Combining Dietary Sulfur Amino Acid Restriction with Polyunsaturated Fatty Acid Intake in Humans: A Randomized Controlled Pilot Trial
Abstract
:1. Introduction
2. Materials and Methods
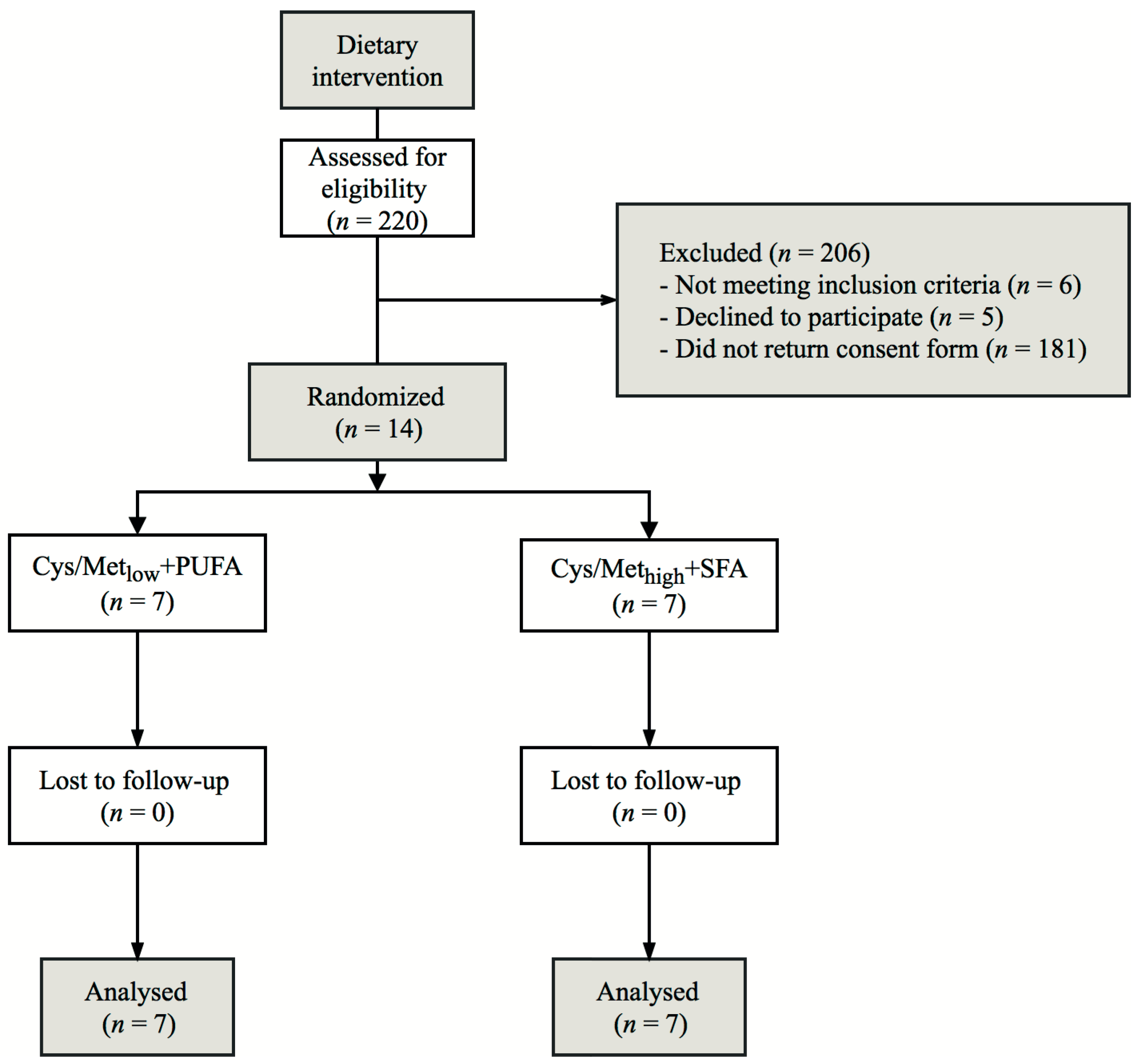
2.1. Participants
2.2. Study Design and Outcomes
2.3. Dietary Interventions
2.4. Data Collection
2.4.1. Lifestyle and Dietary Data
2.4.2. Anthropometric Parameters
2.5. Blood and Urine Sampling and Biochemical Assays
2.5.1. Blood Sampling
2.5.2. Urine Sampling
2.5.3. Clinical Biochemistry
2.5.4. Amino Acid Assays
2.5.5. Fatty Acid Assays
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Changes in Plasma SAAs in Response to the Dietary Interventions
3.3. Changes in Urine SAAs in Response to the Dietary Interventions
3.4. SCD Indices Based on Plasma Total Fatty Acids
3.5. Associations between tCys Fractions and SCD Indices
3.6. Harmful Effects
4. Discussion
Supplementary Materials
Author contributions
Funding
Acknowledgments
Conflicts of Interest
Data availability statement
References
- Koppes, L.L.; Boon, N.; Nooyens, A.C.; van Mechelen, W.; Saris, W.H. Macronutrient distribution over a period of 23 years in relation to energy intake and body fatness. Br. J. Nutr. 2009, 101, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Murtaugh, M.A.; Herrick, J.S.; Sweeney, C.; Baumgartner, K.B.; Guiliano, A.R.; Byers, T.; Slattery, M.L. Diet composition and risk of overweight and obesity in women living in the southwestern United States. J. Am. Diet. Assoc. 2007, 107, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Silventoinen, K.; Hasselbalch, A.L.; Lallukka, T.; Bogl, L.; Pietilainen, K.H.; Heitmann, B.L.; Schousboe, K.; Rissanen, A.; Kyvik, K.O.; Sorensen, T.I.; et al. Modification effects of physical activity and protein intake on heritability of body size and composition. Am. J. Clin. Nutr. 2009, 90, 1096–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinknes, K.J.; de Vogel, S.; Elshorbagy, A.K.; Nurk, E.; Drevon, C.A.; Gjesdal, C.G.; Tell, G.S.; Vollset, S.E.; Refsum, H. Dietary intake of protein is positively associated with percent body fat in middle-aged and older adults. J. Nutr. 2011, 141, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bujnowski, D.; Xun, P.; Daviglus, M.L.; Van Horn, L.; He, K.; Stamler, J. Longitudinal association between animal and vegetable protein intake and obesity among men in the United States: The Chicago Western Electric Study. J. Am. Diet. Assoc. 2011, 111, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.; Appleby, P.; Spencer, E.; Key, T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int. J. Obes. (Lond.) 2006, 30, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef] [PubMed]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [PubMed]
