Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study
Abstract
:1. Introduction
2. Methods
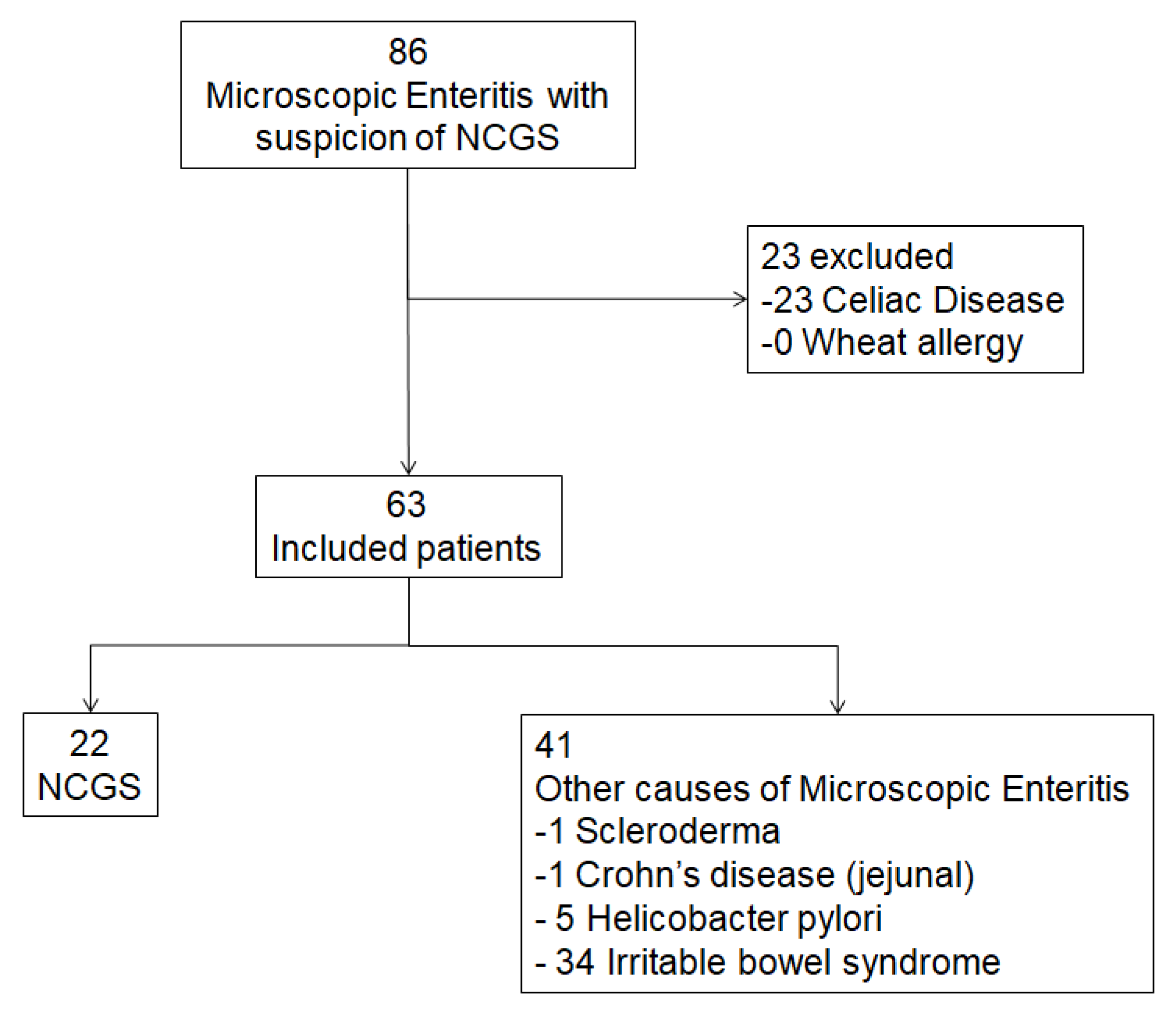
2.1. Patients Selection
2.2. Histology and Immunohistochemistry
2.3. Follow Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictive Factors for NCGS Onset
3.3. Autoimmunity and HLA Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fasano, A.; Sapone, A.; Zevallos, V.; Schuppan, D. Nonceliac gluten sensitivity. Gastroenterology 2015, 148, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Golley, S.; Corsini, N.; Topping, D.; Morell, M.; Mohr, P. Motivations for avoiding wheat consumption in Australia: Results from a population survey. Public Health Nutr. 2015, 18, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Verdu, E.F.; Armstrong, D.; Murray, J.A. Between celiac disease and irritable bowel syndrome: The “no man’s land” of gluten sensitivity. Am. J. Gastroenterol. 2009, 104, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.L.; Biesiekierski, J.R.; Yelland, G.W.; Muir, J.G.; Gibson, P.R. Randomised clinical trial: Gluten may cause depression in subjects with non-coeliac gluten sensitivity—An exploratory clinical study. Aliment. Pharmacol. Ther. 2014, 39, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The salerno experts’ criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Tomba, C.; Branchi, F.; Roncoroni, L.; Lombardo, V.; Bardella, M.T.; Ferretti, F.; Conte, D.; Valiante, F.; Fini, L.; et al. Evidence for the presence of Non-Celiac Gluten Sensitivity in patients with functional gastrointestinal symptoms: Results from a multicenter randomized double-blind placebo-controlled gluten challenge. Nutrients 2016, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Lanzini, A.; Lanzarotto, F.; Ricci, C. Observations on the paper of Carroccio et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: Exploring a new clinical entity. Am. J. Gastroenterol. 2013, 108, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; Tripodo, C.; Florena, A.M. Response to Villanacci et al. Am. J. Gastroenterol. 2013, 108, 620. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Piscitelli, D.; Pezzuto, F.; Fortarezza, F.; Covelli, C.; Marra, A.; Iannone, A.; Amoruso, A.; Principi, M.; Ierardi, E.; et al. T Helper Lymphocyte and Mast Cell Immunohistochemical Pattern in Nonceliac Gluten Sensitivity. Gastroenterol. Res. Pract. 2017, 2017, 5023680. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Piscitelli, D.; Di Leo, A. Novel steps forward in the histopathology of non-celiac gluten sensitivity. Virchows Arch. 2018, 473, 525. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; Iannone, A.; Piscitelli, D.; Amoruso, A.; Barone, M.; Principi, M.; Pisani, A.; Di Leo, A. Lymphocytic duodenitis or microscopic enteritis and gluten-related conditions: What needs to be explored? Ann. Gastroenterol. 