The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function
Abstract
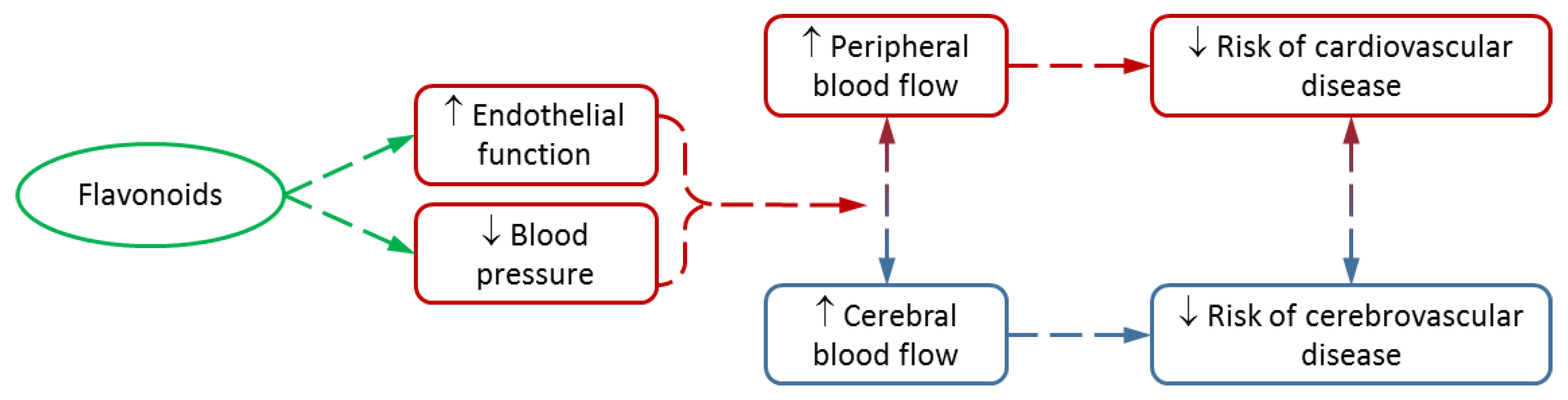
:1. Cardiovascular Health and Flavonoids
2. Epidemiological Evidence
3. Impact of Flavonoid Consumption on Blood Pressure
4. Impact of Flavonoid Consumption on Endothelial Function
5. Impact of Flavonoid Consumption on Cerebral Blood Flow
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation (WHO). Cardiovascular Diseases (CVDs). Available online: http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 5 October 2018).
- O’Brien, J.; Thomas, A. Vascular dementia. Lancet 2015, 386, 1698–1706. [Google Scholar] [CrossRef]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; Dekosky, S.T.; Kuller, L.H. Dementia and alzheimer’s disease incidence in relationship to cardiovascular disease in the cardiovascular health study cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Valderas-Martinez, P.; Chiva-Blanch, G.; Casas, R.; Urpi-Sarda, M.; Lamuela-Raventos, R.M.; Estruch, R. Cardioprotective effects of cocoa: Clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013, 57, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.; Muller, M.; Hornberger, M.; Vauzour, D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Effect of flavonoids on learning, memory and neurocognitive performance: Relevance and potential implications for alzheimer’s disease pathophysiology. J. Sci. Food Agric. 2014, 94, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Beyond antioxidants: The cellular and molecular interactions of flavonoids and how these underpin their actions on the brain. Proc. Nutr. Soc. 2010, 69, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Davey, A.; Alkerwi, A. Cardiovascular health and cognitive function: The maine-syracuse longitudinal study. PLoS ONE 2014, 9, e89317. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Wright, C.B.; Dong, C.; Cheung, K.; DeRosa, J.; Nannery, M.; Stern, Y.; Elkind, M.S.; Sacco, R.L. Ideal cardiovascular health and cognitive aging in the northern manhattan study. J. Am. Heart Assoc. 2016, 5, e002731. [Google Scholar] [CrossRef] [PubMed]
- Samieri, C.; Perier, M.C.; Gaye, B.; Proust-Lima, C.; Helmer, C.; Dartigues, J.F.; Berr, C.; Tzourio, C.; Empana, J.P. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 2018, 320, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.; Boland, L.L.; Mosley, T.; Howard, G.; Liao, D.; Szklo, M.; McGovern, P.; Folsom, A.R. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology 2001, 56, 42–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicario, A.; Cerezo, G.H. At the heart of brain disorders—Preventing cognitive decline and dementia. Eur. Cardiol. Rev. 2015, 10, 60–63. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of us adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of cvd: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; O’Reilly, E.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.P.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Tuomainen, T.P.; Kurl, S.; Salonen, J.T. Flavonoid intake and the risk of ischaemic stroke and cvd mortality in middle-aged finnish men: The kuopio ischaemic heart disease risk factor study. Br. J. Nutr. 2008, 100, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in european and us populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Buijsse, B.; Feskens, E.J.; Kok, F.J.; Kromhout, D. Cocoa intake, blood pressure, and cardiovascular mortality: The zutphen elderly