Zinc and Skin Disorders
Abstract
:1. Introduction
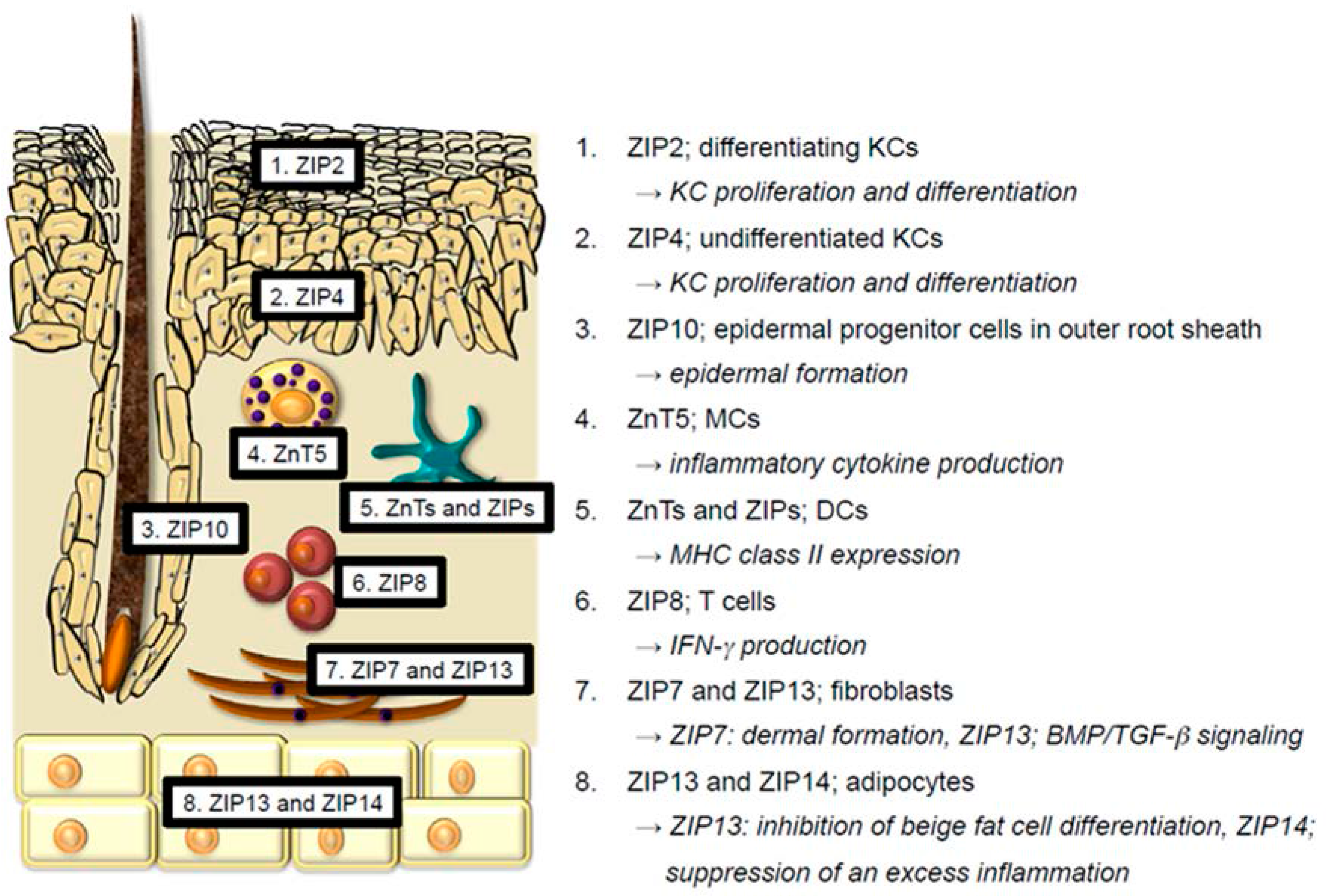
2. Physiological Functions of Zn and Zn Transporters in the Skin
2.1. Keratinocytes
2.2. Langerhans Cells
2.3. Melanocytes
2.4. Mast Cells and Dendritic Cells
2.5. T cells
2.6. Endothelial Cells
2.7. Fibroblasts
2.8. Adipocytes
3. Human Skin Disorders Caused by Mutations of Zn Transporters
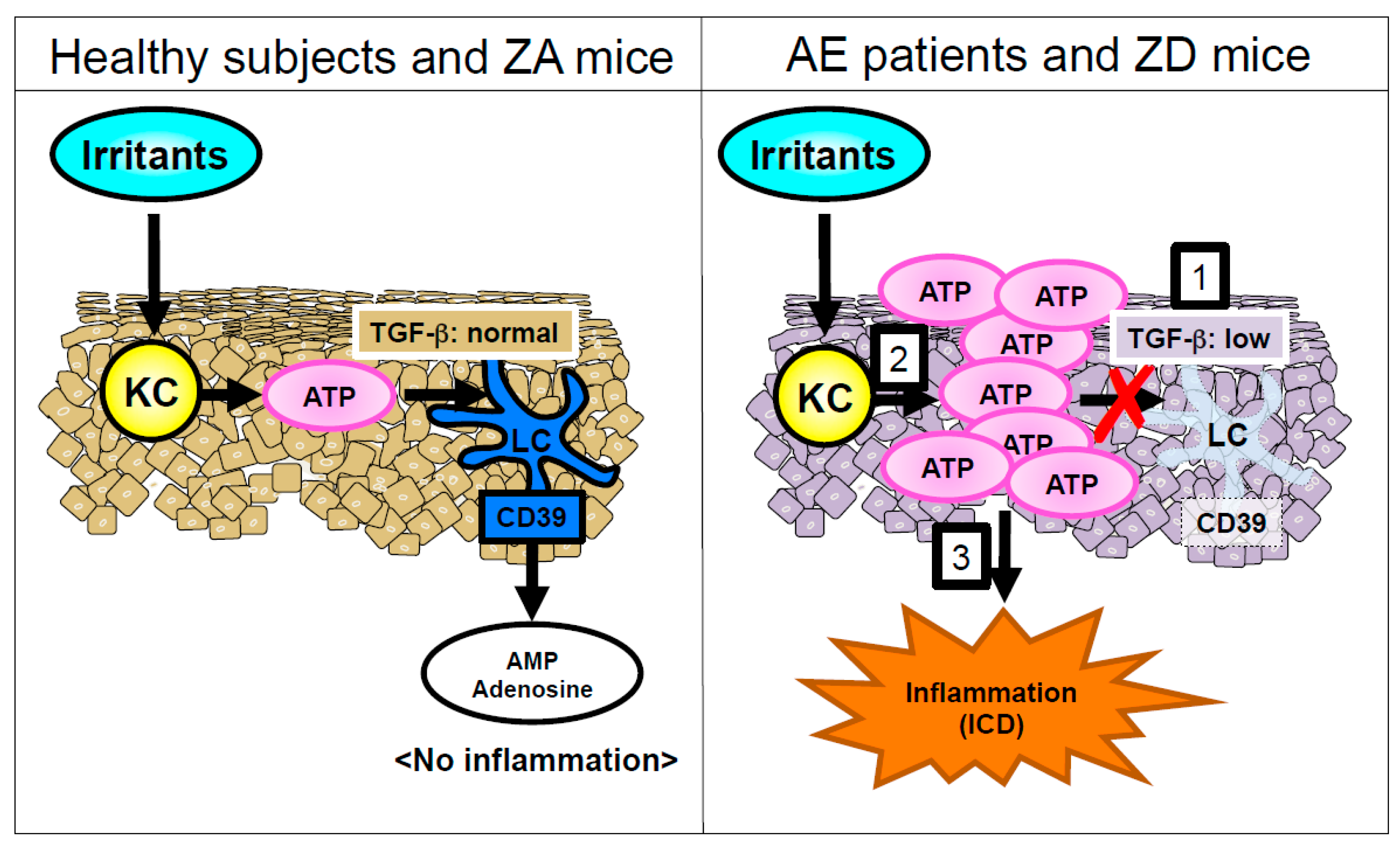
3.1. ZIP4 Mutation; Acrodermatitis Enteropathica (AE; OMIM 201100)
3.2. ZIP13 Mutation; Spondylocheiro Dysplastic Form of Ehlers–Danlos Syndrome (SCD-EDS; OMIM 612350)
3.3. ZnT2 Mutation; Transient Neonatal Zn Deficiency (TNZD; OMIM 608118)
4. Human Skin Disorders Caused by Dysregulation of Zn Transporters
5. Human Skin Disorders Associated with Zn Deficiency
5.1. Nutritional Deficiency Diseases
5.1.1. Necrolytic Migratory Erythema
5.1.2. Pellagra
5.1.3. Biotin Deficiency
5.2. Alopecia
5.2.1. Alopecia in Acrodermatitis Enteropathica
5.2.2. Alopecia Areata
5.3. Cutaneous Wounds and Ulcers
5.4. Other Skin Disorders Associated with Zn Deficiency
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Jackson, M.J. Physiology of zinc: General aspects. In Zinc in Human Biology; Mills, C.F., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1989; pp. 1–14. [Google Scholar]
- Michaelsson, G.; Ljunghall, K.; Danielson, B.G. Zinc in epidermis and dermis in healthy subjects. Acta Derm. Venereol. 1980, 60, 295–299. [Google Scholar] [PubMed]
- Inoue, Y.; Hasegawa, S.; Ban, S.; Yamada, T.; Date, Y. ZIP2 protein, a zinc transporter, is associated with keratinocyte differentiation. J. Biol. Chem. 2014, 289, 21451–21462. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, G.T. Heavy metals in rat mast cell granules. Lab. Investig. 1967, 17, 588–598. [Google Scholar] [PubMed]
- Cowen, T.; Trigg, P.; Eady, R.A. Distribution of mast cells in human dermis: Development of a mapping technique. Br. J. Dermatol. 1979, 100, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Knop, J.; Maurer, M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br. J. Dermatol. 2003, 148, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteins. Pflugers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Eide, D.J. The SLC39 family of metal ion transporters. Pflugers Arch. 2004, 447, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Palmiter, R.D.; Huang, L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004, 447, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kawamura, T.; Shimada, S. Zinc and skin biology. Arch. Biochem. Biophys. 2016, 611, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I.; Cavallaro, G. Minimal functional sites allow a classification of zinc sites in proteins. PLoS ONE 2011, 6, e26325. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I. A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 2012, 111, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.M.; Shi, Y. The galvanization of biology: A growing appreciation for the roles of zinc. Science 1996, 271, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Yamasaki, S.; Nishida, K.; Murakami, M.; Hirano, T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011, 16, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Klug, A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation. Annu. Rev. Biochem. 2010, 79, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Andreini, C.; Banci, L.; Bertini, I.; Rosato, A. Counting the zinc-proteins encoded in the human genome. J. Proteome Res. 2006, 5, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Emri, E.; Miko, E.; Bai, P.; Boros, G.; Nagy, G.; Rózsa, D.; Juhász, T.; Hegedűs, C.; Horkay, I.; Remenyik, É.; et al. Effects of non-toxic zinc exposure on human epidermal keratinocytes. Metallomics 2015, 7, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Truong-Tran, A.Q.; Evdokiou, A. Intracellular zinc depletion induces caspase activation and p21Waf1/Cip1 cleavage in human epithelial cell lines. J. Infect. Dis. 2000, 182 (Suppl. 1), S85–S92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D.; Varigos, G.; Ackland, M.L. Apoptosis may underlie the pathology of zinc-deficient skin. Immunol. Cell Biol. 2006, 84, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.J.; Daniel, J.C.; Gerson, S.J. Effect of zinc deficiency on keratins in buccal epithelium of rats. Arch. Oral Biol. 1991, 36, 759–763. [Google Scholar] [CrossRef]