2017, 30, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Lammers, K.M.; Casolaro, V.; Cammarota, M.; Giuliano, M.T.; De Rosa, M.; Stefanile, R.; Mazzarella, G.; Tolone, C.; Russo, M.I.; et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: Celiac disease and gluten sensitivity. BMC Med. 2011, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Lammers, K.M.; Mazzarella, G.; Mikhailenko, I.; Cartenì, M.; Casolaro, V.; Fasano, A. Differential mucosal IL-17 expression in two gliadin-induced disorders: Gluten sensitivity and the autoimmune enteropathy celiac disease. Int. Arch. Allergy Immunol. 2010, 152, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Uhde, M.; Ajamian, M.; Caio, G.; De Giorgio, R.; Indart, A.; Green, P.H.; Verna, E.C.; Volta, U.; Alaedini, A. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut 2016, 65, 1930–1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroccio, A.; D’Alcamo, A.; Cavataio, F.; Soresi, M.; Seidita, A.; Sciumè, C.; Geraci, G.; Iacono, G.; Mansueto, P. High Proportions of People with Nonceliac Wheat Sensitivity Have Autoimmune Disease or Antinuclear Antibodies. Gastroenterology 2015, 149, 596–603.e1. [Google Scholar] [CrossRef] [PubMed]
- Rostami, K.; Aldulaimi, D.; Holmes, G.; Johnson, M.W.; Robert, M.; Srivastava, A.; Fléjou, J.F.; Sanders, D.S.; Volta, U.; Derakhshan, M.H.; et al. Microscopic enteritis: Bucharest consensus. World J. Gastroenterol. 2015, 21, 2593–2604. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.M.; Kelly, A.B.; Jewell, S.D.; McShane, L.M.; Clark, D.P.; Greenspan, R.; Hayes, D.F.; Hainaut, P.; Kim, P.; Mansfield, E.; et al. Biospecimen reporting for improved study quality (BRISQ). Cancer Cytopathol. 2011, 119, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Piscitelli, D.; Giangaspero, A.; Principi, M.; Buffelli, F.; Giorgio, F.; Montenegro, L.; Sorrentino, C.; Amoruso, A.; Ierardi, E.; et al. Evolution of nonspecific duodenal lymphocytosis over 2 years of follow-up. World J. Gastroenterol. 2015, 21, 7545–7552. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.; Hadjivassiliou, M.; Holdoway, A.; Van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Quigley, E.M. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 2014, 109 (Suppl. 1), S2–S26. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Amoruso, A.; Giorgio, F.; Principi, M.; Losurdo, G.; Piscitelli, D.; Buffelli, F.; Fiore, M.G.; Mongelli, A.; Castellaneta, N.M.; et al. Mucosal molecular pattern of tissue transglutaminase and interferon gamma in suspected seronegative celiac disease at marsh 1 and 0 stages. Saudi J. Gastroenterol. 2015, 21, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; Piscitelli, D.; Giorgio, F.; Sorrentino, C.; Principi, M.; Montenegro, L.; Amoruso, A.; Di Leo, A. Seronegative celiac disease: Where is the specific setting? Gastroenterol. Hepatol. Bed Bench 2015, 8, 110–116. [Google Scholar] [PubMed]
