Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Test Material and Determination of Dose
2.2. Study Design
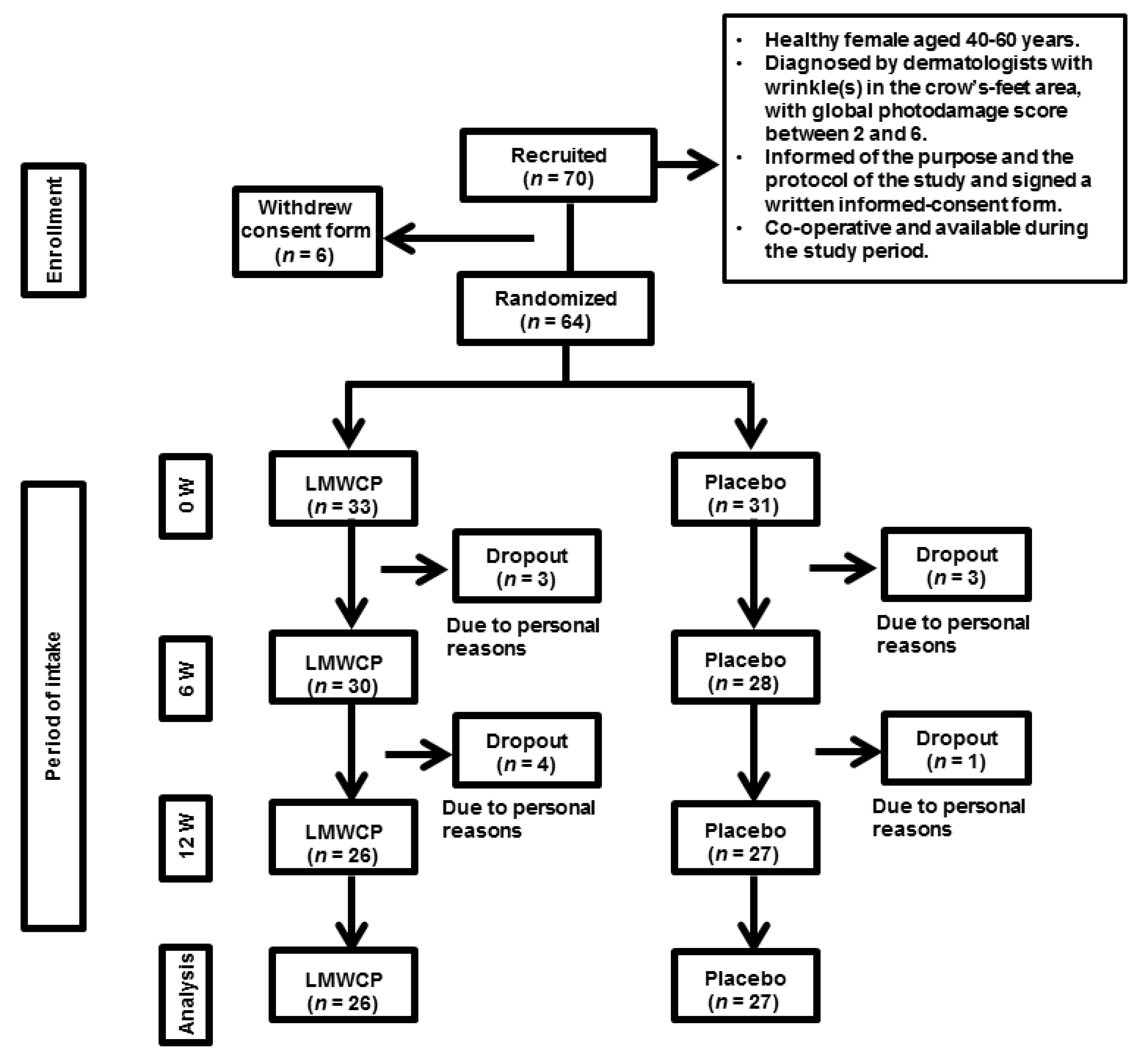
2.3. Study Participants
2.4. Study Schedule
2.5. Measurement of Skin Hydration
2.6. Measurement of Skin Wrinkling
2.7. Measurement of Skin Elasticity
2.8. Selection of Test Area
2.9. Participant Questionnaire
2.10. Safety Assessment
2.11. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Effect of LMWCP on Skin Hydration
3.3. Effect of LMWCP on Skin Wrinkling
3.4. Effect of LMWCP on Skin Elasticity
3.5. Analysis of Laboratory Parameters and Adverse Reactions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the skin. Anti-Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Kelly, R.I.; Pearse, R.; Bull, R.H.; Leveque, J.-L.; de Rigal, J.; Mortimer, P.S. The effects of aging on the cutaneous microvasculature. J. Am. Acad. Dermatol. 1995, 33, 749–756. [Google Scholar] [CrossRef]
- Chung, J.H.; Yano, K.; Lee, M.K.; Youn, C.S.; Seo, J.Y.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Detmar, M. Differential effects of photoaging vs intrinsic aging on the vascularization of human skin. Arch. Dermatol. 2002, 138, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, M.J. Tissue elasticity and the ageing elastic fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, R.E.; Griffiths, C.E.; Craven, N.M.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-rich microfibrils are reduced in photoaged skin. Distribution at the dermal–epidermal junction. J. Investig. Dermatol. 1999, 112, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Long, S.; Bowden, J.; Bastrilles, J.; Barton, S.; Griffiths, C. Repair of photoaged dermal matrix by topical application of a cosmetic ‘antiageing’ product. Br. J. Dermatol. 2008, 158, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Pyun, H.-B.; Kim, M.; Park, J.; Sakai, Y.; Numata, N.; Shin, J.-Y.; Shin, H.-J.; Kim, D.-U.; Hwang, J.-K. Effects of collagen tripeptide supplement on photoaging and epidermal skin barrier in UVB-exposed hairless mice. Prev. Nutr. Food Sci. 2012, 17, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M.; Fonferko, E. Studies in cutaneous aging: I. The elastic fiber network. J. Investig. Dermatol. 1982, 78, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kurdykowski, S.; Mine, S.; Bardey, V.; Danoux, L.; Jeanmaire, C.; Pauly, G.; Brabencova, E.; Wegrowski, Y.; Maquart, F.X. Ultraviolet-B Irradiation Induces Differential Regulations of Hyaluronidase Expression and Activity in Normal Human Keratinocytes. Photochem. Photobiol. 