Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Animals and Diet
2.3. Collection of Serum and Tissue Samples
2.4. Serum Biochemical Analysis
2.5. Histological Analysis
2.6. Cold Tolerance Test and Infrared Imaging of Heat Intensity Measurement
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.8. Western Blot Analysis
2.9. ELISA Assays
2.10. Immunofluorescence
2.11. Statistical Analysis
3. Results
3.1. EGCG Ameliorates Obesity Induced by HFD
3.2. EGCG Enhances BAT Thermogenesis
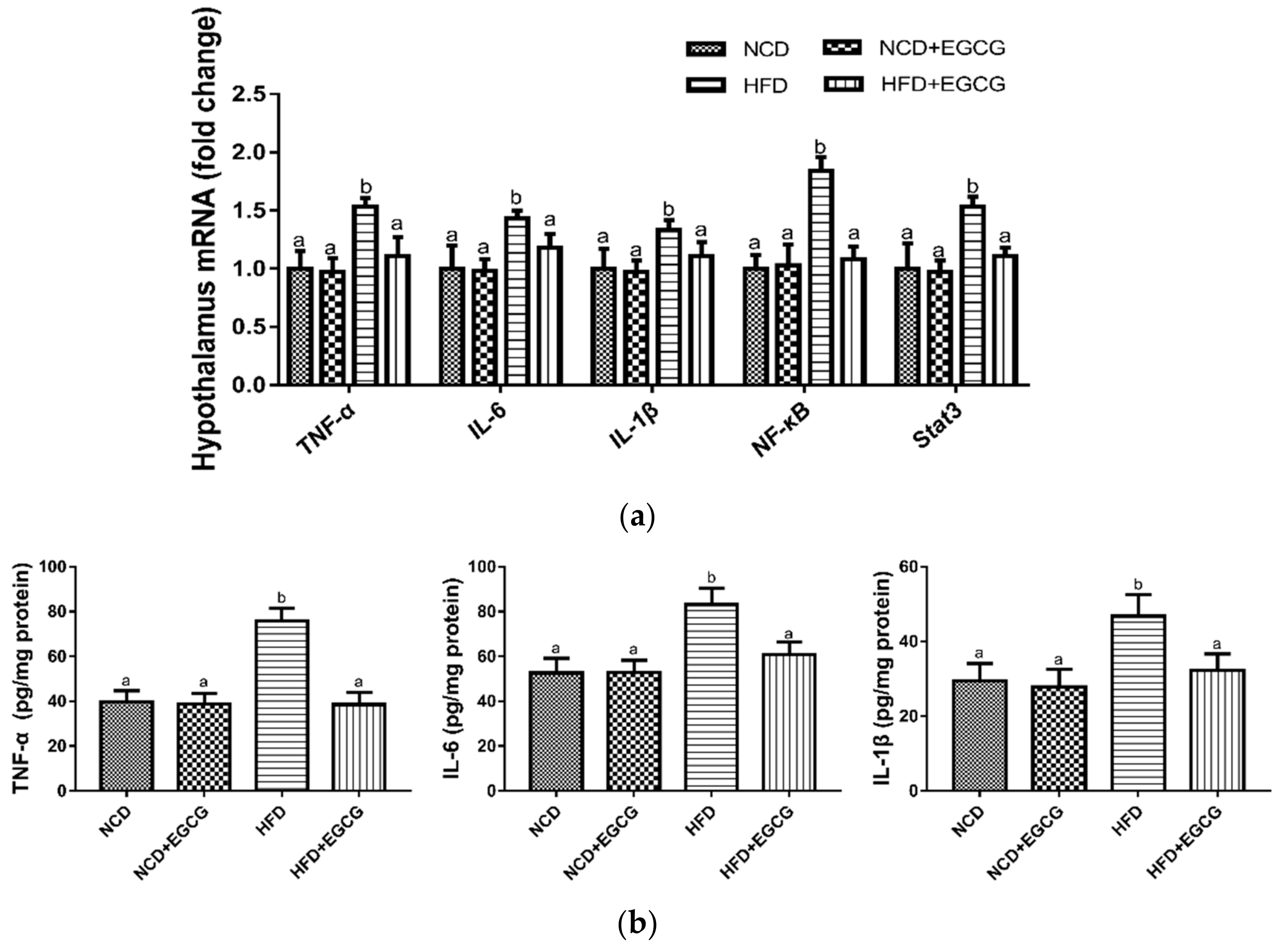
3.3. EGCG Reduces Inflammation in the Hypothalamus
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Narayanaswami, V.; Dwoskin, L.P. Obesity: Current and potential pharmacotherapeutics and targets. Pharmacol. Therapeut. 2017, 170, 116–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blancas-Velazquez, A.; Mendoza, J.; Garcia, A.N.; la Fleur, S.E. Diet-induced obesity and circadian disruption of feeding behavior. Front. Neurosci. 2017, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Majdic, G. Integrative role of brain and hypothalamus in the control of energy balance. Acta Chim. Slov. 2009, 56, 289–296. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Carpentier, A.C.; Dore, G.; Ouellet, V.; Picard, F. Determinants of brown adipocyte development and thermogenesis. Int. J. Obes. 2010, 34, S59–S66. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.Y.; Zhang, W.; Luk, C.T.; Sivasubramaniyam, T.; Brunt, J.J.; Schroer, S.A.; Desai, H.R.; Majerski, A.; Woo, M.N. Jak2 promotes brown adipose tissue function and is required for diet- and cold-induced thermogenesis in mice. Diabetologia 2016, 59, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nakamura, K. Central regulation of brown adipose tissue thermogenesis and energy homeostasis dependent on food availability. Pflug. Arch. Eur. J. Phys. 2018, 470, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Beerens, S.; Adan, R.A.H. Role of leptin in energy expenditure: The hypothalamic perspective. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R938–R947. [Google Scholar] [CrossRef] [PubMed]
- Morselli, L.L.; Claflin, K.E.; Cui, H.; Grobe, J.L. Control of energy expenditure by agrp neurons of the arcuate nucleus: Neurocircuitry, signaling pathways, and angiotensin. Curr. Hypertens. Rep. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Chitravanshi, V.C.; Kawabe, K.; Sapru, H.N. Stimulation of the hypothalamic arcuate nucleus increases brown adipose tissue nerve activity via hypothalamic paraventricular and dorsomedial nuclei. Am. J. Physiol.-Heart C 2016, 311, H433–H444. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.A.; Herkenham, M. Arcuate nucleus neurons that project to the hypothalamic paraventricular nucleus—Neuropeptidergic identity and consequences of adrenalectomy on messenger-rna levels in the rat. J. Comp. Neurol. 1995, 358, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Kawabe, K.; Sapru, H.N. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: Role of the hypothalamic paraventricular nucleus. PLoS ONE 2012, 7, e45180. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.S. Neuroinflammation in overnutrition-induced diseases. Obesity 2013, 91, 195–218. [Google Scholar]
- Valdearcos, M.; Xu, A.W.; Koliwad, S.K. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 2015, 77, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Perry, V.H. Microglial physiology: Unique stimuli, specialized responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Therapeut. 2015, 154, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Beumer, W.; Gibney, S.M.; Drexhage, R.C.; Pont-Lezica, L.; Doorduin, J.; Klein, H.C.; Steiner, J.; Connor, T.J.; Harkin, A.; Versnel, M.A.; et al. The immune theory of psychiatric diseases: A key role for activated microglia and circulating monocytes. J. Leukocyte Biol. 2012, 92, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cronk, J.C.; Kipnis, J. The role of microglia in brain maintenance: Implications for rett syndrome. Trends Immunol. 2013, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab. 2017, 26, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, C.S.; Tu, T.H.; Kim, M.S.; Goto, T.; Kawada, T.; Choi, M.S.; Park, T.; Sung, M.K.; Yun, J.W.; et al. Quercetin protects obesity-induced hypothalamic inflammation by reducing microglia-mediated inflammatory responses via ho-1 induction. Nutrients 2017, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Klaus, S.; Pultz, S.; Thone-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Delzenne, N.M. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Fang, B.; Huang, Y.; Tao, S.; Sun, B.; Guan, S.; Jin, Y. Epigallocatechin-3-gallate protects against 1,3-dichloro-2-propanol-induced lipid accumulation in c57bl/6j mice. Life Sci. 2018, 209, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Liu, X.; Tian, H.; Liu, H.; Li, J.; Qi, G.; Liu, X. Egcg stimulates the recruitment of brite adipocytes, suppresses adipogenesis and counteracts tnf-alpha-triggered insulin resistance in adipocytes. Food Funct. 2018, 9, 3374–3386. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Chung, C.S.; Lee, H.G.; Kim, T.G.; Choi, Y.J.; Cho, C.S. Inhibitory effect of (−)-epigallocatechin-3-gallate on lipid accumulation of 3t3-l1 cells. Obesity 2007, 15, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Raederstorff, D.; Wang, Y.; Teixeira, S.R.; Elste, V.; Weber, P. Teavigo (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann. Nutr. Metab. 