Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals and Diets
2.2. Experimental Design
2.3. Lipoprotein Isolation
2.4. Plasma Lipid Analysis and Lipoprotein Composition
2.5. Extraction and Analysis of RNA and Quantification by Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Protein Expression by Western-Blot Analysis
2.7. Statistical Analyses
3. Results
3.1. Feed Consumption, Body Weight, Adipose Tissue Weight, Fecal Excretion, and Diet Digestibility
3.2. Plasma and Liver Lipids and Atherogenic Index
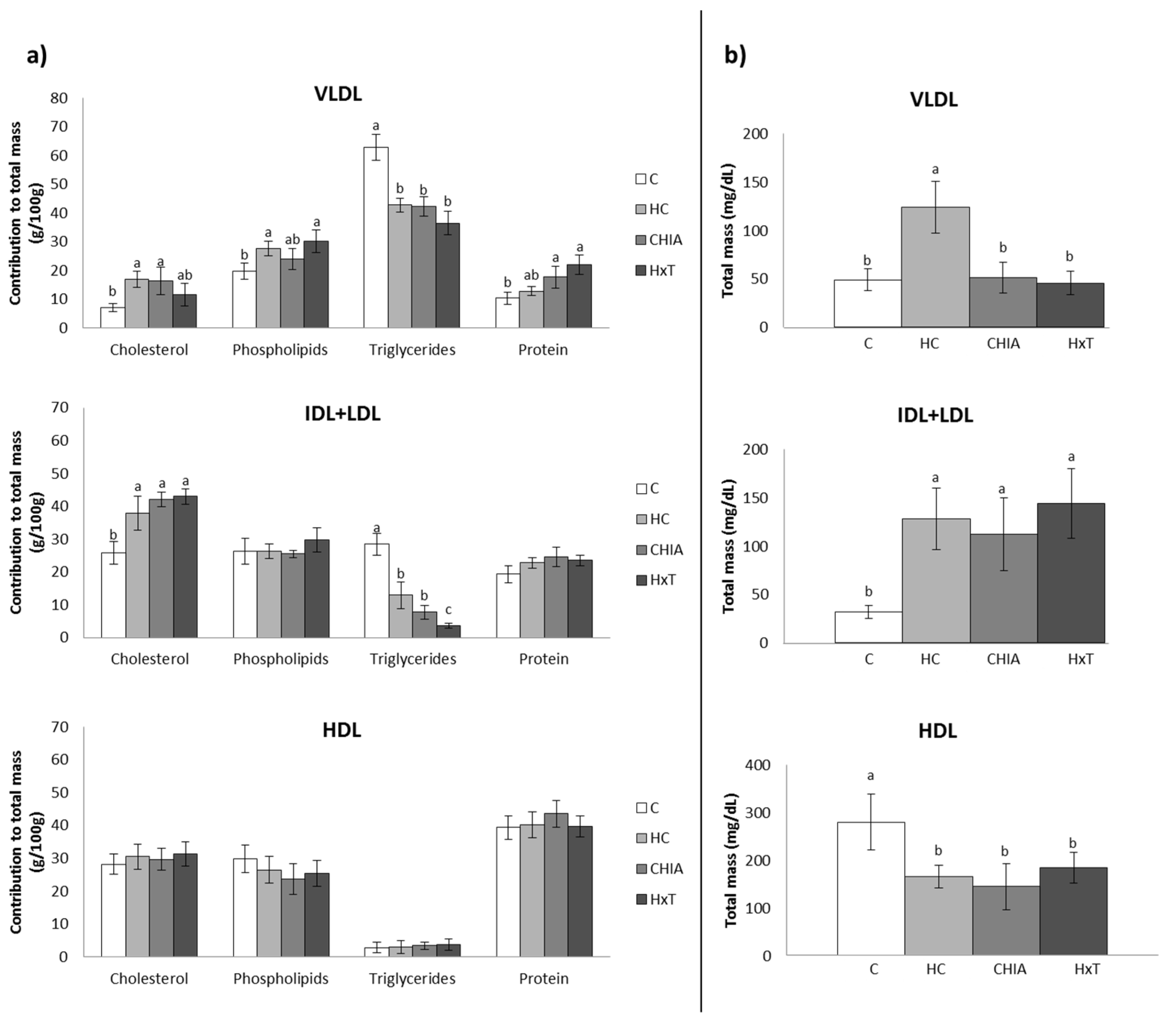
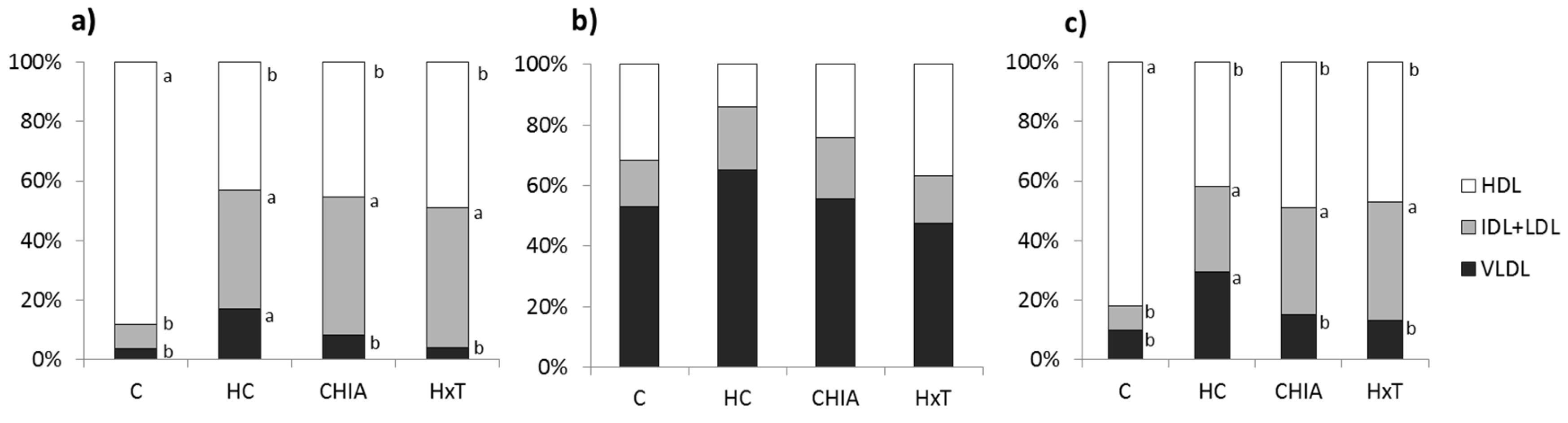
3.3. Plasma Lipoprotein Composition and Profile
3.4. Liver Ldlr Gene and SREBP-1c Protein Expressions
3.5. Pearson Product-Moment Correlation Between Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Celada, P.; Bastida, S.; Sánchez-Muniz, F.J. To eat or not to eat meat. That is the question. Nutr. Hosp. 2016, 33, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Colmenero, F.; Sánchez-Muniz, F.J.; Olmedilla-Alonso, B. Design and development of meat-based functional foods with walnut: Technological, nutritional and health impact. Food Chem. 2010, 123, 959–967. [Google Scholar] [CrossRef] [Green Version]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F.J. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Celada, P.; Olmedilla-Alonso, B.; Delgado-Pando, G.; Raposo, R.; Jimenez-Colmenero, F.; Garcimartin, A.; Sánchez-Muniz, F.J. Coagulation, thrombogenesis, and insulin resistance markers in increased-cardiovascular-risk subjects consuming improved-fat meat products. J. Am. Coll. Nutr. 2018, 95, 919–930. [Google Scholar]
- Schultz, A.R.; Olivero-David, R.; Vázquez-Velasco, M.; González-Torres, L.; Benedí, J.; Bastida, S.; Sánchez-Muniz, F.J. Protective effects of sea spaghetti-enriched restructured pork against dietary cholesterol: Effects on arylesterase and lipoprotein profile and composition of growing rats. J. Med. Food 2014, 17, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Alvarez, S.M.; Illesca, P.; Giménez, M.S.; Lombardo, Y.B. Dietary salba (Salvia hispanica L.) ameliorates the adipose tissue dysfunction of dyslipemic insulin-resistant rats through mechanisms involving oxidative stress, inflammatory cytokines and peroxisome proliferator-activated receptor γ. Eur. J. Nutr. 2018, 57, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Creus, A.; Ferreira, M.R.; Oliva, M.E.; Lombardo, Y.B. Mechanisms