- Gueniche, A.; Viac, J.; Lizard, G.; Charveron, M.; Schmitt, D. Protective effect of zinc on keratinocyte activation markers induced by interferon or nickel. Acta Derm. Venereol. 1995, 75, 19–23. [Google Scholar] [PubMed]
- Yamaoka, J.; Kume, T.; Akaike, A.; Miyachi, Y. Suppressive effect of zinc ion on iNOS expression induced by interferon-γ or tumor necrosis factor-α in murine keratinocytes. J. Dermatol. Sci. 2000, 23, 27–35. [Google Scholar] [CrossRef]
- Wessels, I.; Maywald, M.; Rink, L. Zinc as a gatekeeper of immune function. Nutrients 2017, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Kim, N.H.; Lee, S.-H.; Jung, H.-S.; Seo, J.; Kim, D.-K.; Hwang, D.; Fukada, T.; Lee, A.-Y.; et al. An acrodermatitis enteropathica-associated Zn transporter, ZIP4, Regulates human epidermal homeostasis. J. Investig. Dermatol. 2017, 137, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Takaishi, M.; Toyoshima, K.-E.; Kawamata, S.; Ito, K.; Hara, T.; Watanabe, T.; Irié, T.; Takagishi, T.; et al. Requirement of zinc transporter ZIP10 for epidermal development: Implication of the ZIP10-p63 axis in epithelial homeostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 12243–12248. [Google Scholar] [CrossRef] [PubMed]
- Nartey, N.O.; Banerjee, D.; Cherian, M.G. Immunohistochemical localization of metallothionein in cell nucleus and cytoplasm of fetal human liver and kidney and its changes during development. Pathology 1987, 19, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Thirumoorthy, N.; Shyam Sunder, A.; Manisenthil Kumar, K.; Senthil kumar, M.; Ganesh, G.N.K.; Chatterjee, M. A review of metallothionein isoforms and their role in pathophysiology. World J. Surg. Oncol. 2011, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Van den Oord, J.J.; De Ley, M. Distribution of metallothionein in normal and pathological human skin. Arch. Dermatol. Res. 1994, 286, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zamirska, A.; Matusiak, L.; Dziegiel, P.; Szybejko-Machaj, G.; Szepietowski, J.C. Expression of metallothioneins in cutaneous squamous cell carcinoma and actinic keratosis. Pathol. Oncol. Res. 2012, 18, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Sawamura, D.; Hashimoto, I.; Kida, K.; Naganuma, A. Epidermal proliferation of the skin in metallothionein-null mice. J. Investig. Dermatol. 1998, 110, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Slusser, A.; Zheng, Y.; Zhou, X.D.; Somji, S.; Sens, D.A.; Sens, M.A.; Garrett, S.H. Metallothionein isoform 3 expression in human skin, related cancers and human skin derived cell cultures. Toxicol. Lett. 2015, 232, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pula, B.; Tazbierski, T.; Zamirska, A.; Werynska, B.; Bieniek, A.; Szepietowski, J.; Rys, J.; Dziegiel, P.; Podhorska-Okolow, M. Metallothionein 3 expression in normal skin and malignant skin lesions. Pathol. Oncol. Res. 2015, 21, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Quaife, C.J.; Findley, S.D.; Erickson, J.C.; Froelick, G.J.; Kelly, E.J.; Zambrowicz, B.P.; Palmiter, R.D. Induction of a new metallothionein isoform (MT-IV) occurs during differentiation of stratified squamous epithelia. Biochemistry 1994, 33, 7250–7259. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Ginhoux, F.; Collin, M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008, 8, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Maverakis, E.; Fung, M.A.; Lynch, P.J.; Draznin, M.; Michael, D.J.; Ruben, B.; Fazel, N. Acrodermatitis enteropathica and an overview of zinc metabolism. J. Am. Acad. Dermatol. 2007, 56, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kasana, S.; Din, J.; Maret, W. Genetic causes and gene-nutrient interactions in mammalian zinc deficiencies: Acrodermatitis enteropathica and transient neonatal zinc deficiency as examples. J. Trace Elem. Med. Biol. 2015, 29, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Ogawa, Y.; Nakamura, Y.; Nakamizo, S.; Ohta, Y.; Nakano, H.; Kabashima, K.; Katayama, I.; Koizumi, S.; Kodama, T.; et al. Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J. Clin. Investig. 2012, 122, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, T.A.; Letterio, J.J.; Farr, A.G.; Udey, M.C. A role for endogenous transforming growth factor β1 in Langerhans cell biology: The skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 1996, 184, 2417–2422. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hu, W.; Xu, R.; Hou, B.; Zhang, L.; Zhang, W. ZNF580, a novel C2H2 zinc-finger transcription factor, interacts with the TGF-β signal molecule Smad2. Cell Biol. Int. 2011, 35, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, E.; Rudolf, K. Increases in intracellular zinc enhance proliferative signaling as well as mitochondrial and endolysosomal activity in human melanocytes. Cell. Physiol. Biochem. 2017, 43, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Weinlich, G.; Bitterlich, W.; Mayr, V.; Fritsch, P.O.; Zelger, B. Metallothionein-overexpression as a prognostic factor for progression and survival in melanoma. A prospective study on 520 patients. Br. J. Dermatol. 2003, 149, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Emri, E.; Egervari, K.; Varvolgyi, T.; Rozsa, D.; Miko, E.; Dezso, B.; Veres, I.; Mehes, G.; Emri, G.; Remenyik, E. Correlation among metallothionein expression, intratumoural macrophage infiltration and the risk of metastasis in human cutaneous malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, e320–e327. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T. Roles of zinc signaling in the immune system. J. Immunol. Res. 2016, 2016, 6762343. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hasegawa, A.; Nakae, S.; Oboki, K.; Saito, H.; Yamasaki, S.; Hirano, T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J. Exp. Med. 2009, 206, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Kabu, K.; Yamasaki, S.; Kamimura, D.; Ito, Y.; Hasegawa, A.; Sato, E.; Kitamura, H.; Nishida, K.; Hirano, T. Zinc is required for FcεRI-mediated mast cell activation. J. Immunol. 2006, 177, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Morikawa, H.; Kamon, H.; Iguchi, M.; Hojyo, S.; Fukada, T.; Yamashita, S.; Kaisho, T.; Akira, S.; Murakami, M.; et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat. Immunol. 2006, 7, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Dowd, P.S.; Kelleher, J.; Guillou, P.J. T-lymphocyte subsets and interleukin-2 production in zinc-deficient rats. Br. J. Nutr. 1986, 55, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Nair, M.; Onoe, K.; Tanaka, T.; Floyd, R.; Good, R.A. Impairment of cell-mediated immunity functions by dietary zinc deficiency in mice. Proc. Natl. Acad. Sci. USA 1979, 76, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Golden, M.H.; Jackson, A.A.; Golden, B.E. Effect of zinc on thymus of recently malnourished children. Lancet 1977, 2, 1057–1059. [Google Scholar] [CrossRef]
- DePasquale-Jardieu, P.; Fraker, P.J. The role of corticosterone in the loss in immune function in the zinc-deficient A/J mouse. J. Nutr. 1979, 109, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Liuzzi, J.P.; McClellan, S.; Cousins, R.J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-γ expression in activated human T cells. J. Leukoc. Biol. 2009, 86, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Costarelli, L.; Giacconi, R.; Basso, A.; Piacenza, F.; Pierpaoli, E.; Provinciali, M.; Ogo, O.A.; Ford, D. Changes in Zn homeostasis during long term culture of primary endothelial cells and effects of Zn on endothelial cell senescence. Exp. Gerontol. 2017, 99, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Shearier, E.R.; Bowen, P.K.; He, W.; Drelich, A.; Drelich, J.; Goldman, J.; Zhao, F. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc. ACS Biomater. Sci. Eng. 2016, 2, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Tada-Oikawa, S.; Ichihara, G.; Suzuki, Y.; Izuoka, K.; Wu, W.; Yamada, Y.; Mishima, T.; Ichihara, S. Zn(II) released from zinc oxide nano/micro particles suppresses vasculogenesis in human endothelial colony-forming cells. Toxicol. Rep. 2015, 2, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Schulkens, I.A.; Castricum, K.C.; Weijers, E.M.; Koolwijk, P.; Griffioen, A.W.; Thijssen, V.L. Expression, regulation and function of human metallothioneins in endothelial cells. J. Vasc. Res. 2014, 51, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Shay, N.F. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J. Nutr. 2001, 131, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Fukada, T.; Hosaka, T.; Yamasaki, S.; Ohashi, W.; Hojyo, S.; Miyai, T.; Nishida, K.; Yokoyama, S.; Hirano, T. Biochemical characterization of human ZIP13 protein: A homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 2011, 286, 40255–40265. [Google Scholar] [CrossRef] [PubMed]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-β signaling pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-function analysis of HKE4, A member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2004, 377, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Bhin, J.; Seo, J.; Kim, S.Y.; Lee, E.; Park, K.; Choi, D.H.; Takagishi, T.; Hara, T.; Hwang, D.; et al. Requirement of zinc transporter SLC39A7/ZIP7 for dermal development to fine-tune endoplasmic reticulum function by regulating protein disulfide isomerase. J. Investig. Dermatol. 2017, 137, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Bin, B.H.; Hojyo, S.; Hosaka, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. Molecular pathogenesis of spondylocheirodysplastic Ehlers-Danlos syndrome caused by mutant ZIP13 proteins. EMBO Mol. Med. 2014, 6, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Spiegelman, B.M.; Seale, P. Brown and beige fat: Physiological roles beyond heat generation. Cell Metab. 2015, 22, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Fukada, T.; Bhin, J.; Suzuki, L.; Tsuzuki, T.; Takamine, Y.; Bin, B.H.; Yoshihara, T.; Ichinoseki-Sekine, N.; Naito, H.; et al. Zinc transporter ZIP13 suppresses beige adipocyte biogenesis and energy expenditure by regulating C/EBP-β expression. PLoS Genet. 2017, 13, e1006950. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Lapoint, K.; Martinez, K.; Kennedy, A.; Boysen Sandberg, M.; McIntosh, M.K. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology 2006, 147, 5340–5351. [Google Scholar] [CrossRef] [PubMed]
- Liuzzi, J.P.; Lichten, L.A.; Rivera, S.; Blanchard, R.K.; Aydemir, T.B.; Knutson, M.D.; Ganz, T.; Cousins, R.J. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA 2005, 102, 6843–6848. [Google Scholar] [CrossRef] [PubMed]
- Troche, C.; Aydemir, T.B.; Cousins, R.J. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E258–E268. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, L.; Zhou, X.; Du, M.; Jiang, Z.; Hausman, G.J.; Bergen, W.G.; Zan, L.; Dodson, M.V. Emerging roles of zinc finger proteins in regulating adipogenesis. Cell. Mol. Life Sci. 2013, 70, 4569–4584. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Kury, S.; Giraud, M.; Dreno, B.; Kharfi, M.; Bezieau, S. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum. Mutat. 2009, 30, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Boucher, R.C.; Harden, T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003, 64, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Fujishita, K.; Inoue, K.; Shigemoto-Mogami, Y.; Tsuda, M.; Inoue, K. Ca2+ waves in keratinocytes are transmitted to sensory neurons: The involvement of extracellular ATP and P2Y2 receptor activation. Biochem. J. 2004, 380, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, N.; Kumamoto, T.; Robson, S.C.; Sevigny, J.; Matsue, H.; Enjyoji, K.; Takashima, A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: Modulatory roles in inflammation and immune responsiveness. Nat. Med. 2002, 8, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Yang, C.Y.; Lin, W.J.; Lin, C.H. Ecto-nucleoside triphosphate diphosphohydrolase 2 modulates local ATP-induced calcium signaling in human HaCaT keratinocytes. PLoS ONE 2013, 8, e57666. [Google Scholar] [CrossRef] [PubMed]
- Giunta, C.; Elcioglu, N.H.; Albrecht, B.; Eich, G.; Chambaz, C.; Janecke, A.R.; Yeowell, H.; Weis, M.; Eyre, D.R.; Kraenzlin, M.; et al. Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome—An autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 2008, 82, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, N.; Yamada, M.; Kan-no, T.; Kojima, T.; Kaneko, T.; Yonekubo, A. Macronutrient, mineral and trace element composition of breast milk from Japanese women. J. Trace Elem. Med. Biol. 2005, 19, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Gitschier, J. A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat. Genet. 1997, 17, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Lonnerdal, B.; Kelleher, S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006, 281, 39699–39707. [Google Scholar] [CrossRef] [PubMed]