2005, 49, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Yu, L.; Chen, Z.Y.; Zhu, J.S.; Hua, R.; Qin, X.; Cao, J.L.; Zhang, Y.M. Activation of corticotropin-releasing factor neurons and microglia in paraventricular nucleus precipitates visceral hypersensitivity induced by colorectal distension in rats. Brain Behav. Immun. 2016, 55, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by prdm16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Kusminski, C.M.; Luby-Phelps, K.; Spurgin, S.B.; An, Y.A.; Wang, Q.A.; Holland, W.L.; Scherer, P.E. Brown adipose tissue derived vegf-a modulates cold tolerance and energy expenditure. Mol. Metab. 2014, 3, 474–483. [Google Scholar] [CrossRef] [PubMed]
- You, Y.L.; Han, X.; Guo, J.L.; Guo, Y.; Yin, M.W.; Liu, G.J.; Huang, W.D.; Zhan, J.C. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods 2018, 41, 62–71. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, Y.; Jung, S.; Kim, Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr. Res. 2017, 61, 1325307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokoeva, M.V.; Yin, H.L.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Wisse, B.E.; Schwartz, M.W. Does hypothalamic inflammation cause obesity? Cell Metab. 2009, 10, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Rachid, B.; van de Sande-Lee, S.; Rodovalho, S.; Folli, F.; Beltramini, G.C.; Morari, J.; Amorim, B.J.; Pedro, T.; Ramalho, A.F.; Bombassaro, B.; et al. Distinct regulation of hypothalamic and brown/beige adipose tissue activities in human obesity. Int. J. Obes. 2015, 39, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Zoheir, K.M.A.; Ansari, M.A.; Korashy, H.M.; Abdel-Hamied, H.E.; Ashour, A.E.; Abd-Allah, A.R.A. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of nf-kappa b, stat3 and pro-inflammatory mediators and enhancement of i kappa b alpha and anti-inflammatory cytokines. Inflammation 2015, 38, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassarddoulcier, A.M.; Miroux, B.; Raimbault, S.; Gelly, C.; Klaus, S.; Champigny, O.; Levimeyrueis, C.; Frossard, V.; Bouillaud, F.; Ricquier, D. Molecular studies of the mitochondrial uncoupling protein of brown adipocytes. J. Cell. Biochem. 1994, 18A, 155. [Google Scholar]