- Nimni, M.E.; Han, B.; Cordoba, F. Are we getting enough sulfur in our diet? Nutr. Metab. 2007, 4, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnan, J.T.; Brosnan, M.E. The sulfur-containing amino acids: An overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Smith, A.D.; Kozich, V.; Refsum, H. Cysteine and obesity. Obesity 2012, 20, 473–481. [Google Scholar] [CrossRef] [PubMed]
- El-Khairy, L.; Ueland, P.M.; Nygard, O.; Refsum, H.; Vollset, S.E. Lifestyle and cardiovascular disease risk factors as determinants of total cysteine in plasma: The Hordaland Homocysteine Study. Am. J. Clin. Nutr. 1999, 70, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Nurk, E.; Gjesdal, C.G.; Tell, G.S.; Ueland, P.M.; Nygard, O.; Tverdal, A.; Vollset, S.E.; Refsum, H. Homocysteine, cysteine, and body composition in the Hordaland Homocysteine Study: Does cysteine link amino acid and lipid metabolism? Am. J. Clin. Nutr. 2008, 88, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Refsum, H.; Smith, A.D.; Graham, I.M. The association of plasma cysteine and gamma-glutamyltransferase with BMI and obesity. Obesity 2009, 17, 1435–1440. [Google Scholar] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Graham, I.M.; Palma Reis, R.; Sales Luis, A.; Smith, A.D.; Refsum, H. The association of fasting plasma sulfur-containing compounds with BMI, serum lipids and apolipoproteins. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Refsum, H.; Butte, N. The association of cysteine with obesity, inflammatory cytokines and insulin resistance in Hispanic children and adolescents. PLoS ONE 2012, 7, e44166. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Kozich, V.; Smith, A.D.; Refsum, H. Cysteine and obesity: Consistency of the evidence across epidemiologic, animal and cellular studies. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Mattocks, D.A.; Plummer, J.D.; Smith, A.D.; Drevon, C.A.; Refsum, H.; Perrone, C.E. Cysteine supplementation reverses methionine restriction effects on rat adiposity: Significance of stearoyl-coenzyme A desaturase. J. Lipid Res. 2011, 52, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Valdivia-Garcia, M.; Refsum, H.; Smith, A.D.; Mattocks, D.A.; Perrone, C.E. Sulfur amino acids in methionine-restricted rats: Hyperhomocysteinemia. Nutrition 2010, 26, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Elshorbagy, A.K.; Church, C.; Valdivia-Garcia, M.; Smith, A.D.; Refsum, H.; Cox, R. Dietary cystine level affects metabolic rate and glycaemic control in adult mice. J. Nutr. Biochem. 