involved in the improvement of lipotoxicity and impaired lipid metabolism by dietary alpha-linolenic acid rich Salvia hispanica L. (salba) seed in the heart of dyslipemic insulin-resistant rats. J. Clin. Med. 2016, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Barrera, C.; González-Astorga, M.; Sanhueza, J.; Valenzuela, A. Alpha linolenic acid (ALA) from rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014, 5, 1564–1572. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Effect of dietary α-linolenic fatty acid derived from chia when fed as ground seed, whole seed and oil on lipid content and fatty acid composition of rat plasma. Ann. Nutr. Metab. 2007, 51, 27–34. [Google Scholar] [CrossRef]
- Chicco, A.G.; D’Alessandro, M.E.; Hein, G.J.; Oliva, M.E.; Lombardo, Y.B. Dietary chia seed (Salvia hispanica L.) rich in α-linolenic acid improves adiposity and normalises hypertriacylglycerolaemia and insulin resistance in dyslipaemic rats. Br. J. Nutr. 2009, 101, 41–50. [Google Scholar] [CrossRef]
- Rossi, A.S.; Oliva, M.E.; Ferreira, M.R.; Chicco, A.; Lombardo, Y.B. Dietary chia seed induced changes in hepatic transcription factors and their target lipogenic and oxidative enzyme activities in dyslipidaemic insulin-resistant rats. Br. J. Nutr. 2013, 109, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Pazos, M.; Alonso, A.; Sanchez, I.; Medina, I. Hydroxytyrosol prevents oxidative deterioration in foodstuffs rich in fish lipids. J. Agric. Food Chem. 2008, 56, 3334–3340. [Google Scholar] [CrossRef] [PubMed]
- Cofrades, S.; Santos-López, J.A.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.J.; Jiménez-Colmenero, F. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Santos-López, J.A.; Garcimartín, A.; Merino, P.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Effects of silicon vs. hydroxytyrosol-enriched restructured pork on liver oxidation status of aged rats fed high-saturated/high-cholesterol diets. PLoS ONE 2016, 11, e0147469. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.Y.; Shi, Y. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Rad. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Muniz, F.J.; Bastida, S.; Viejo, J.M.; Terpstra, A.H.M. Small supplements of n-3 fatty acids change serum low density lipoprotein composition by decreasing phospholipid and apolipoprotein B concentrations in young adult women. Eur. J. Nutr. 1999, 38, 20–27. [Google Scholar] [CrossRef]