study. Arch. Intern. Med. 2006, 166, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Boekholdt, S.M.; Lentjes, M.A.; Loke, Y.K.; Luben, R.N.; Yeong, J.K.; Wareham, N.J.; Myint, P.K.; Khaw, K.T. Habitual chocolate consumption and risk of cardiovascular disease among healthy men and women. Heart 2015, 101, 1279–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dower, J.I.; Geleijnse, J.M.; Hollman, P.; Soedamah-Muthu, S.S.; Kromhout, D. Dietary epicatechin intake and 25-y risk of cardiovascular mortality: The zutphen elderly study. Am. J. Clin. Nutr. 2016, 104, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Sansone, R.; Rodriguez-Mateos, A.; Heuel, J.; Falk, D.; Schuler, D.; Wagstaff, R.; Kuhnle, G.G.; Spencer, J.P.; Schroeter, H.; Merx, M.W.; et al. Cocoa flavanol intake improves endothelial function and framingham risk score in healthy men and women: A randomised, controlled, double-masked trial: The flaviola health study. Br. J. Nutr. 2015, 114, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Sansone, R.; Karimi, H.; Krabbe, M.; Schuler, D.; Rodriguez-Mateos, A.; Kraemer, T.; Cortese-Krott, M.M.; Kuhnle, G.G.; Spencer, J.P.; et al. Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: A randomized, controlled, double-masked trial. Age (Dordr.) 2015, 37, 9794. [Google Scholar] [CrossRef] [PubMed]
- Faridi, Z.; Njike, V.Y.; Dutta, S.; Ali, A.; Katz, D.L. Acute dark chocolate and cocoa ingestion and endothelial function: A randomized controlled crossover trial. Am. J. Clin. Nut. 2008, 88, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Davison, K.; Berry, N.M.; Misan, G.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Dose-related effects of flavanol-rich cocoa on blood pressure. J. Hum. Hypertens. 2010, 24, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schomig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: The cocoa, cognition, and aging (cocoa) study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The cocoa, cognition, and aging (cocoa) study--a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Massee, L.A.; Ried, K.; Pase, M.; Travica, N.; Yoganathan, J.; Scholey, A.; Macpherson, H.; Kennedy, G.; Sali, A.; Pipingas, A. The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: A randomized, controlled trial. Fron. Pharmacol. 2015, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Kroon, P.A.; Philo, M.; Mensink, M.; Kromhout, D.; Hollman, P.C. Does epicatechin contribute to the acute vascular function effects of dark chocolate? A randomized, crossover study. Mol. Nutr. Food Res. 2016, 60, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.D.; Hollenberg, N.K. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006, 24, 1575–1580. [Google Scholar] [CrossRef] [PubMed]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C. Effects of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind, placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, N.P.; Bondonno, C.P.; Blekkenhorst, L.C.; Considine, M.J.; Maghzal, G.; Stocker, R.; Woodman, R.J.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Flavonoid-rich apple improves endothelial function in individuals at risk for cardiovascular disease: A randomized controlled clinical trial. Mol. Nutr. Food Res. 2017, 62. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Mulder, T.P.; Draijer, R.; Desideri, G.; Molhuizen, H.O.; Ferri, C. Black tea consumption dose-dependently improves flow-mediated dilation in healthy males. J. Hypertens. 