- Lasry, I.; Seo, Y.A.; Ityel, H.; Shalva, N.; Pode-Shakked, B.; Glaser, F.; Berman, B.; Berezovsky, I.; Goncearenco, A.; Klar, A.; et al. A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J. Biol. Chem. 2012, 287, 29348–29361. [Google Scholar] [CrossRef] [PubMed]
- Itsumura, N.; Inamo, Y.; Okazaki, F.; Teranishi, F.; Narita, H.; Kambe, T.; Kodama, H. Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: A novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE 2013, 8, e64045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miletta, M.C.; Bieri, A.; Kernland, K.; Schoni, M.H.; Petkovic, V.; Fluck, C.E.; Eble, A.; Mullis, P.E. Transient neonatal zinc deficiency caused by a heterozygous G87R mutation in the Zinc transporter ZnT-2 (SLC30A2) Gene in the mother highlighting the importance of Zn2+ for normal growth and development. Int. J. Endocrinol. 2013, 2013, 259189. [Google Scholar] [CrossRef] [PubMed]
- Lazarczyk, M.; Pons, C.; Mendoza, J.A.; Cassonnet, P.; Jacob, Y.; Favre, M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 2008, 205, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Orth, G. Host defenses against human papillomaviruses: Lessons from epidermodysplasia verruciformis. Curr. Top. Microbiol. Immunol. 2008, 321, 59–83. [Google Scholar] [PubMed]
- Ramoz, N.; Taieb, A.; Rueda, L.A.; Montoya, L.S.; Bouadjar, B.; Favre, M.; Orth, G. Evidence for a nonallelic heterogeneity of epidermodysplasia verruciformis with two susceptibility loci mapped to chromosome regions 2p21-p24 and 17q25. J. Investig. Dermatol. 2000, 114, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Ramoz, N.; Rueda, L.A.; Bouadjar, B.; Montoya, L.S.; Orth, G.; Favre, M. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat. Genet. 2002, 32, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Offord, E.A.; Beard, P. A member of the activator protein 1 family found in keratinocytes but not in fibroblasts required for transcription from a human papillomavirus type 18 promoter. J. Virol. 1990, 64, 4792–4798. [Google Scholar] [PubMed]
- Stammers, A.L.; Lowe, N.M.; Medina, M.W.; Patel, S.; Dykes, F.; Perez-Rodrigo, C.; Serra-Majam, L.; Nissensohn, M.; Moran, V.H. The relationship between zinc intake and growth in children aged 1–8 years: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.F.; Miller, L.V.; Hambidge, K.M. Zinc deficiency in infants and children: A review of its complex and synergistic interactions. Paediatr. Int. Child Health 2014, 34, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Penny, M.E. Zinc supplementation in public health. Ann. Nutr. Metab. 2013, 62 (Suppl. 1), 31–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hu, Y.F.; Hao, J.H.; Chen, Y.H.; Su, P.Y.; Wang, Y.; Yu, Z.; Fu, L.; Xu, Y.Y.; Zhang, C.; et al. Maternal zinc deficiency during pregnancy elevates the risks of fetal growth restriction: A population-based birth cohort study. Sci. Rep. 2015, 5, 11262. [Google Scholar] [CrossRef] [PubMed]
- Mullans, E.A.; Cohen, P.R. Iatrogenic necrolytic migratory erythema: A case report and review of nonglucagonoma-associated necrolytic migratory erythema. J. Am. Acad. Dermatol. 1998, 38, 866–873. [Google Scholar] [CrossRef]
- Alexander, E.K.; Robinson, M.; Staniec, M.; Dluhy, R.G. Peripheral amino acid and fatty acid infusion for the treatment of necrolytic migratory erythema in the glucagonoma syndrome. Clin. Endocrinol. 2002, 57, 827–831. [Google Scholar] [CrossRef]
- Tierney, E.P.; Badger, J. Etiology and pathogenesis of necrolytic migratory erythema: Review of the literature. MedGenMed 2004, 6, 4. [Google Scholar] [PubMed]
- Van Beek, A.P.; de Haas, E.R.; van Vloten, W.A.; Lips, C.J.; Roijers, J.F.; Canninga-van Dijk, M.R. The glucagonoma syndrome and necrolytic migratory erythema: A clinical review. Eur. J. Endocrinol. 2004, 151, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Walker, N.P. Atypical necrolytic migratory erythema in association with a jejunal adenocarcinoma. J. R. Soc. Med. 1982, 75, 134–135. [Google Scholar] [PubMed]
- Kelly, C.P.; Johnston, C.F.; Nolan, N.; Keeling, P.W.; Weir, D.G. Necrolytic migratory erythema with elevated plasma enteroglucagon in celiac disease. Gastroenterology 1989, 96, 1350–1353. [Google Scholar] [CrossRef]
