, J. The evaluation of pulse wave velocity using arteriograph and complior apparatus across multiple cohorts of cardiovascular-related diseases. Int. J. Cardiol. 2013, 168, 4890–4892. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Naqvi, T.Z.; Gardin, J.M.; Gerhard-Herman, M.; Jaff, M.; Mohler, E. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification. Vasc. Med. 2006, 11, 201–211. [Google Scholar] [CrossRef] [PubMed]
- van Wissen, S.; Trip, M.D.; Smilde, T.J.; de Graaf, J.; Stalenhoef, A.F.; Kastelein, J.J. Differential HS-CRP reduction in patients with familial hypercholesterolemia treated with aggressive or conventional statin therapy. Atherosclerosis 2002, 165, 361–366. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the framingham heart study. Circulation 2008, 117, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Creager, M.A.; Libby, P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA 2002, 287, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, R.-X.; Cao, X.-L.; Huang, F.; Zhou, Y.-J.; Chen, W.-X. Angptl4 variants and their haplotypes are associated with serum lipid levels, the risk of coronary artery disease and ischemic stroke and atorvastatin cholesterol-lowering responses. Nutr. Metab. 2018, 15, 70. [Google Scholar] [CrossRef] [PubMed]



| Variable | CG | ARG | DAG | Total |
|---|---|---|---|---|
| N = 30 | N = 25 | N = 29 | N = 84 | |
| Gender | ||||
| Male | 5 (5.95%) | 3 (3.57%) | 6 (7.14%) | 14 (16.6%) |
| Female | 25 (29.76%) | 22 (26.19%) | 23 (27.38%) | 70 (83.3) |
| Age, years | 41.8 ± 6.8 | 50.12 ± 6.6 | 55.8 ± 6.7 | 48.21 ± 8.3 |
| BMI, kg/m2 | 30.6 ± 6.9 | 31.8 ± 4.8 | 33.6 ± 5.9 | 31.9 ± 6 |
| WHR, cm | 0.86 ± 0.08 | 0.95 ± 0.07 | 0.94 ± 0.13 | 0.91 ± 0.107 |
| Variable | CG | ARG | DAG | Total | p |
|---|---|---|---|---|---|
| N = 30 | N = 25 | N = 29 | N = 84 | ||
| Use Statin | 0.001 ** | ||||
| Yes | 0 (0%) | 10 (11.9%) | 10 (11.9%) | 20 (23.8%) | |
| No | 30 (35.7%) | 15 (17.85%) | 19 (22.6%) | 64 (76.2%) | |
| TG, mg/dL | 0.010 ** | ||||
| Median (IQR) | 93 (74) | 145 (93) | 128 (99) | 119.5 (82) | |
| TC, mg/dL | 0.072 | ||||
| Mean ± SD | 198.8 ± 32.3 | 182.3 ± 45.98 | 178.9 ± 40.59 | 187 ± 40.15 | |
| HDL, mg/dL | 0.676 | ||||
| Median (IQR) | 48.5 (20) | 43 (12) | 45 (19) | 45.5 (16) | |
| LDL, mg/dL | 0.004 ** | ||||
| Mean ± SD | 131.9 ± 27.65 | 112.4 ± 41.5 | 105.1 ± 34.7 | 116.86 ± 36.17 | |
| CRP, mg/dL | 0.045 | ||||
| Median (IQR) | 0.15 (0.99) | 0.50 (0.745) | 0.000 (0.712) | 0.41 (0.87) | |
| FBG, mg/dL | 0.0001 ** | ||||
| Median (IQR) | 90.5 (12) | 162 (135) | 119 (51) | 104 (57) | |
| P-SBP, mmHg | 0.0001 ** | ||||
| Median (IQR) | 121 (20) | 131.5 (14) | 137 (23) | 130.5 (18) | |
| P-DBP, mmHg | 0.020 * | ||||
| Mean ± SD | 68.47 ± 9.86 | 73.9 ± 8.94 | 75.48 ± 10.32 | 72.5 ± 10.137 | |
| C-SBP, mmHg | 0.0001 ** | ||||
| Median (IQR) | 106 (15) | 117 (21) | 123 (34) | 113 (22) | |
| CR-PWV, m/s | 0.071 | ||||
| Median (IQR) | 7.1 (7.6) | 10.8 (8.3) | 8.9 (6.5) | 9.2 (8.1) | |
| HR, bpm | 0.007 ** | ||||
| Mean ± SD | 69.5 ± 10.35 | 79.25 ± 11.45 | 73.74 ± 15.6 | 73.86 ± 13.17 | |
| IMT, mm | 0.0001 ** | ||||
| Median (IQR) | 0.49 (0.18) | 0.556 (0.19) | 0.696 (0.35) | 0.575 (0.25) | |
| IAD, mm | 0.004 ** | ||||
| Mean ± SD | 6.32 ± 0.62 | 6.61 ± 0.68 | 7.03 ± 0.94 | 6.65 ± 0.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azhar, W.; Buczkowski, B.; Smith, C.; Onambele-Pearson, G. Impact of Circulating Triglycerides Concentration on Atherosclerotic Disease Status in Middle-Aged Saudi Arabian Dwellers. Nutrients 2018, 10, 1642. https://doi.org/10.3390/nu10111642
Azhar W, Buczkowski B, Smith C, Onambele-Pearson G. Impact of Circulating Triglycerides Concentration on Atherosclerotic Disease Status in Middle-Aged Saudi Arabian Dwellers. Nutrients. 2018; 10(11):1642. https://doi.org/10.3390/nu10111642
Chicago/Turabian StyleAzhar, Wedad, Bartek Buczkowski, Christopher Smith, and Gladys Onambele-Pearson. 2018. "Impact of Circulating Triglycerides Concentration on Atherosclerotic Disease Status in Middle-Aged Saudi Arabian Dwellers" Nutrients 10, no. 11: 1642. https://doi.org/10.3390/nu10111642
APA StyleAzhar, W., Buczkowski, B., Smith, C., & Onambele-Pearson, G. (2018). Impact of Circulating Triglycerides Concentration on Atherosclerotic Disease Status in Middle-Aged Saudi Arabian Dwellers. Nutrients, 10(11), 1642. https://doi.org/10.3390/nu10111642