2009, 27, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, J.M.; Puddey, I.B.; Woodman, R.J.; Mulder, T.P.; Fuchs, D.; Scott, K.; Croft, K.D. Effects of black tea on blood pressure: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Draijer, R.; Desideri, G.; Mulder, T.; Ferri, C. Black tea lowers blood pressure and wave reflections in fasted and postprandial conditions in hypertensive patients: A randomised study. Nutrients 2015, 7, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Gosmanov, A.R.; Smiley, D.D.; Robalino, G.; Siquiera, J.; Khan, B.; Le, N.A.; Patel, R.S.; Quyyumi, A.A.; Peng, L.; Kitabchi, A.E.; et al. Effects of oral and intravenous fat load on blood pressure, endothelial function, sympathetic activity, and oxidative stress in obese healthy subjects. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E953–E958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgson, J.M.; Burke, V.; Puddey, I.B. Acute effects of tea on fasting and postprandial vascular function and blood pressure in humans. J. Hypertens. 2005, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Barona, J.; Aristizabal, J.C.; Blesso, C.N.; Volek, J.S.; Fernandez, M.L. Grape polyphenols reduce blood pressure and increase flow-mediated vasodilation in men with metabolic syndrome. J. Nutr. 2012, 142, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; Dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Holbrook, M.; Hamburg, N.M.; Shenouda, S.M.; Chung, W.B.; Titas, M.; Kluge, M.A.; Wang, N.; Palmisano, J.; Milbury, P.E.; et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011, 93, 934–940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Dubray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Dong, H.; Saunders, C.; Harkness, L.; Blaze, M.; Hou, Y.; Belanger, R.L.; Corona, G.; Lovegrove, J.A.; Spencer, J.P. Flavanone-rich citrus beverages counteract the transient decline in postprandial endothelial function in humans: A randomised, controlled, double-masked, cross-over intervention study. Br. J. Nutr. 2016, 116, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, V.; Verny, M.A.; Milenkovic, D.; Barber-Chamoux, N.; Mazur, A.; Dubray, C.; Morand, C. Flavanones protect from arterial stiffness in postmenopausal women consuming grapefruit juice for 6 mo: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2015, 102, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Litterio, M.C.; Prince, P.D.; Calabró, V.; Piotrkowski, B.; Galleano, M. Cocoa flavanols: Effects on vascular nitric oxide and blood pressure. J. Clin. Biochem. Nutr. 2011, 48, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Luscher, T.F.; Barton, M. Biology of the endothelium. Clin. Cardiol. 1997, 20, 3–10. [Google Scholar]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The assessment of endothelial function—From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Anderson, T.J. Fundamentals of endothelial function for the clinical cardiologist. Circulation 2002, 105, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Luscher, T.F. Three decades of endothelium research: From the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med. Wkly. 2010, 140, w13122. [Google Scholar] [CrossRef] [PubMed]