- Aziz, I.; Sanders, D.S. The irritable bowel syndrome-celiac disease connection. Gastrointest. Endosc. Clin. N. Am. 2012, 22, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.P.; Tosco, A.; Izzo, V.; Tucci, F.; Troncone, R.; Auricchio, R.; Romanos, J.; Trynka, G.; Auricchio, S.; Jabri, B.; et al. Potential celiac patients: A model of celiac disease pathogenesis. PLoS ONE 2011, 6, e21281. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, F.; Principi, M.; Losurdo, G.; Piscitelli, D.; Iannone, A.; Barone, M.; Amoruso, A.; Ierardi, E.; Di Leo, A. Seronegative Celiac Disease and Immunoglobulin Deficiency: Where to Look in the Submerged Iceberg? Nutrients 2015, 7, 7486–7504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montenegro, L.; Piscitelli, D.; Giorgio, F.; Covelli, C.; Fiore, M.G.; Losurdo, G.; Iannone, A.; Ierardi, E.; Di Leo, A.; Principi, M. Reversal of IgM deficiency following a gluten-free diet in seronegative celiac disease. World J. Gastroenterol. 2014, 20, 17686–17689. [Google Scholar] [CrossRef] [PubMed]
- Carroccio, A.; Mansueto, P.; D’alcamo, A.; Iacono, G. Non-celiac wheat sensitivity as an allergic condition: Personal experience and narrative review. Am. J. Gastroenterol. 2013, 108, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.; Rajaguru Paramaguru, D.A.; Augustine, P. Dermatitis Herpetiformis as the Initial Presentation of Primary Biliary Cholangitis in a Male with Gluten Sensitivity. Cureus 2017, 9, e1247. [Google Scholar] [CrossRef] [PubMed]
- Bonciolini, V.; Bianchi, B.; Del Bianco, E.; Verdelli, A.; Caproni, M. Cutaneous Manifestations of Non-Celiac Gluten Sensitivity: Clinical Histological and Immunopathological Features. Nutrients 2015, 7, 7798–7805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isasi, C.; Tejerina, E.; Morán, L.M. Non-celiac gluten sensitivity and rheumatic diseases. Reumatol. Clin. 2016, 12, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Lanzarotto, F.; Villanacci, V.; Carabellese, N.; Ricci, C.; Lanzini, A. Clinical expression of lymphocytic duodenosis in “mild enteropathy” celiac disease and in functional gastrointestinal syndromes. Scand. J. Gastroenterol. 2014, 49, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Di Leo, A.; Ierardi, E. Letter: Helicobacter-negative gastritis—A distinct condition? Aliment. Pharmacol. Ther. 2015, 41, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Bibbò, S.; Bruno, G.; Ricci, R.; Arena, V.; Gasbarrini, A.; Cammarota, G. Prior Misdiagnosis of Celiac Disease Is Common among Patients Referred to a Tertiary Care Center: A Prospective Cohort Study. Clin. Transl. Gastroenterol. 2016, 7, e139. [Google Scholar] [CrossRef] [PubMed]