2011, 87, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinol. 2012, 4, 253–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkinson, I. Molecular components of the extracellular matrix. J. Wound Care 1992, 1, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.B.; Chongchitnant, N.; Sanchez, J.; Guo, Y.; Bennin, B.; Reichel, M.; Randall, M.B. Histologic Diagnosis of Inflammatory Skin Diseases: An Algorithmic Method Based on Pattern Analysis; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1997. [Google Scholar]
- Meigel, W.N.; Gay, S.; Weber, L. Dermal architecture and collagen type distribution. Arch. Dermatol. Res. 1977, 259, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kielty, C.M.; Sherratt, M.J.; Shuttleworth, C.A. Elastic fibres. J. Cell Sci. 2002, 115, 2817–2828. [Google Scholar] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Lee, S.H.; Youn, C.S.; Park, B.J.; Kim, K.H.; Park, K.C.; Cho, K.H.; Eun, H.C. Cutaneous photodamage in Koreans: Influence of sex, sun exposure, smoking, and skin color. Arch. Dermatol. 2001, 137, 1043–1051. [Google Scholar] [PubMed]
- Hwang, E.; Sun, Z.-W.; Lee, T.H.; Shin, H.-S.; Park, S.-Y.; Lee, D.-G.; Cho, B.-G.; Sohn, H.; Kwon, O.W.; Kim, S.Y. Enzyme-processed Korean Red Ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice. J. Ginseng Res. 2013, 37, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yotsawimonwat, S.; Rattanadechsakul, J.; Rattanadechsakul, P.; Okonogi, S. Skin improvement and stability of Echinacea purpurea dermatological formulations. Int. J. Cosmet. Sci. 2010, 32, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Grove, G.L.; Grove, M.J.; Leyden, J.J.; Lufrano, L.; Schwab, B.; Perry, B.H.; Thorne, E.G. Skin replica analysis of photodamaged skin after therapy with tretinoin emollient cream. J. Am. Acad. Dermatol. 1991, 25, 231–237. [Google Scholar] [CrossRef]
- Ryu, H.S.; Joo, Y.H.; Kim, S.O.; Park, K.C.; Youn, S.W. Influence of age and regional differences on skin elasticity as measured by the Cutometer®. Skin Res. Technol. 2008, 14, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Hasegawa, T.; Taguchi, Y.; Morimatsu, F.; Sato, K.; Nakamura, Y.; Higashi, A.; Kido, Y.; Nakabo, Y.; Ohtsuki, K. Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. J. Agric. Food Chem. 2005, 53, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Deguchi, K.; Onuma, M.; Numata, N.; Sakai, Y. Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans. Biol. Pharm. Bull. 2016, 39, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, M.; Ito, Y.; Yamada, M.; Goulas, S.; Teramoto, S.; Nakaya, M.