- Sitia, S.; Tomasoni, L.; Atzeni, F.; Ambrosio, G.; Cordiano, C.; Catapano, A.; Tramontana, S.; Perticone, F.; Naccarato, P.; Camici, P.; et al. From endothelial dysfunction to atherosclerosis. Autoimmun. Rev. 2010, 9, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Joannides, R.; Haefeli, W.E.; Linder, L.; Richard, V.; Bakkali, E.H.; Thuillez, C.; Luscher, T.F. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation 1995, 91, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, T.H.; Eijsvogels, T.M.; Greyling, A.; Draijer, R.; Hopman, M.T.; Thijssen, D.H. Effect of black tea consumption on brachial artery flow-mediated dilation and ischaemia-reperfusion in humans. Appl. Physiol. Nutr. Metab. 2014, 39, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Drouin, A.; Thorin, E. Flow-induced dilation is mediated by akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke 2009, 40, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; Dawson, E.A.; Groenewoud, H.M.; Jones, H.; Thijssen, D.H. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension 2014, 63, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.E.; Carter, H.H.; Guelfi, K.J.; Smith, K.J.; Pike, K.E.; Naylor, L.H.; Green, D.J. Brachial and cerebrovascular functions are enhanced in postmenopausal women after ingestion of chocolate with a high concentration of cocoa. J. Nutr. 2017, 147, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: A meta-analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Saarenhovi, M.; Salo, P.; Scheinin, M.; Lehto, J.; Lovro, Z.; Tiihonen, K.; Lehtinen, M.J.; Junnila, J.; Hasselwander, O.; Tarpila, A.; et al. The effect of an apple polyphenol extract rich in epicatechin and flavan-3-ol oligomers on brachial artery flow-mediated vasodilatory function in volunteers with elevated blood pressure. Nutr. J. 2017, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Jochmann, N.; Lorenz, M.; Krosigk, A.; Martus, P.; Bohm, V.; Baumann, G.; Stangl, K.; Stangl, V. The efficacy of black tea in ameliorating endothelial function is equivalent to that of green tea. Br. J. Nutr. 2008, 99, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.J.; Keaney, J.F., Jr.; Holbrook, M.; Gokce, N.; Swerdloff, P.L.; Frei, B.; Vita, J.A. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation 2001, 104, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Draijer, R.; Schalkwijk, C.; Desideri, G.; D’Angeli, A.; Francavilla, S.; Mulder, T.; Ferri, C. Black tea increases circulating endothelial progenitor cells and improves flow mediated dilatation counteracting deleterious effects from a fat load in hypertensive patients: A randomized controlled study. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- van Mierlo, L.A.; Zock, P.L.; van der Knaap, H.C.; Draijer, R. Grape polyphenols do not affect vascular function in healthy men. J. Nutr. 2010, 140, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Keevil, J.G.; Wiebe, D.A.; Aeschlimann, S.; Folts, J.D. Purple grape juice improves endothelial function and reduces the susceptibility of ldl cholesterol to oxidation in patients with coronary artery disease. Circulation 1999, 100, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C.; Aliev, G. Inhibition of vascular nitric oxide after rat chronic brain hypoperfusion: Spatial memory and immunocytochemical changes. J. Cereb. Blood Flow Metab. 2005, 25, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Palacios, H.H.; Lipsitt, A.E.; Fischbach, K.; Lamb, B.T.; Obrenovich, M.E.; Morales, L.; Gasimov, E.; Bragin, V. Nitric oxide as an initiator of brain lesions during the development of alzheimer disease. Neurotox. Res. 2009, 16, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. USA 2007, 104, 5638–5643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010, 104 (Suppl. 