- Garcimartin, A.; López-Oliva, M.E.; Sántos-López, J.A.; García-Fernández, R.A.; Macho-González, A.; Bastida, J.; Benedí, J.; Sánchez-Muniz, F.J. Silicon alleviates nonalcoholic steatohepatitis by reducing apoptosis in aged Wistar rats fed a high-saturated fat, high-cholesterol diet. J. Nutr. 2017, 147, 1104–1112. [Google Scholar] [CrossRef]
- Santos-López, J.A.; Garcimartín, A.; López-Oliva, M.E.; Bautista-Avila, M.; González-Muñoz, M.J.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Chia oil–enriched restructured pork effects on oxidative and inflammatory status of aged rats fed high cholesterol/high fat diets. J. Med. Food 2017, 20, 526–534. [Google Scholar] [CrossRef]
- Christian, M.S.; Sharper, V.A.; Hoberman, A.M.; Seng, J.E.; Fu, L.; Covell, D.; Crea, R. The toxicity profile of hydrolyzed aqueous olive pulp extract. Drug Chem. Toxicol. 2004, 27, 309–330. [Google Scholar] [CrossRef]
- Lee, O.H.; Lee, B.Y.; Lee, J.; Lee, H.B.; Son, J.Y.; Park, C.S.; Kim, Y.C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Wakame and Nori in restructured meats included in cholesterol-enriched diets affect the antioxidant enzyme gene expressions and activities in Wistar rats. Plant Foods Hum. Nutr. 2010, 65, 290–298. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Velasco, M.; Díaz, L.E.; Lucas, R.; Gómez-Martínez, S.; Bastida, S.; Marcos, A.; Sánchez-Muniz, F.J. Effects of hydroxytyrosol-enriched sunflower oil consumption on CVD risk factors. Br. J. Nutr. 2011, 105, 1448–1452. [Google Scholar] [CrossRef]
- Terpstra, A.H.M.; Sánchez-Muniz, F.J.; West, C.E.; Woodward, C.J.H. The density profile and cholesterol concentration of serum lipoproteins in domestic and laboratory animals. Comp. Biochem. Physiol. B 1982, 71, 669–673. [Google Scholar] [CrossRef]
- Olivero-David, R.; Schultz-Moreira, A.; Vázquez-Velasco, M.; González-Torres, L.; Bastida, S.; Benedí, J.; Sánchez-Reus, M.I.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Effects of Nori-and Wakame-enriched meats with or without supplementary cholesterol on arylesterase activity, lipaemia and lipoproteinaemia in growing Wistar rats. Br. J. Nutr. 2011, 106, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Nus, M.; Sánchez-Montero, J.M.; Sánchez-Muniz, F.J. Effect of seaweed and cholesterol-enriched diets on postprandial lipoproteinaemia in rats. Br. J. Nutr. 2009, 102, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nesic, D.M.; Stevanovic, D.M.; Stankovic, S.D.; Milosevic, V.L.; Trajkovic, V.; Starcevic, V.P.; Severs, W.B. Age-dependent modulation of central ghrelin effects on food intake and lipid metabolism in rats. Eur. J. Pharmacol. 2013, 710, 85–91. [Google Scholar] [CrossRef]