2012, 23, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshorbagy, A.K.; Jerneren, F.; Scudamore, C.L.; McMurray, F.; Cater, H.; Hough, T.; Cox, R.; Refsum, H. Exploring the Lean Phenotype of Glutathione-Depleted Mice: Thiol, Amino Acid and Fatty Acid Profiles. PLoS ONE 2016, 11, e0163214. [Google Scholar] [CrossRef] [PubMed]
- Perrone, C.E.; Mattocks, D.A.; Jarvis-Morar, M.; Plummer, J.D.; Orentreich, N. Methionine restriction effects on mitochondrial biogenesis and aerobic capacity in white adipose tissue, liver, and skeletal muscle of F344 rats. Metabolism 2010, 59, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Vinknes, K.J.; Dekker, J.M.; Drevon, C.A.; Refsum, H.; Nurk, E.; Nijpels, G.; Stehouwer, C.D.; Teerlink, T.; Tell, G.S.; Nygard, O.; et al. Plasma sulfur amino acids and stearoyl-CoA desaturase activity in two caucasian populations. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Vinknes, K.J.; Elshorbagy, A.K.; Nurk, E.; Drevon, C.A.; Gjesdal, C.G.; Tell, G.S.; Nygard, O.; Vollset, S.E.; Refsum, H. Plasma stearoyl-CoA desaturase indices: Association with lifestyle, diet, and body composition. Obesity 2013, 21, E294–E302. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Ohrvall, M.; Vessby, B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Miyazaki, M.; Ntambi, J.M. Dietary cholesterol opposes PUFA-mediated repression of the stearoyl-CoA desaturase-1 gene by SREBP-1 independent mechanism. J. Lipid Res. 2002, 43, 1750–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahagi, N.; Shimano, H.; Hasty, A.H.; Amemiya-Kudo, M.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Shionoiri, F.; Ohashi, K.; Osuga, J.; et al. A crucial role of sterol regulatory element-binding protein-1 in the regulation of lipogenic gene expression by polyunsaturated fatty acids. J. Biol. Chem. 1999, 274, 35840–35844. [Google Scholar] [CrossRef] [PubMed]
- Nordic Council of Ministers. Nordic Nutrition Recommendations 2012: Integrating Nutrition and Physical Activity; Nordisk Ministerråd: Copenhagen, Denmark, 2012. [Google Scholar]
- Mansoor, M.A.; Bergmark, C.; Svardal, A.M.; Lonning, P.E.; Ueland, P.M. Redox status and protein binding of plasma homocysteine and other aminothiols in patients with early-onset peripheral vascular disease. Homocysteine and peripheral vascular disease. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Baarholm, O.A.; Van-Assche, T.; Cunnington, C.; Pillai, R.; Ratnatunga, C.; Tousoulis, D.; Stefanadis, C.; et al. MTHFR 677 C>T Polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation 2009, 119, 2507–2515. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Yim, J.; Lim, Y.; Kim, J.H. Validation of a liquid chromatography tandem mass spectrometry method to measure oxidized and reduced forms of glutathione in whole blood and verification in a mouse model as an indicator of oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1019, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Demmelmair, H.; Koletzko, B. High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS ONE 2010, 5, e12045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wainwright, P.E.; Leatherdale, S.T.; Dubin, J.A. Advantages of mixed effects models over traditional ANOVA models in developmental studies: A worked example in a mouse model of fetal alcohol syndrome. Dev. Psychobiol. 2007, 49, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Raguso, C.A.; Regan, M.M.; Young, V.R. Cysteine kinetics and oxidation at different intakes of methionine and cystine in young adults. Am. J. Clin. Nutr. 2000, 71, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukagawa, N.K.; Yu, Y.M.; Young, V.R. Methionine and cysteine kinetics at different intakes of methionine and cysteine in elderly men and women. Am. J. Clin. Nutr. 1998, 68, 380–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiramatsu, T.; Fukagawa, N.K.; Marchini, J.S.; Cortiella, J.; Yu, Y.M.; Chapman, T.E.; Young, V.R. Methionine and cysteine kinetics at different intakes of cystine in healthy adult men. Am. J. Clin. Nutr. 1994, 60, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Park, Y.; Gletsu-Miller, N.; Liang, Y.; Yu, T.; Accardi, C.J.; Ziegler, T.R. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition 2011, 27, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bella, D.L.; Hahn, C.; Stipanuk, M.H. Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am. J. Physiol. 1999, 277, E144–E153. [Google Scholar] [CrossRef] [PubMed]
- Bella, D.L.; Hirschberger, L.L.; Hosokawa, Y.; Stipanuk, M.H. Mechanisms involved in the regulation of key enzymes of cysteine metabolism in rat liver in vivo. Am. J. Physiol. 1999, 276, E326–E335. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, M.J.; Hoffer, L.J. Effect of protein restriction on sulfur amino acid catabolism in insulin-dependent diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E382–E389. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingenbleek, Y. Lean Body Mass Harbors Sensing Mechanisms that Allow Safeguarding of Methionine Homeostasis. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sinha, R.; Richie, J.P., Jr. Disease prevention and delayed aging by dietary sulfur amino acid restriction: Translational implications. Ann. N. Y. Acad. Sci. 2018, 1418, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, E.P.; Greenway, F.L.; Boudreau, A.; Hill, K.L.; Johnson, W.D.; Krajcik, R.A.; Perrone, C.E.; Orentreich, N.; Cefalu, W.T.; Gettys, T.W. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E836–E840. [Google Scholar] [CrossRef] [PubMed]
- Velliquette, R.A.; Gillies, P.J.; Kris-Etherton, P.M.; Green, J.W.; Zhao, G.; Vanden Heuvel, J.P. Regulation of human stearoyl-CoA desaturase by omega-3 and omega-6 fatty acids: Implications for the dietary management of elevated serum triglycerides. J. Clin. Lipidol. 2009, 3, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Riserus, U.; Gustafsson, I.B.; Mohsen, R.; Cederholm, T.; Vessby, B. Effects of saturated and unsaturated fatty acids on estimated desaturase activities during a controlled dietary intervention. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 683–690. [Google Scholar] [CrossRef] [PubMed]
| Cys/Metlow + PUFA | Cys/Methigh + SFA | p | |
|---|---|---|---|
| Female, n | 5 | 5 | - |
| Male, n | 2 | 2 | - |
| Age, y | 31 (20–37) | 24 (21–38) | 0.70 |
| Weight, kg | 66.8 (59.2–83.9) | 65.7 (59.5–80.2) | 0.90 |
| Height, m | 1.71 (1.66–1.89) | 1.73 (1.59–1.84) | 0.75 |
| BMI, kg/m2 | 22.6 (21.1–25.0) | 22.3 (20.7–26.2) | 1.00 |
| Total cholesterol, mmol/L | 4.4 (4.0–4.8) | 3.8 (2.9–5.4) | 0.04 |
| HDL cholesterol, mmol/L | 1.6 (1.3–2.1) | 1.5 (1.2–2.3) | 0.84 |
| LDL cholesterol, mmol/L | 2.5 (2.0–2.6) | 2.0 (1.4–2.9) | 0.60 |
| Triglycerides, mmol/L | 0.7 (0.5–1.0) | 0.8 (0.4–1.0) | 1.00 |
| Amino Acids | Group | Baseline | Day 3 | Day 7 | pint |
|---|---|---|---|---|---|
| Methionine 1 | Cys/Metlow + PUFA | 23.6 (21.2, 26) | 22.4 (20.1, 24.8) | 21.1 (18.7, 23.5) | 0.044 |
| Cys/Methigh + SFA | 22.2 (19.8, 24.6) | 21.8 (19.5, 24.2) | 23 (20.6, 25.3) | ||
| SAM 1 | Cys/Metlow + PUFA | 102 (92.9, 110) | 110 (101, 119) | 110 (101, 119) | 0.27 |
| Cys/Methigh + SFA | 104 (95.3, 113) | 100 (91.8, 109) | 105 (95.9, 113) | ||
| SAH 1 | Cys/Metlow + PUFA | 12.8 (8.8, 16.8) | 14.2 (10.2, 18.2) | 14 (10, 18) | < 0.01 |
| Cys/Methigh + SFA | 15.3 (11.3, 19.3) | 15.2 (11.2, 19.2) | 13.1 (9.14, 17.1) | ||
| SAM/SAH | Cys/Metlow + PUFA | 8.06 (6.56, 9.57) | 7.94 (6.44, 9.45) | 7.98 (6.47, 9.49) | 0.25 |
| Cys/Methigh + SFA | 7.8 (6.29, 9.3) | 7.6 (6.1, 9.11) | 8.42 (6.91, 9.92) | ||
| tHcy 1 | Cys/Metlow + PUFA | 10.1 (8.77, 11.5) | 11.6 (10.3, 13) | 11.2 (9.83, 12.5) | < 0.01 |