3), S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Kritchevsky, J.; Hargett, K.; Feller, K.; Klobusnik, R.; Song, B.J.; Cooper, B.; Jouni, Z.; Ferruzzi, M.G.; Janle, E.M. Plasma bioavailability and regional brain distribution of polyphenols from apple/grape seed and bilberry extracts in a young swine model. Mol. Nutr. Food Res. 2015, 59, 2432–2447. [Google Scholar] [CrossRef] [PubMed]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral blood flow and autoregulation: Current measurement techniques and prospects for noninvasive optical methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grolimund, P.; Seiler, R.W. Age dependence of the flow velocity in the basal cerebral arteries—A transcranial doppler ultrasound study. Ultrasound. Med. Biol. 1988, 14, 191–198. [Google Scholar] [CrossRef]
- Dai, W.; Lopez, O.L.; Carmichael, O.T.; Becker, J.T.; Kuller, L.H.; Michael Gach, H. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 2008, 39, 349–354. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc. Psychiatry Neurol. 2012, 2012, 367516–367531. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dysfunction in the pathogenesis of alzheimer’s disease--a review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.T.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fmri response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47 (Suppl. 2), S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Dodd, G.F. The acute effects of flavonoid-rich blueberries on cognitive function in healthy younger and older adults. Ph.D. Thesis, University of Reading, Reading, UK, March 2012. [Google Scholar]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effects of flavanone-rich citrus juice on cognitive function and cerebral blood flow: An acute, randomised, placebo-controlled cross-over trial in healthy, young adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Dye, L.; Wightman, J.D.; Lawton, C.L. The effects of flavonoid and other polyphenol consumption on cognitive performance: A systematic research review of human experimental and epidemiological studies. Nutr. Ageing 2012, 1, 5–25. [Google Scholar]
- Lamport, D.J.; Pal, D.; Moutsiana, C.; Field, D.T.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The effect of flavanol-rich cocoa on cerebral perfusion in healthy older adults during conscious resting state: A placebo controlled, crossover, acute trial. Psychopharmacology 2015, 232, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Hollenberg, N.K.; Panych, L.P.; Fisher, N.D. Brain blood flow and velocity: Correlations between magnetic resonance imaging and transcranial doppler sonography. J. Ultrasound. Med. 2010, 29, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Brickman, A.M.; Khan, U.A.; Provenzano, F.A.; Yeung, L.K.; Suzuki, W.; Schroeter, H.; Wall, M.; Sloan, R.P.; Small, S.A. Enhancing dentate gyrus function with dietary flavanols improves cognition in older adults. Nat. Neurosci. 2014, 17, 1798–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.; Adlam, A.R.; Fulford, J. Enhanced task related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Haskell, C.F.; Forster, J.S.; Veasey, R.C.; Kennedy, D.O. Epigallocatechin gallate, cerebral blood flow parameters, cognitive performance and mood in healthy humans: A double-blind, placebo-controlled, crossover investigation. Hum. Psychopharmacol. 2012, 27, 177–186. [Google Scholar] [CrossRef] [PubMed]