- Chapman, I.M. The anorexia of aging. Clin. Geriatr. Med. 2007, 23, 735–756. [Google Scholar] [CrossRef] [PubMed]
- Beynen, A.C.; Boogaard, A.; Van Laack, H.L.; Katan, M.B. Cholesterol metabolism in two strains of rats with high or low response of serum cholesterol to a cholesterol-rich diet. J. Nutr. 1984, 114, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- González-Torres, L.; Churruca, I.; Schultz-Moreira, A.R.; Bastida, S.; Benedí, J.; Portillo, M.P.; Sánchez-Muniz, F.J. Effects of restructured pork containing Himanthalia elongata on adipose tissue lipogenic and lipolytic enzyme expression of normo- and hypercholesterolemic rats. Lifestyle Genom. 2012, 5, 158–167. [Google Scholar]
- Erdinçler, D.S.; Seven, A.; Inci, F.; Beǧer, T.; Candan, G. Lipid peroxidation and antioxidant status in experimental animals: Effects of aging and hypercholesterolemic diet. Clin. Chim. Acta 1997, 265, 77–84. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; Bastida, S. Do not use the Friedewald formula to calculate LDL-cholesterol in hypercholesterolaemic rats. Eur. J. Lipid Sci. Technol. 2008, 110, 295–301. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Muniz, F.J.; Cava, F.; Viejo, J.M.; Higón, E.; Cuesta, C. Olive oil-and sunflower oil-fried sardines in the prevention of rat hypercholesterolemia. Z. Ernährungswiss 1995, 34, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Pereira-da-Silva, B.; Morais-Dias, D.; Castro-Moreira, M.E.; Lopez-Toledo, R.C.; Pinto-da-Matta, S.L.; Mattos-Della-Lucia, C.; Duarte-Martino, H.S.; Pinheiro-Sant’Ana, H.M. Chia seed shows good protein quality, hypoglycemic effect and improves the lipid profile and liver and intestinal morphology of Wistar rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid. Based Complement. Altern. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef]
- Pallottini, V.; Martini, C.; Cavallini, G.; Donati, A.; Bergamini, E.; Notarnicola, M.; Caruso, M.G.; Trentalance, A. Modified HMG-CoA reductase and LDLr regulation is deeply involved in age-related hypercholesterolemia. J. Cell. Biochem. 2006, 98, 1044–1053. [Google Scholar] [CrossRef]