- Bibbò, S.; Pes, G.M.; Usai-Satta, P.; Salis, R.; Soro, S.; Colosso, B.M.; Dore, M.P. Chronic autoimmune disorders are increased in coeliac disease: A case-control study. Medicine (Baltimore) 2017, 96, e8562. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H.J. Endocrine manifestations in celiac disease. World J. Gastroenterol. 2016, 22, 8472–8479. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Jeremias, P.; Matthias, T. Gut-thyroid axis and celiac disease. Endocr. Connect. 2017, 6, R52–R58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, A.; Aminov, R.; Matthias, T. Transglutaminases in dysbiosis as potential environmental drivers of autoimmunity. Front. Microbiol. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Ierardi, E.; Di Leo, A. The interaction between celiac disease and intestinal microbiota. J. Clin. Gastroenterol. 2016, 50 (Suppl. 2), S145–S147. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Leone, M.C.; Casato, M.; Nicoli, M.; Granata, G.; Carlesimo, M. High prevalence of gluten sensitivity in a cohort of patients with undifferentiated connective tissue disease. Eur. Ann. Allergy Clin. Immunol. 2015, 47, 54–57. [Google Scholar] [PubMed]
- Volta, U.; Tovoli, F.; Cicola, R.; Parisi, C.; Fabbri, A.; Piscaglia, M.; Fiorini, E.; Caio, G. Serological tests in gluten sensitivity (nonceliac gluten intolerance). J. Clin. Gastroenterol. 2012, 46, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Giorgio, F.; Piscitelli, D.; Montenegro, L.; Covelli, C.; Fiore, M.G.; Giangaspero, A.; Iannone, A.; Principi, M.; Amoruso, A.; et al. May the assessment of baseline mucosal molecular pattern predict the development of gluten related disorders among microscopic enteritis? World J. Gastroenterol. 2016, 22, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Smigoc Schweiger, D.; Mendez, A.; Kunilo Jamnik, S.; Bratanic, N.; Bratina, N.; Battelino, T.; Brecelj, J.; Vidan-Jeras, B. High-risk genotypes HLA-DR3-DQ2/DR3-DQ2 and DR3-DQ2/DR4-DQ8 in co-occurrence of type 1 diabetes and celiac disease. Autoimmunity 2016, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Spadaccino, A.C.; Basso, D.; Chiarelli, S.; Albergoni, M.P.; D’Odorico, A.; Plebani, M.; Pedini, B.; Lazzarotto, F.; Betterle, C. Celiac disease in North Italian patients with autoimmune thyroid diseases. Autoimmunity 2008, 41, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Volta, U.; Bardella, M.T.; Calabrò, A.; Troncone, R.; Corazza, G.R. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC Med. 2014, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Macchia, D.; Pagliai, G.; Gori, A.M.; Cesari, F.; Marcucci, R.; Sofi, F.; Casini, A. Symptomatic efficacy of buckwheat products in Non-Celiac Gluten Sensitivity (NCGS). Asia Pac. J. Clin. Nutr. 2017, 26, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, P.; Alberti, A.; Baldi, A.; Coppola, F.; Rossi, C.; Pierguidi, L.; Floridi, A.; Calabresi, P. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006, 46, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Green, P.H.; Alaedini, A.; Sander, H.W.; Brannagan, T.; Latov, N.; Chin, R.L. Mechanisms underlying celiac disease and its neurologic manifestations. Cell. Mol. Life Sci. 2005, 62, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, E.; Losurdo, G.; Piscitelli, D.; Giorgio, F.; Amoruso, A.; Iannone, A.; Principi, M.; Di Leo, A. Biological markers for non celiac gluten sensitivity: A question awaiting for a convincing answer. Gastroenterol. Hepatol. Bed Bench 2018, 11, 203–208. [Google Scholar] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]



| Non Gluten-Related Microscopic Enteritis (n = 41) | NCGS (n = 22) | p Value | |
|---|---|---|---|
| Age (mean ± standard deviation) | 36.0 ± 13.3 | 30.6 ± 8.7 | 0.09 |
| Female sex, n (%) | 31 (75.6) | 19 (86.4) | 0.51 |
Marsh classification
| 24 (58.5) 17 (41.5) | 7 (31.8) 15 (68.2) | 0.07 |
| AGA, n (%) | 1 (2.5) | 6 (27.3) | 0.006 |
| ANA, n (%) | 5 (12.2) | 10 (45.4) | 0.005 |
| Familiarity for celiac disease, n (%) | 0 (0) | 2 (9.1) | 0.12 |
| Autoimmune thyroiditis, n (%) | 6 (14.6) | 9 (40.1) | 0.03 |
| Other autoimmune diseases, n (%) | 2 (4.9) | 2 (9.1) | 0.61 |
| Anemia, n (%) | 7 (17.1) | 3 (13.6) | 0.70 |
| Folate deficit, n (%) | 6 (14.6) | 7 (31.8) | 0.19 |
HLA, n (%)
| 24 (58.5) 17 (41.5) | 21 (95.4) 1 (4.6) | 0.002 |
| Weight loss, n (%) | 8 (19.5) | 10 (45.4) | 0.04 |
| Bloating, n (%) | 22 (53.6) | 17 (77.3) | 0.10 |
| Diarrhea, n (%) | 7 (17.1) | 9 (40.1) | 0.08 |
| Tiredness, n (%) | 7 (17.1) | 8 (36.4) | 0.12 |
| Headache, n (%) | 0 (0) | 11 (50) | <0.001 |
| HR (95% CI) | p Value | |
|---|---|---|
| Headache | 4.5 (1.7–11.8) | 0.002 |
| Weight loss | 1.7 (0.4–7.2) | 0.45 |
| HLA DQ2-8 | 6.6 (0.8–53.5) | 0.07 |
| AGA | 2.7 (1.1–7.1) | 0.04 |
| ANA | 2.4 (1.1–5.7) | 0.04 |
| Autoimmune thyroiditis | 2.4 (0.9–5.8) | 0.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losurdo, G.; Principi, M.; Iannone, A.; Giangaspero, A.; Piscitelli, D.; Ierardi, E.; Di Leo, A.; Barone, M. Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study. Nutrients 2018, 10, 2001. https://doi.org/10.3390/nu10122001
Losurdo G, Principi M, Iannone A, Giangaspero A, Piscitelli D, Ierardi E, Di Leo A, Barone M. Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study. Nutrients. 2018; 10(12):2001. https://doi.org/10.3390/nu10122001
Chicago/Turabian StyleLosurdo, Giuseppe, Mariabeatrice Principi, Andrea Iannone, Antonio Giangaspero, Domenico Piscitelli, Enzo Ierardi, Alfredo Di Leo, and Michele Barone. 2018. "Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study" Nutrients 10, no. 12: 2001. https://doi.org/10.3390/nu10122001
APA StyleLosurdo, G., Principi, M., Iannone, A., Giangaspero, A., Piscitelli, D., Ierardi, E., Di Leo, A., & Barone, M. (2018). Predictivity of Autoimmune Stigmata for Gluten Sensitivity in Subjects with Microscopic Enteritis: A Retrospective Study. Nutrients, 10(12), 2001. https://doi.org/10.3390/nu10122001