| Author | Flavonoid Source and Dose | Duration | Sample | Effects |
|---|---|---|---|---|
| Sansone et al. (2015) [32] | Cocoa 900 mg flavanols | 1 month | Healthy subjects (n = 100) | −4.4 mmHg SBP, −3.9 mmHg DBP |
| Grassi et al. (2015) [33] | Cocoa 80, 200, 500, 800 mg flavonoids | 1 week | Healthy subjects (n = 20) | −4.8 mmHg SBP, −3 mmHg DBP |
| Heiss et al. (2015) [34] | Cocoa 450 mg flavanols twice/daily | Acute and 2 weeks | Healthy younger (aged <35 year, n = 22) and older males (aged 50−80 year, n = 20) | −5 mmHg SBP (acute) and −6 mmHg SBP (chronic) in older group |
| Faridi et al. (2008) [35] | Dark chocolate 821 mg flavanols | Acute | Overweight subjects (n = 45) | −3.2 mmHg SBP, −1.4 mmHg DBP |
| Cocoa 805 mg flavanols (sugar-free), 605 mg flavanols (sugared) | Acute | Overweight subjects (n = 45) | −2.1 mmHg SBP, −1.2 mmHg DBP; no effect of sugared cocoa | |
| Davison et al. (2010) [36] | Cocoa 33, 372, 712, 1052 mg flavanols | 6 weeks | Mildly hypertensive subjects (n = 52) | −5.3 mmHg SBP, −3 mmHg DBP at highest dose, no other effects |
| Taubert et al. (2007) [37] | Cocoa 30 mg total polyphenols | 18 weeks | Mildly hypertensive subjects (n = 44) | −2.9 mmHg SBP, −1.9 mmHg DBP |
| Desideri et al. (2012) [38] | Cocoa 48, 520, 993 mg flavanols | 8 weeks | Elderly subjects with MCI (n = 90) | −10 mmHg SBP, −4.8 mmHg DBP |
| Mastroiacovo et al. (2015) [39] | Cocoa 48, 520, 993 mg flavanols | 8 weeks | Elderly subjects (n = 90) | −7.8 mmHg SBP, −4.8 mmHg DBP |
| Massee et al. (2015) [40] | Cocoa 250 mg polyphenols | Acute and 4 weeks | Healthy subjects (n = 40) | No significant effect |
| Engler et al. (2004) [41] | Cocoa 213 mg procyanidins, 48 mg epicatechin | 2 weeks | Healthy subjects (n = 22) | No significant effect |
| Dower et al. (2016) [42] | Dark chocolate 150 mg epicatechin, 100 mg pure epicatechin with white chocolate | Acute | Healthy males (n = 20) | No significant effect |
| Fisher and Hollenberg (2006) [43] | Cocoa 821 mg flavanols | 4−6 days | Healthy younger (aged<50 year, n = 15), and older subjects (>50 year, n = 19) | No significant effect |
| Dower et al. (2015) [44] | Pure epicatechin 100 mg | 4 weeks | Healthy subjects (n = 37) | No significant effect |
| Bondonno et al. (2012) [45] | Apple 180 mg epicatechin, 184 mg quercetin | Acute | Healthy subjects (n = 30) | −3.3 mmHg SBP, no significant effect on DBP |
| Bondonno et al. (2017) [46] | Apple 48 mg epicatechin, 306 mg total polyphenols | Acute and 4 weeks | Subjects at risk of CVD (n = 30) | No significant effect |
| Grassi et al. (2009) [47] | Black tea 100, 200, 400, 800 mg flavonoids | 1 week | Healthy males (n = 19) | −2.6 mmHg SBP, −2.2 mmHg DBP |
| Hodgson et al. (2012) [48] | Black tea 429 mg total polyphenols | 6 months | Healthy to mildly hypertensive subjects (n = 95) | −2.7 mmHg SBP, −2.3 mmHg DBP |
| Grassi et al. (2015) [49] | Black tea 258 mg flavonoids | 1 week | Hypertensive subjects (n = 19) | −3.2 mmHg SBP, −2.6 mmHg DBP |
| Barona et al. (2012) [52] | Grape 35 mg anthocyanins, 267 mg total polyphenols | 1 month | Subjects with metabolic syndrome (n = 24) | −6 mmHg SBP, no significant effect on DBP |
| Rodriguez-Mateos et al. (2013) [53] | Blueberry 766, 1278, 1791 mg polyphenols | Acute | Healthy males (n = 10) | No significant effect |
| Rodrigues-Mateos et al. (2016) [54] | Cranberry 409, 787, 1238, 1534, 1910 mg total polyphenols | Acute | Healthy males (n = 10) | No significant effect |