- Blackford, S.; Wright, S.; Roberts, D.L. Necrolytic migratory erythema without glucagonoma: The role of dietary essential fatty acids. Br. J. Dermatol. 1991, 125, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Kerleau, J.M.; Levesque, H.; Cailleux, N.; Gancel, A.; Boullie, M.C.; Courtois, H. Isolated zinc deficiency and necrolytic migratory erythema. Apropos of a case. Rev. Med. Interne 1993, 14, 784–787. [Google Scholar] [CrossRef]
- Thorisdottir, K.; Camisa, C.; Tomecki, K.J.; Bergfeld, W.F. Necrolytic migratory erythema: A report of three cases. J. Am. Acad. Dermatol. 1994, 30, 324–329. [Google Scholar] [CrossRef]
- Marinkovich, M.P.; Botella, R.; Datloff, J.; Sangueza, O.P. Necrolytic migratory erythema without glucagonoma in patients with liver disease. J. Am. Acad. Dermatol. 1995, 32, 604–609. [Google Scholar] [CrossRef]
- Delaporte, E.; Catteau, B.; Piette, F. Necrolytic migratory erythema-like eruption in zinc deficiency associated with alcoholic liver disease. Br. J. Dermatol. 1997, 137, 1027–1028. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.A.; Reynolds, N.J. Necrolytic migratory erythema and zinc deficiency. Br. J. Dermatol. 1997, 136, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Al-Rikabi, A.C.; Al-Homsi, H.I. Propionic acidemia and zinc deficiency presenting as necrolytic migratory erythema. Saudi Med. J. 2004, 25, 660–662. [Google Scholar] [PubMed]
- Topham, E.J.; Child, F.J. Exfoliative erythema of malnutrition with zinc and essential amino acid deficiency. Clin. Exp. Dermatol. 2005, 30, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Komine, M.; Sasaki, K.; Mitsui, H.; Fujimoto, M.; Ihn, H.; Asahina, A.; Kikuchi, K.; Tamaki, K. Necrolytic migratory erythema without glucagonoma in a patient with short bowel syndrome. J. Dermatol. 2006, 33, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Healy, E.; Scanlain, N.I.; Barnes, L. Necrolytic migratory erythema due to zinc deficiency. Br. J. Dermatol. 1992, 127, 57–58. [Google Scholar] [CrossRef]
- Rokunohe, D.; Nakano, H.; Ikenaga, S.; Umegaki, N.; Kaneko, T.; Matsuhashi, Y.; Tando, Y.; Toyoki, Y.; Hakamada, K.; Kusumi, T.; et al. Reduction in epidermal Langerhans cells in patients with necrolytic migratory erythema. J. Dermatol. Sci. 2008, 50, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K. Pellagra in the United States: A historical perspective. South. Med. J. 2000, 93, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Chick, H. The aetiology of pellagra: A review of current theories. J. Trop. Med. Hyg. 1951, 54, 207–213. [Google Scholar] [CrossRef]
- Sugita, K.; Ikenouchi-Sugita, A.; Nakayama, Y.; Yoshioka, H.; Nomura, T.; Sakabe, J.; Nakahigashi, K.; Kuroda, E.; Uematsu, S.; Nakamura, J.; et al. Prostaglandin E2 is critical for the development of niacin-deficiency-induced photosensitivity via ROS production. Sci. Rep. 2013, 3, 2973. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, H.; Favaro, R.M.; Cunha, D.F.; Marchini, J.S. Assessment of zinc nutritional status of pellagra patients. Alcohol Alcohol. 1995, 30, 297–302. [Google Scholar] [PubMed]
- Yamaguchi, S.; Miyagi, T.; Sogabe, Y.; Yasuda, M.; Kanazawa, N.; Utani, A.; Izaki, S.; Uezato, H.; Takahashi, K. Depletion of Epidermal Langerhans Cells in the Skin Lesions of Pellagra Patients. Am. J. Dermatopathol. 2017, 39, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Wijeratne, S.S.; Hassan, Y.I. Biotin. Biofactors 2009, 35, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mock, D.M. Skin manifestations of biotin deficiency. Semin. Dermatol. 1991, 10, 296–302. [Google Scholar] [PubMed]
- Mock, D.M.; Johnson, S.B.; Holman, R.T. Effects of biotin deficiency on serum fatty acid composition: Evidence for abnormalities in humans. J. Nutr. 1988, 118, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Mock, D.M.; Mock, N.I.; Johnson, S.B.; Holman, R.T. Effects of biotin deficiency on plasma and tissue fatty acid composition: Evidence for abnormalities in rats. Pediatr. Res. 1988, 24, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Kramer, T.R.; Briske-Anderson, M.; Johnson, S.B.; Holman, R.T. Effects of biotin deficiency on polyunsaturated fatty acid metabolism in rats. J. Nutr. 1984, 114, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Suchy, S.F.; Rizzo, W.B.; Wolf, B. Effect of biotin deficiency and supplementation on lipid metabolism in rats: Saturated fatty acids. Am. J. Clin. Nutr. 1986, 44, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Matsusue, S.; Kashihara, S.; Takeda, H.; Koizumi, S. Biotin deficiency during total parenteral nutrition: Its clinical manifestation and plasma nonesterified fatty acid level. JPEN J. Parenter. Enter. Nutr. 1985, 9, 760–763. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Mizukoshi, M.; Koyama, H.; Kitano, N.; Koike, M. Intractable diaper dermatitis as an early sign of biotin deficiency. Acta Paediatr. 1998, 87, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Lagier, P.; Bimar, P.; Seriat-Gautier, S.; Dejode, J.M.; Brun, T.; Bimar, J. Zinc and biotin deficiency during prolonged parenteral nutrition in the infant. Presse Med. 1987, 16, 1795–1797. [Google Scholar] [PubMed]
- Khalidi, N.; Wesley, J.R.; Thoene, J.G.; Whitehouse, W.M., Jr.; Baker, W.L. Biotin deficiency in a patient with short bowel syndrome during home parenteral nutrition. JPEN J. Parenter. Enter. Nutr. 1984, 8, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, R.; Noda, E.; Koyama, Y.; Shirai, T.; Horino, A.; Juri, T.; Koike, M. Biotin deficiency in an infant fed with amino acid formula and hypoallergenic rice. Acta Paediatr. 1996, 85, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, W.; Inaoki, M.; Fukui, T.; Inoue, Y.; Kuhara, T. Biotin deficiency in an infant fed with amino acid formula. J. Dermatol. 2005, 32, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.R.; Kossard, S. Acquired scalp alopecia. Part I: A review. Australas. J. Dermatol. 1998, 39, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Finner, A.M. Nutrition and hair: Deficiencies and supplements. Dermatol. Clin. 2013, 31, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Karashima, T.; Tsuruta, D.; Hamada, T.; Ono, F.; Ishii, N.; Abe, T.; Ohyama, B.; Nakama, T.; Dainichi, T.; Hashimoto, T. Oral zinc therapy for zinc deficiency-related telogen effluvium. Dermatol. Ther. 2012, 25, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Kil, M.S.; Kim, C.W.; Kim, S.S. Analysis of serum zinc and copper concentrations in hair loss. Ann. Dermatol. 2013, 25, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Amador, M.; Pena, M.; Garcia-Miranda, A.; Gonzalez, A.; Hermelo, M. Letter: Low hair-zinc concentrations in acrodermatitis enteropathica. Lancet 1975, 1, 1379. [Google Scholar] [CrossRef]
- Traupe, H.; Happle, R.; Grobe, H.; Bertram, H.P. Polarization microscopy of hair in acrodermatitis enteropathica. Pediatr. Dermatol. 1986, 3, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Dupre, A.; Bonafe, J.L.; Carriere, J.P. The hair in acrodermatitis interopathica—A disease indicator? Acta Derm. Venereol. 1979, 59, 177–178. [Google Scholar] [PubMed]
- Follis, R.H.; Day, H.G.; McCollum, E.V. Histological studies of the tissues of rats fed a diet extremely low in zinc. J. Nutr. 1941, 22, 223–237. [Google Scholar] [CrossRef]
- Hsu, J.N. Zinc as Related to Cystine Metabolism; Academic Press: London, UK, 1976. [Google Scholar]
- Wilson, R.H.; Lewis, H.B. The cystine content of hair and other epidermal tissues. J. Biol. Chem. 1927, 73, 543–553. [Google Scholar]
- Day, H.G.; Skidmore, B.E. Some effects of dietary zinc deficiency in the mouse. J. Nutr. 1946, 33, 27–38. [Google Scholar] [CrossRef]
- Swenerton, H.; Hurley, L.S. Severe zinc deficiency in male and female rats. J. Nutr. 1968, 95, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Todd, W.R.; Elvehjem, C.A.; Hart, E.B. Zinc in the nutrition of the rat. Am. J. Physiol. 1933, 107, 146–156. [Google Scholar] [CrossRef]
- Shaw, N.A.; Dickey, H.C.; Brugman, H.H.; Blamberg, D.L.; Witter, J.F. Zinc deficiency in female rabbits. Lab. Anim. 1974, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Freyschmidt-Paul, P.; McElwee, K.J.; Hoffmann, R.; Sundberg, J.P.; Vitacolonna, M.; Kissling, S.; Zoller, M. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br. J. Dermatol. 2006, 155, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, C.W.; Kim, S.S.; Park, C.W. The therapeutic effect and the changed serum zinc level after zinc supplementation in alopecia areata patients who had a low serum zinc level. Ann. Dermatol. 2009, 21, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Y.J.; Manzoor, S.; Khan, A.R.; Qayoom, S. Trace element levels in alopecia areata. Indian J. Dermatol. Venereol. Leprol. 