- Oelkrug, R.; Polymeropoulos, E.T.; Jastroch, M. Brown adipose tissue: Physiological function and evolutionary significance. J. Comp. Physiol. B 2015, 185, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.L.; Revelo, X.; Shao, W.J.; Tian, L.L.; Zeng, K.J.; Lei, H.; Sun, H.S.; Woo, M.; Winer, D.; Jin, T. Dietary curcumin intervention targets mouse white adipose tissue inflammation and brown adipose tissue ucp1 expression. Obesity 2018, 26, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.X.; Wei, G.; You, Y.L.; Huang, Y.Y.; Lee, H.J.; Dong, M.; Lin, J.; Hu, T.; Zhang, H.L.; Zhang, C.H.; et al. Rutin ameliorates obesity through brown fat activation. FASEB J. 2017, 31, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.Y.; Mi, Y.S.; Liu, Z.G.; Fan, R.; Qiao, Q.L.; Sun, Y.L.; Ren, B.; Liu, X.B. Dietary tea polyphenols ameliorate metabolic syndrome and memory impairment via circadian clock related mechanisms. J. Funct. Foods 2017, 34, 168–180. [Google Scholar] [CrossRef]
- Atrens, D.M.; Menendez, J.A. Somatostatin and the paraventricular hypothalamus—Modulation of energy-balance. Brain Res. 1993, 630, 238–244. [Google Scholar] [CrossRef]
- Valdearcos, M.; Robblee, M.M.; Benjamin, D.I.; Nomura, D.K.; Xu, A.W.; Koliwad, S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014, 9, 2124–2138. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.H.; Nie, Y.; Wu, J.J.; Huang, Q.; Cheng, Y.Q. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing tlr4/nf-kappa b signaling pathway in high-fat diet rats. Biochem. Biophys. Res. Commun. 2015, 462, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Tang, C.H.; Chen, Y.H.; Wei, I.H. Berberine suppresses neuroinflammatory responses through amp-activated protein kinase activation in bv-2 microglia. J. Cell. Biochem. 2010, 110, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Park, E.J.; Joe, E.H.; Jou, I. Curcumin suppresses janus kinase-stat inflammatory signaling through activation of src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J. Immunol. 2003, 171, 6072–6079. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Tong, Q.; Ye, C.; Koda, S.; Fuller, P.M.; Krashes, M.J.; Vong, L.; Ray, R.S.; Olson, D.P.; Lowell, B.B. Gabaergic rip-cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 2012, 151, 645–657. [Google Scholar] [CrossRef] [PubMed]





| Ingredient (g) | Group | |||
|---|---|---|---|---|
| NCD | NCD + EGCG | HFD | HFD + EGCG | |
| Casein | 200.00 | 200.00 | 200.00 | 200.00 |
| L-Cystine | 3.00 | 3.00 | 3.00 | 3.00 |
| Corn Starch | 506.20 | 506.20 | 0.00 | 0.00 |
| Maltodextrin | 125.00 | 119.73 | 125.00 | 121.13 |
| Sucrose | 68.80 | 63.52 | 68.80 | 64.93 |
| Cellulose | 50.00 | 50.00 | 50.00 | 50.00 |
| Soybean Oil | 25.00 | 25.00 | 25.00 | 25.00 |
| Lard | 20.00 | 20.00 | 245.00 | 245.00 |
| Mineral Mix S10026 | 10.00 | 10.00 | 10.00 | 10.00 |
| DiCalcium Phosphate | 13.00 | 13.00 | 13.00 | 13.00 |
| Calcium Carbonate | 5.50 | 5.50 | 5.50 | 5.50 |