| Dohadwala et al. (2011) [55] | Cranberry 94 mg anthocyanins, 835 mg total polyphenols | 4 weeks | Subjects with CAD (n = 44) | No significant effect |
| Curtis et al. (2009) [56] | Elderberry 500 mg anthocyanins | 12 weeks | Postmenopausal women (n = 52) | No significant effect |
| Morand et al. (2011) [57] | Orange juice 292 mg hesperidin | 4 weeks | Overweight males (n = 24) | −5.5 mmHg DBP, no significant effect on SBP |
| Rendeiro et al. (2016) [58] | Orange juice 128, 272, 452 mg total flavonoids | Acute | Healthy males (n = 28) | No significant effect |
| Habauzit et al. (2015) [59] | Grapefruit 210 mg naringenin | 6 months | Postmenopausal women (n = 48) | No significant effect |
| Study | Flavonoid Source and Dose | Duration | Sample | Effects |
|---|---|---|---|---|
| Faridi et al. (2008) [35] | Dark chocolate 821 mg flavanols | Acute | Overweight subjects (n = 45) | 4.3% increase in FMD |
| Marsh et al. (2017) [73] | Chocolate 395 mg (dark), 200 mg (milk) total polyphenols | Acute | Postmenopausal women (n = 12) | 2.4% increase in FMD following dark chocolate, no significant effect of milk chocolate |
| Schroeter et al. (2006) [72] | Cocoa 917 mg flavanols | Acute | Healthy subjects (n = 10) | Increase in FMD |
| Pure epicatechin 1 mg/kg, 2 mg/kg body weight | Acute | Healthy subjects (n = 3) | Increase in FMD | |
| Dower et al. (2015) [44] | Pure epicatechin 100 mg | 4 weeks | Healthy subjects (n = 37) | No significant effect |
| Dower et al. (2016) [42] | Dark chocolate 150 mg epicatechin, 100 mg pure epicatechin with white chocolate | Acute | Healthy males (n = 20) | 0.96% increase in FMD, no significant effect of pure epicatechin |
| Engler et al. (2004) [41] | Chocolate 259 mg total flavonoids | 2 weeks | Healthy subjects (n = 22) | 1.3% increase in FMD |
| Sansone et al. (2015) [32] | Cocoa 900 mg flavanols | 1 month | Healthy subjects (n = 100) | 1.2% increase in FMD |
| Fisher and Hollenberg (2006) [43] | Cocoa 821 mg flavanols | 4–6 days | Healthy younger (aged <50 year, n = 15), and older subjects (>50 year, n = 19) | 3.5% (younger) and 4.5% increase in FMD (older) |
| Bondonno et al. (2012) [45] | Apples 180 mg epicatechin, 184 mg quercetin | Acute | Healthy subjects (n = 30) | 1.1% increase in FMD |
| Bondonno et al. (2017) [46] | Apples 48 mg epicatechin, 306 mg total polyphenols | Acute and 4 weeks | Subjects at risk of CVD (n = 30) | 0.8% (acute) and 0.5% (chronic) increase in FMD |
| Saarenhovi et al. (2017) [76] | Apple 100 mg epicatechin | Acute and 4 weeks | Borderline hypertensive subjects (n = 60) | No significant effect |
| Grassi et al. (2009) [47] | Black tea 100, 200, 400, 800 mg flavonoids | 1 week | Healthy males (n = 19) | 2.5% increase in FMD |
| Schreuder et al. (2014) [69] | Black tea 1800 mg total polyphenols | Acute and 1 week | Healthy subjects (n = 20) | 1.4% increase in FMD |
| Jochmann et al. (2008) [77] | Black and green tea 560 mg (black), 1012 mg (green) total catechins | Acute | Postmenopausal women (n = 24) | 4.4% (black) and 5% (green) increase in FMD |
| Duffy et al. (2001) [77] | Black tea 964 mg total flavonoids | Acute and 4 weeks | Subjects with CAD (n = 50) | 4.8% increase in FMD (acute-on-chronic) |
| Grassi et al. (2016) [79] | Black tea 150 mg polyphenols twice/day | Acute and 8 days | Hypertensive subjects (n = 19) | 1% (acute) and 1.8% (chronic) increase in FMD |
| van Mierlo et al. (2010) [81] | Wine and grape seed 800 mg total polyphenols | 3 weeks | Healthy males (n = 35) | No significant effect |