2009, 75, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Abdel Fattah, N.S.; Atef, M.M.; Al-Qaradaghi, S.M. Evaluation of serum zinc level in patients with newly diagnosed and resistant alopecia areata. Int. J. Dermatol. 2016, 55, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Agren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Marsh, R.G.; Draelos, Z.D. Zinc and skin health: Overview of physiology and pharmacology. Dermatol. Surg. 2005, 31, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.A.; Hawke, C.I. Does oral zinc aid the healing of chronic leg ulcers? A systematic literature review. Arch. Dermatol. 1998, 134, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Stromberg, H.E. Topical treatment of pressure ulcers. A randomized comparative trial of Varidase and zinc oxide. Scand. J. Plast. Reconstr. Surg. 1985, 19, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S. Studies on zinc in wound healing. Acta Derm. Venereol. Suppl. 1990, 154, 1–36. [Google Scholar]
- Popovics, P.; Stewart, A.J. GPR39, a Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell. Mol. Life Sci. 2011, 68, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Fuchs, E. Skin and its regenerative powers: An alliance between stem cells and their niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Qiao, J.; Zhang, S.; Zhang, H.; Lei, X.; Wang, X.; Deng, Z.; Ning, L.; Cao, Y.; Guo, Y.; et al. GPR39 marks specific cells within the sebaceous gland and contributes to skin wound healing. Sci. Rep. 2015, 5, 7913. [Google Scholar] [CrossRef] [PubMed]
- David, T.J.; Wells, F.E.; Sharpe, T.C.; Gibbs, A.C. Low serum zinc in children with atopic eczema. Br. J. Dermatol. 1984, 111, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Yoo, S.R.; Jeong, M.G.; Ko, J.Y.; Ro, Y.S. Hair zinc levels and the efficacy of oral zinc supplementation in patients with atopic dermatitis. Acta Derm. Venereol. 2014, 94, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Mehdipour, M.; Najafi, S.; Bahramian, A.; Garjani, S.; Khoeini Poorfar, H. Evaluation of the serum zinc level in erosive and non-erosive oral lichen planus. J. Dent. 2014, 15, 52–56. [Google Scholar]
- Dogan, P.; Dogan, M.; Klockenkamper, R. Determination of trace elements in blood serum of patients with Behcet disease by total reflection X-ray fluorescence analysis. Clin. Chem. 1993, 39, 1037–1041. [Google Scholar] [PubMed]
- Saglam, K.; Serce, A.F.; Yilmaz, M.I.; Bulucu, F.; Aydin, A.; Akay, C.; Sayal, A. Trace elements and antioxidant enzymes in Behcet’s disease. Rheumatol. Int. 2002, 22, 93–96. [Google Scholar] [PubMed]
- Yazdanpanah, M.J.; Ghayour-Mobarhan, M.; Taji, A.; Javidi, Z.; Pezeshkpoor, F.; Tavallaie, S.; Momenzadeh, A.; Esmaili, H.; Shojaie-Noori, S.; Khoddami, M.; et al. Serum zinc and copper status in Iranian patients with pemphigus vulgaris. Int. J. Dermatol. 2011, 50, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Hanada, K.; Hashimoto, I. Analyses of serum copper and zinc levels and copper/zinc ratios in skin diseases. J. Dermatol. 1993, 20, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Ingen-Housz-Oro, S.; Blanchet-Bardon, C.; Vrillat, M.; Dubertret, L. Vitamin and trace metal levels in recessive dystrophic epidermolysis bullosa. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.D.; Tamura, T.; Johnson, L. Blood vitamin and trace metal levels in epidermolysis bullosa. Arch. Dermatol. 1989, 125, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.M.; Safavi, A.N.; Iranparvar, A.M.; Maleki, N.; Aghabalaei, D.M. Evaluation of the serum zinc level in adult patients with melasma: Is there a relationship with serum zinc deficiency and melasma? J. Cosmet. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fraker, P.J.; King, L.E. Reprogramming of the immune system during zinc deficiency. Annu. Rev. Nutr. 2004, 24, 277–298. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and Skin Disorders. Nutrients 2018, 10, 199. https://doi.org/10.3390/nu10020199
Ogawa Y, Kinoshita M, Shimada S, Kawamura T. Zinc and Skin Disorders. Nutrients. 2018; 10(2):199. https://doi.org/10.3390/nu10020199
Chicago/Turabian StyleOgawa, Youichi, Manao Kinoshita, Shinji Shimada, and Tatsuyoshi Kawamura. 2018. "Zinc and Skin Disorders" Nutrients 10, no. 2: 199. https://doi.org/10.3390/nu10020199
APA StyleOgawa, Y., Kinoshita, M., Shimada, S., & Kawamura, T. (2018). Zinc and Skin Disorders. Nutrients, 10(2), 199. https://doi.org/10.3390/nu10020199