| Potassium Citrate, 1 H2O | 16.50 | 16.50 | 16.50 | 16.50 |
| Vitamin Mix V10001 | 10.00 | 10.00 | 10.00 | 10.00 |
| Choline Bitartrate | 2.00 | 2.00 | 2.00 | 2.00 |
| FD&C Yellow Dye #5 | 0.04 | 0.04 | 0.00 | 0.00 |
| FD&C Blue Dye #1 | 0.01 | 0.01 | 0.05 | 0.05 |
| EGCG | 0.00 | 10.55 | 0.00 | 7.74 |
| Total | 1055.05 | 1055.05 | 773.85 | 773.85 |
| Total energy | ||||
| Energy, Kcal/g | 3.85 | 3.81 | 5.24 | 5.20 |
| Protein, % | 20.0 | 20.0 | 20.0 | 20.2 |
| Carbohydrate, % | 70.0 | 69.9 | 20.0 | 19.5 |
| Fat, % | 10.0 | 10.1 | 60.0 | 60.3 |
| Gene | Forward Primer (5′ to 3′) | Reverse Primer (3′ to 5′) |
|---|---|---|
| UCP-1 | CCAAAGTCCGCCTTCAGATC | TCTGTAGGCTGCCCAATGAA |
| PGC-1α | GTCGTGTTCCCGATCACCATAT | CTTTGCGGTATTCATCCCTCTT |
| PRDM16 | CACGTCTACGGTGAACGGAA | ATGGGATCCATGAAGAACGGT |
| TNF-α | GACCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| IL-6 | TCTACTCGGCAAACCTAGTGCGTTA | TTCTGACCACAGTGAGGAATGTCCA |
| IL-1β | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
| GAPDH | GAAGGTCGGTGTGAACGGATTTG | CATGTAGACCATGTAGTTGAGGTCA |
| NCD | NCD + EGCG | HFD | HFD + EGCG | |
|---|---|---|---|---|
| Initial body weight (g) | 21.89 ± 0.87 a | 21.92 ± 0.78 a | 22.05 ± 1.16 a | 22.12 ± 1.02 a |
| Final body weight (g) | 24.82 ± 0.38 a | 24.80 ± 1.06 a | 27.60 ± 0.83 b | 25.09 ± 1.27 a |
| Body weight gain (g) | 2.93 ± 0.61 a | 2.88 ± 0.50 a | 5.55 ± 0.97 b | 2.97 ± 0.47 a |
| Food intake (g/day) | 2.93 ± 0.60 a | 3.16 ± 0.40 a | 4.61 ± 0.68b | 4.99 ± 1.05 b |
| Energy intake (kcal/day) | 11.29 ± 2.32 a | 12.05 ± 1.52 a | 24.14 ± 3.58 b | 25.93 ± 5.46 b |
| Energy efficiency (g gain/kcal consumption) | 0.26 ± 0.05 a | 0.24 ± 0.04 a | 0.23 ± 0.04 a | 0.11 ± 0.04 b |
| Glucose (mmol/L) | 4.75 ± 0.47 a | 4.29 ± 0.51 a | 6.92 ± 0.56 b | 5.08 ± 0.30 c |
| TG (mmol/L) | 0.74 ± 0.05 a | 0.66 ± 0.09 a | 1.01 ± 0.11 b | 0.82 ± 0.17 a |
| TC (mmol/L) | 2.69 ± 0.19 a | 2.63 ± 0.13 a | 2.93 ± 0.14 b | 2.86 ± 0.12 b |
| HDL (mmol/L) | 2.81 ± 0.17 a | 2.87 ± 0.15 a | 2.91 ± 0.13 a | 2.86 ± 0.17 a |
| LDL (mmol/L) | 0.43 ± 0.02 a | 0.42 ± 0.08 a | 0.40 ± 0.04 a | 0.41 ± 0.08 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1681. https://doi.org/10.3390/nu10111681
Zhou J, Mao L, Xu P, Wang Y. Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients. 2018; 10(11):1681. https://doi.org/10.3390/nu10111681
Chicago/Turabian StyleZhou, Jihong, Limin Mao, Ping Xu, and Yuefei Wang. 2018. "Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet" Nutrients 10, no. 11: 1681. https://doi.org/10.3390/nu10111681
APA StyleZhou, J., Mao, L., Xu, P., & Wang, Y. (2018). Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients, 10(11), 1681. https://doi.org/10.3390/nu10111681