- Dietschy, J.M. Dietary fatty acids and the regulation of plasma low density lipoprotein cholesterol concentrations. J. Nutr. 1998, 128, 444S–448S. [Google Scholar] [CrossRef] [PubMed]
- Viejo, J.; Bastida, S.; Sanchez-Muniz, F.J.; Garcia-Linares, M.C.; Garcia-Arias, M.T. Effect of olive oil-fried sardine consumption on liver lipid composition and fatty acid cholesterol esterification in hypercholesterolemic rats. Food Sci. Technol. Int. 2003, 9, 329–338. [Google Scholar] [CrossRef]
- Vázquez-Velasco, M.; González-Torres, L.; Olivero-David, R.; Bastida, S.; Benedí, J.; Sánchez-Reus, M.I.; González-Muñoz, M.J.; Sánchez-Muniz, F.J. Lipoproteinemia and arylesterase activity in Zucker fa/fa rats fed glucomannan/spirulina-enriched squid-surimi. Eur. J. Lipid Sci. Technol. 2013, 115, 1274–1283. [Google Scholar] [CrossRef]
- Sánchez-Muniz, F.J.; García-Linares, M.C.; García-Arias, M.T.; Bastida, S.; Viejo, J. Fat and protein from olive-oil-fried sardines interact to normalize serum lipoproteins and reduce liver lipids in hypercholesterolemic rats. J. Nutr. 2003, 133, 2302–2308. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W. Atherogenic hyperlipoproteinemia: The cellular and molecular biology of plasma lipoproteins altered by dietary fat and cholesterol. Med. Clin. North Am. 1982, 66, 375–402. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Z.; Silver, D.L.; Jiang, X.C. Cholesteryl ester transfer protein (CETP) expression enhances HDL cholesteryl ester liver delivery, which is independent of scavenger receptor BI, LDL receptor related protein and possibly LDL receptor. Biochim. Biophys. Acta 2006, 1761, 1482–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayerza, R.; Coates, W. An ω-3 fatty acid enriched chia diet: Influence on egg fatty acid composition, cholesterol and oil content. Can. J. Anim. Sci. 1999, 79, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Poudyal, H.; Panchal, S.K.; Waanders, J.; Ward, L.; Brown, L. Lipid redistribution by α-linolenic acid-rich chia seed inhibits stearoyl-CoA desaturase-1 and induces cardiac and hepatic protection in diet-induced obese rats. J. Nutr. Biochem. 2012, 23, 153–162. [Google Scholar] [CrossRef]

| C | HC | CHIA | HxT | |
|---|---|---|---|---|
| Protein (%) | 21.6 | 21.6 | 21.6 | 21.6 |
| Fat (%) | 16.9 | 16.6 | 16.6 | 16.6 |
| Cholesterol (g/kg) | 0.14 | 16.3 | 16.3 | 16.3 |