| Cys/Methigh + SFA | 6.79 (5.44, 8.14) | 6.44 (5.09, 7.79) | 6.28 (4.93, 7.63) | ||
| Cystathionine 2 | Cys/Metlow + PUFA | 249 (186, 311) | 104 (40.8, 166) | 78.3 (15.5, 141) | 0.041 |
| Cys/Methigh + SFA | 110 (46.7, 172) | 117 (53.8, 179) | 126 (63.4, 189) | ||
| tCys 1 | Cys/Metlow + PUFA | 249 (221, 276) | 268 (240, 295) | 262 (234, 289) | 0.35 |
| Cys/Methigh + SFA | 241 (214, 269) | 251 (223, 278) | 246 (218, 273) | ||
| GSH 1 | Cys/Metlow + PUFA | 5.5 (4.75, 6.25) | 6.29 (5.54, 7.04) | 4.91 (4.16, 5.66) | 0.51 |
| Cys/Methigh + SFA | 5.62 (4.87, 6.37) | 5.96 (5.21, 6.71) | 4.63 (3.88, 5.38) | ||
| Taurine 1 | Cys/Metlow + PUFA | 87.2 (69.2, 105) | 107 (89.1, 125) | 74.5 (56.4, 92.5) | 0.46 |
| Cys/Methigh + SFA | 77.5 (59.5, 95.6) | 82.4 (64.4, 100) | 75.7 (57.6, 93.7) |
| Amino Acids | Group | Baseline | Day 3 | Day 7 | pint |
|---|---|---|---|---|---|
| Free hcy 1 | Cys/Metlow + PUFA | 2.77 (2.47, 3.08) | 3.00 (2.70, 3.31) | 2.95 (2.64, 3.26) | 0.023 |
| Cys/Methigh + SFA | 1.96 (1.66, 2.27) | 1.83 (1.52, 2.14) | 1.89 (1.58, 2.2) | ||
| Protein-bound hcy 1 | Cys/Metlow + PUFA | 7.34 (6.25, 8.43) | 8.61 (7.53, 9.7) | 8.23 (7.14, 9.31) | < 0.01 |
| Cys/Methigh + SFA | 4.82 (3.74, 5.91) | 4.61 (3.52, 5.7) | 4.39 (3.3, 5.48) | ||
| Red hcy 2 | Cys/Metlow + PUFA | 141 (93, 189) | 156 (108, 204) | 184 (134, 234) | 0.29 |
| Cys/Methigh + SFA | 172 (124, 220) | 136 (88.3, 184) | 174 (126, 222) | ||
| Homocystine 2 | Cys/Metlow + PUFA | 22.9 (17.7, 28.2) | 30.3 (25.1, 35.6) | 29.6 (24.1, 35.1) | < 0.01 |
| Cys/Methigh + SFA | 12.8 (7.49, 18) | 11.4 (6.09, 16.6) | 11.3 (6.08, 16.6) | ||
| Reduced hcy/homocystine | Cys/Metlow + PUFA | 6.71 (3.48, 9.95) | 5.5 (2.27, 8.74) | 6.5 (3.12, 9.88) | 0.48 |
| Cys/Methigh + SFA | 14.1 (10.9, 17.4) | 11.8 (8.57, 15) | 15.6 (12.4, 18.8) | ||
| Free cysteine 1 | Cys/Metlow + PUFA | 118 (106, 131) | 121 (108, 134) | 122 (109, 134) | 0.55 |
| Cys/Methigh + SFA | 115 (102, 128) | 116 (103, 129) | 121 (108, 134) | ||
| Protein-bound cysteine 1 | Cys/Metlow + PUFA | 130 (114, 147) | 147 (130, 163) | 140 (124, 157) | 0.21 |
| Cys/Methigh + SFA | 126 (110, 143) | 135 (118, 151) | 125 (108, 141) | ||
| Reduced cysteine 1 | Cys/Metlow + PUFA | 10.1 (8.44, 11.8) | 9.65 (7.96, 11.3) | 10.2 (8.46, 12) | 0.42 |
| Cys/Methigh + SFA | 12.5 (10.8, 14.2) | 11.2 (9.55, 12.9) | 13.7 (12, 15.4) | ||
| Cystine 1 | Cys/Metlow + PUFA | 35.5 (30.8, 40.1) | 37.4 (32.7, 42) | 39 (34.2, 43.8) | 0.06 |
| Cys/Methigh + SFA | 36.1 (31.5, 40.8) | 36.7 (32.1, 41.4) | 35.5 (30.8, 40.2) | ||