| Barona et al. (2012) [52] | Grape 35 mg anthocyanins, 267 mg total polyphenols | 1 month | Subjects with metabolic syndrome (n = 24) | 1.7% increase in FMD |
| Stein et al. (1999) [82] | Grape | 14 days | Subjects with CAD (n = 15) | 4.2% increase in FMD |
| Rodriguez-Mateos et al. (2013) [53] | Blueberry 766, 1278, 1791 mg polyphenols | Acute | Healthy males (n = 10) | 2.4% increase in FMD |
| Rodriguez-Mateos et al. (2016) [54] | Cranberry 409, 787, 1238, 1534, 1910 mg total polyphenols | Acute | Healthy males (n = 10) | 2.6% increase in FMD |
| Dohadwala et al. (2011) [55] | Cranberry 835 mg total polyphenols | 4 weeks | Subjects with CAD (n = 44) | No significant effect |
| Rendeiro et al. (2016) [58] | Orange 128, 272, 452 mg total flavonoids | Acute | Healthy males (n = 28) | Recovery in % FMD to baseline levels following a high fat meal |
| Rizza et al. (2011) [83] | Hesperidin 500 mg hesperidin | 3 weeks | Subjects with metabolic syndrome (n = 24) | 2.5% increase in FMD |
| Habauzit et al. (2015) [59] | Grapefruit 210 mg naringenin | 6 months | Postmenopausal women (n = 48) | No significant effect |
| Study | Flavonoid Source and Dose | Duration | Sample | Effects |
|---|---|---|---|---|
| Francis et al. (2006) [98] | Cocoa 516 mg flavanols | Acute | Healthy adults (aged 24–31 years, n = 4) | Increase in CBF across grey matter |
| Lamport et al. (2015) [102] | Cocoa 494 mg flavanols | Acute | Healthy older adults (aged 50–65 years, n = 18) | Increase in regional perfusion (anterior cingulate cortex, central opercular cortex) |
| Sorond et al. (2008) [104] | Cocoa 450 mg flavanols | 1 week | Healthy older adults (aged 59–83 years, n = 21) | Increase in cerebral blood flow velocity |
| Brickman et al. (2014) [105] | Cocoa 900 mg flavanols | 3 months | Healthy older adults (aged 50–69 years, n = 41) | Increase in cerebral blood volume in the dentate gyrus |
| Marsh et al. (2017) [73] | Chocolate 395 mg (dark), 200 mg (milk) total polyphenols | Acute | Postmenopausal women (n = 12) | Reduction in cerebral blood flow velocity with both dark and milk chocolate |
| Massee et al. (2015) [40] | Cocoa 250 mg catechin polyphenols | Acute and 4 weeks | Healthy younger adults (aged 18–40 years, n = 40) | No significant effect |
| Dodd et al. (2012) [99] | Blueberry 579 mg flavonoids | Acute | Healthy younger adults (aged 18–25 years, n = 19) | Increase in regional perfusion (occipital cortex, frontal lobe, angular gyrus) |
| Bowtell et al. (2017) [106] | Blueberry 387 mg anthocyanins | 12 weeks | Healthy older adults (aged >65 year, n = 26) | Increase in regional perfusion (parietal lobe, occipital lobe) |
| Lamport et al. (2016) [100] | Citrus 70.5 mg flavanones | Acute | Healthy young subjects (aged 18–30 years, n = 24) | Increase in regional perfusion (inferior and middle right frontal gyrus) |
| Wightman et al. (2012) [107] | EGCG 135 mg, 270 mg | Acute | Healthy adults (aged 18–30 years, n = 27) | Reduction in CBF to frontal cortex (135 mg), no effect of 270 mg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. https://doi.org/10.3390/nu10121852
Rees A, Dodd GF, Spencer JPE. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients. 2018; 10(12):1852. https://doi.org/10.3390/nu10121852
Chicago/Turabian StyleRees, Amy, Georgina F. Dodd, and Jeremy P. E. Spencer. 2018. "The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function" Nutrients 10, no. 12: 1852. https://doi.org/10.3390/nu10121852