| SFA/MUFA/PUFA | 2.36/2.46/1 | 2.36/2.46/1 | 0.27/0.33/1 | 2.36/2.46/1 |
| Energy content, (MJ/kg) 1 | 18.6 | 18.3 | 18.3 | 18.3 |
| Ingredients (g/kg) | ||||
| Cornstarch | 226.1 | 211.0 | 211.0 | 211.0 |
| Casein | 125.6 | 125.6 | 125.6 | 125.6 |
| Maltodextrine | 86.0 | 86.0 | 86.0 | 86.0 |
| Sucrose | 217.8 | 217.8 | 217.8 | 217.8 |
| Soybean oil | 44.0 | 44.0 | 44.0 | 44.0 |
| Cellulose | 31.4 | 31.4 | 31.4 | 31.4 |
| AIN-93MX mineral mix 2 | 44.0 | 44.0 | 44.0 | 44.0 |
| AIN-93VX vitamin mix 3 | 6.3 | 6.3 | 6.3 | 6.3 |
| L-Cystine | 1.9 | 1.9 | 1.9 | 1.9 |
| Cholesterol | 0 | 12.6 | 12.6 | 12.6 |
| Cholic acid | 0 | 2.5 | 2.5 | 2.5 |
| Restructured pork 4 | 217.0 | 217.0 | 217.0 | 217.0 |
| C | HC | CHIA | HxT | ANOVA | |
|---|---|---|---|---|---|
| Initial body weight (g) | 539 ± 21.6 | 510 ± 39.1 | 493 ± 51.68 | 431 ± 34.2 | 0.094 |
| Final body weight (g) | 657 ± 55.1 a | 541 ± 29.4 b | 522 ± 16.3 b | 455 ± 44.2 c | <0.001 |
| Percent of body weight gain (% g/g) | 21.9 ± 3.57 a | 6.08 ± 0.85 b | 5.89 ± 0.32 b | 5.81 ± 0.89 b | <0.001 |
| Adipose tissue weight (g) * | 47.3 ± 9.96 a | 32.8 ± 4.53 b | 26.7 ± 5.38 b | 23.4 ± 5.52 b | <0.001 |
| Adiposomatic index 1 | 7.20 ± 1.08 | 6.06 ± 1.28 | 5.11 ± 0.98 | 5.14 ± 0.88 | 0.096 |
| Feed intake (g/week) | 119 ± 2.45 | 116 ± 7.85 | 120 ± 9.97 | 110 ± 7.79 | 0.058 |
| Cholesterol intake (g/week) | 0.08 ± 0.01 b | 1.6 ± 0.13 a | 1.6 ± 0.13 a | 1.6 ± 0.13 a | <0.001 |
| Fecal excretion (g/week) | 5.22 ± 1.42 b | 8.90 ± 0.72 a | 8.07 ± 1.49 a | 8.43 ± 0.92 a | <0.001 |
| Fecal fat (mg/g dry matter) | 181 ± 66.9 b | 249 ± 42.5 b | 671 ± 37.6 a | 199 ± 31.0 b | <0.001 |
| Fecal cholesterol (mg/g dry matter) | 1.81 ± 0.88 b | 44.5 ± 4.88 a | 40.3 ± 12.12 a | 44.3 ± 3.51 a | <0.001 |
| Dietary digestibility 2 | 0.94 ± 0.01 a | 0.91 ± 0.01 b | 0.92 ± 0.01 a,b | 0.89 ± 0.01 b | <0.001 |
| C | HC | CHIA | HxT | ANOVA | |
|---|---|---|---|---|---|
| Plasma total cholesterol (mmol/L) | 2.52 ± 0.29 b | 3.30 ± 0.29 a,c | 2.66 ± 0.36 b,c | 3.01 ± 0.24 a | <0.001 |
| Plasma triglycerides (mmol/L) | 0.72 ± 0.11 b | 0.98 ± 0.06 a | 0.48 ± 0.06 c | 0.47 ± 0.04 c | <0.001 |
| Plasma phospholipids (mmol/L) | 1.58 ± 0.07 b | 1.69 ± 0.19 a | 1.14 ± 0.18 c | 1.46 ± 0.06 b | <0.001 |
| Plasma total lipids (mg/dL) 1 | 282 ± 28.8 b | 336 ± 22.8 a | 235 ± 27.9 b | 287 ± 9.3 b | <0.001 |
| Plasma free fatty acids (mmol/L) | 0.28 ± 0.06 b | 0.35 ± 0.05 a | 0.15 ± 0.03 c | 0.16 ± 0.05 c | <0.001 |