| Reduced cysteine/cystine | Cys/Metlow + PUFA | 0.291 (0.24, 0.34) | 0.262 (0.21, 0.31) | 0.265 (0.21, 0.32) | 0.07 |
| Cys/Methigh + SFA | 0.348 (0.30, 0.40) | 0.31 (0.26, 0.36) | 0.389 (0.34, 0.44) |
| Amino Acids, μmol/mmol Creatinine | Baseline | Day 3 | Day 7 | pint | |
|---|---|---|---|---|---|
| Methionine | Cys/Metlow + PUFA | 0.83 (0.63, 1.04) | 0.44 (0.20, 0.67) | 0.38 (0.18, 0.58) | 0.047 |
| Cys/Methigh + SFA | 0.80 (0.60, 1.00) | 0.65 (0.45, 0.85) | 0.73 (0.53, 0.93) | ||
| tHcy | Cys/Metlow + PUFA | 0.31 (0.17, 0.45) | 0.34 (0.18, 0.52) | 0.29 (0.16, 0.43) | 0.316 |
| Cys/Methigh + SFA | 0.22 (0.09, 0.36) | 0.32 (0.19, 0.46) | 0.33 (0.19, 0.46) | ||
| Cystathionine | Cys/Metlow + PUFA | 4.27 (2.72, 5.81) | 0.18 (−1.65, 2.01) | 0.182 (−1.37, 1.73) | 0.043 |
| Cys/Methigh + SFA | 0.86 (−0.68, 2.42) | 0.68 (−0.86, 2.24) | 0.93 (−0.61, 2.48) | ||
| tCys | Cys/Metlow + PUFA | 21.2 (16.2, 26.2) | 18.1 (12.5, 23.6) | 17.9 (12.9, 22.9) | 0.001 |
| Cys/Methigh + SFA | 21.1 (16.1, 26.1) | 30.4 (25.4, 35.4) | 32.2 (27.2, 37.2) | ||
| Glutathione | Cys/Metlow + PUFA | 0.12 (0.06, 0.18) | 0.10 (0.03, 0.17) | 0.09 (0.03, 0.15) | 0.786 |
| Cys/Methigh + SFA | 0.21 (0.15, 0.27) | 0.21 (0.15, 0.27) | 0.17 (0.11, 0.22) | ||
| Taurine | Cys/Metlow + PUFA | 42.6 (23.8, 61.3) | 23.8 (2.28, 45.4) | 8.72 (−10.0, 27.5) | 0.009 |
| Cys/Methigh + SFA | 15.9 (−2.81, 34.7) | 19.9 (1.12, 38.6) | 23.6 (4.82, 42.3) | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olsen, T.; Øvrebø, B.; Turner, C.; Bastani, N.E.; Refsum, H.; Vinknes, K.J. Combining Dietary Sulfur Amino Acid Restriction with Polyunsaturated Fatty Acid Intake in Humans: A Randomized Controlled Pilot Trial. Nutrients 2018, 10, 1822. https://doi.org/10.3390/nu10121822
Olsen T, Øvrebø B, Turner C, Bastani NE, Refsum H, Vinknes KJ. Combining Dietary Sulfur Amino Acid Restriction with Polyunsaturated Fatty Acid Intake in Humans: A Randomized Controlled Pilot Trial. Nutrients. 2018; 10(12):1822. https://doi.org/10.3390/nu10121822
Chicago/Turabian StyleOlsen, Thomas, Bente Øvrebø, Cheryl Turner, Nasser E. Bastani, Helga Refsum, and Kathrine J. Vinknes. 2018. "Combining Dietary Sulfur Amino Acid Restriction with Polyunsaturated Fatty Acid Intake in Humans: A Randomized Controlled Pilot Trial" Nutrients 10, no. 12: 1822. https://doi.org/10.3390/nu10121822
APA StyleOlsen, T., Øvrebø, B., Turner, C., Bastani, N. E., Refsum, H., & Vinknes, K. J. (2018). Combining Dietary Sulfur Amino Acid Restriction with Polyunsaturated Fatty Acid Intake in Humans: A Randomized Controlled Pilot Trial. Nutrients, 10(12), 1822. https://doi.org/10.3390/nu10121822