| Atherogenic index 2 | 0.14 ± 0.13 b | 1.36 ± 0.31 a | 1.42 ± 0.43 a | 1.22 ± 0.41 a | <0.001 |
| Total liver cholesterol (µmol) | 277 ± 69.2 c | 771 ± 108 a | 665 ± 135 a | 456 ± 108 b | <0.001 |
| C | HC | CHIA | HxT | ANOVA | |
|---|---|---|---|---|---|
| VLDL | |||||
| Cholesterol (mmol/L) | 0.09 ± 0.01 b | 0.55 ± 0.21 a | 0.22 ± 0.13 a,b | 0.14 ± 0.09 b | <0.001 |
| Triglycerides (mmol/L) | 0.35 ± 0.09 b | 0.56 ± 0.11 a | 0.25 ± 0.09 b | 0.18 ± 0.04 b | <0.001 |
| Phospholipids (mmol/L) | 0.13 ± 0.03 b | 0.46 ± 0.08 a | 0.16 ± 0.08 b | 0.19 ± 0.11 b | <0.001 |
| Total lipids (mg/dL) 1 | 43.8 ± 9.23 b | 108 ± 25.57 a | 42.6 ± 1.52 b | 35.6 ± 9.61 b | <0.001 |
| Protein (mg/dL) | 5.17 ± 0.21 c | 15. 5 ± 1.44 a | 8.63 ± 1.53 b | 10.0 ± 3.29 b | <0.001 |
| IDL + LDL | |||||
| Cholesterol (mmol/L) | 0.20 ± 0.04 b | 1.30 ± 0.49 a | 1.23 ± 0.27 a | 1.60 ± 0.18 a | <0.001 |
| Triglycerides (mmol/L) | 0.10 ± 0.05 a,b | 0.18 ± 0.04 a | 0.09 ± 0.03 b | 0.06 ± 0.01 b | <0.001 |
| Phospholipids (mmol/L) | 0.11 ± 0.03 b | 0.45 ± 0.11 a | 0.38 ± 0.07 a | 0.58 ± 0.11 a | <0.001 |
| Total lipids (mg/dL) 1 | 25.5 ± 5.99 b | 99.2 ± 26.3 a | 85.0 ± 6.52 a | 110 ± 3.63 a | <0.001 |
| Protein (mg/dL) | 6.11 ± 1.32 b | 28.9 ± 5.53 a | 27.5 ± 5.21 a | 33.8 ± 2.88 a | <0.001 |
| HDL | |||||
| Cholesterol (mmol/L) | 2.2 ± 0.25 a | 1.4 ± 0.31 b | 1.21 ± 0.39 b | 1.62 ± 0.29 b | <0.001 |
| Triglycerides (mmol/L) | 0.21 ± 0.10 | 0.12 ± 0.04 | 0.11 ± 0.06 | 0.14 ± 0.05 | 0.059 |
| Phospholipids (mmol/L) | 1.1 ± 0.13 a | 0.65 ± 0.10 b | 0.52 ± 0.17 b | 0.68 ± 0.09 b | <0.001 |
| Total lipids (mg/dL) 1 | 158 ± 51.8 a | 93.3 ± 17.8 b | 76.7 ± 26.6 b | 105 ± 17.2 b | <0.001 |
| Protein (mg/dL) | 121 ± 37.4 a | 71.7 ± 14.1 b | 68.7 ± 17.4 b | 78.9 ± 17.1 b | 0.016 |
| C | HC | CHIA | HxT | ANOVA | |
|---|---|---|---|---|---|
| Ldlr | 1.00 ± 0.07 a | 0.33 ± 0.15 b | 0.48 ± 0.14 b | 0.44 ± 0.13 b | <0.001 |
| SREBP-1c | 1.00 ± 0.19 b | 1.33 ± 0.14 a | 0.55 ± 0.05 c | 0.65 ± 0.06 c | <0.05 |
| Cholesterol Intake (g/week) | Fecal Fat (mg/g Dry Matter) | Fecal Cholesterol (mg/g Dry Matter) | Plasma Total Cholesterol (mmol/L) | Total Liver Cholesterol (µmol) | Plasma Triglycerides (mmol/L) | Plasma Phospholipids (mmol/L) | Plasma Total Lipids (mg/dL) | Plasma Free Fatty Acids (mmol/L) | Atherogenic Index | Ldlr Gene Expression | SREBP-1c Protein Expression | VLDL Total Mass (mg/dL) | IDL + LDL Total Mass (mg/dL) | HDL Total Mass (mg/dL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percent of body weight gain (% g/g) | −0.503 ** | −0.632 *** | −0.524 ** | 0.607 *** | |||||||||||
| Adipose tissue weight (g) | −0.730 *** | −0.766 *** | 0.525 ** | 0.539 ** | 0.620 *** | −0.593 *** | 0.744 *** | 0.521 ** | −0.675 *** | 0.700 *** | |||||
| Cholesterol intake (g/week) | −0.952 *** | 0.533 ** | 0.676 *** | −0.707 *** | −0.857 *** | −0.870 *** | −0.724 *** | ||||||||
| Fecal fat (mg/g dry matter) | −0.800 *** | −0.732 *** | |||||||||||||
| Fecal cholesterol (mg/g dry matter) | 0.697 *** | −0.872 *** | −0.807 *** | −0.711 *** | |||||||||||
| Plasma total cholesterol (mmol/L) | 0.561 ** | −0.807 *** | |||||||||||||
| Total liver cholesterol (µmol) | -0.629 *** | −0.532 ** | |||||||||||||
| Plasma triglycerides (mmol/L) | 0.657 *** | 0.745 *** | 0.766 *** | 0.761 *** | 0.814 *** | ||||||||||
| Plasma phospholipids (mmol/L) | 0.878 *** | 0.641 ** | 0.712 *** | ||||||||||||
| Plasma total lipids (mg/dL) | 0.648 ** | 0.679 *** | 0.598 ** | ||||||||||||
| Plasma free fatty acids (mmol/L) | 0.762 *** | ||||||||||||||
| Atherogenic index | 0.627 ** | −0.713 *** | |||||||||||||
| SREBP-1c protein expression | 0.697 *** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-López, J.A.; Garcimartín, A.; Bastida, S.; Bautista-Ávila, M.; González-Muñoz, M.J.; Benedí, J.; Sánchez-Muniz, F.J. Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets. Nutrients 2018, 10, 1830. https://doi.org/10.3390/nu10121830
Santos-López JA, Garcimartín A, Bastida S, Bautista-Ávila M, González-Muñoz MJ, Benedí J, Sánchez-Muniz FJ. Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets. Nutrients. 2018; 10(12):1830. https://doi.org/10.3390/nu10121830
Chicago/Turabian StyleSantos-López, Jorge Arturo, Alba Garcimartín, Sara Bastida, Mirandeli Bautista-Ávila, María José González-Muñoz, Juana Benedí, and Francisco José Sánchez-Muniz. 2018. "Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets" Nutrients 10, no. 12: 1830. https://doi.org/10.3390/nu10121830
APA StyleSantos-López, J. A., Garcimartín, A., Bastida, S., Bautista-Ávila, M., González-Muñoz, M. J., Benedí, J., & Sánchez-Muniz, F. J. (2018). Lipoprotein Profile in Aged Rats Fed Chia Oil- or Hydroxytyrosol-Enriched Pork in High Cholesterol/High Saturated Fat Diets. Nutrients, 10(12), 1830. https://doi.org/10.3390/nu10121830