-A.; Ohno, S.; Yamaguchi, K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017, 65, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Kamiyama, M.; Shimizu, M.; Kamiyama, S.; Taguchi, Y.; Sone, H.; Morimatsu, F.; Shirakawa, H.; Furukawa, Y.; Komai, M. Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. J. Agric. Food Chem. 2009, 58, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, Y.; Iwai, K.; Morimatsu, F.; Iwamoto, T.; Mori, T.; Oda, C.; Taira, T.; Park, E.Y.; Nakamura, Y.; Sato, K. Effect of prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin. J. Agric. Food Chem. 2009, 57, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Makpol, S.; Jam, F.A.; Yusof, Y.A.M.; Ngah, W.Z.W. Modulation of collagen synthesis and its gene expression in human skin fibroblasts by tocotrienol-rich fraction. Arch. Med. Sci. AMS 2011, 7, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Turakainen, H.; Larjava, H.; Saarni, H.; Penttinen, R. Synthesis of hyaluronic acid and collagen in skin fibroblasts cultures from patients with osteogenesis imperfecta. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1980, 628, 388–397. [Google Scholar] [CrossRef]
- Ohara, H.; Ichikawa, S.; Matsumoto, H.; Akiyama, M.; Fujimoto, N.; Kobayashi, T.; Tajima, S. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J. Dermatol. 2010, 37, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, T.; Ishikawa, O.; Miyachi, Y. Measurement of skin elastic properties with a new suction device (I): Relationship to age, sex and the degree of obesity in normal individuals. J. Dermatol. 1995, 22, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Mandal, M.; Das, S.; Mandal, A.; Chakraborti, T. Regulation of matrix metalloproteinases: An overview. Mol. Cell. Biochem. 2003, 253, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Taddese, S.; Weiss, A.S.; Neubert, R.H.; Schmelzer, C.E. Mapping of macrophage elastase cleavage sites in insoluble human skin elastin. Matrix Biol. 2008, 27, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.L.; Murphy, G.; Rock, M.J.; Sherratt, M.J.; Shapiro, S.D.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Biochem. J. 1999, 340 Pt 1, 171–181. [Google Scholar] [CrossRef]
- Tsuruga, E.; Irie, K.; Yajima, T. Fibrillin-2 degradation by matrix metalloproteinase-2 in periodontium. J. Dent. Res. 2007, 86, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Gosline, J.; Lillie, M.; Carrington, E.; Guerette, P.; Ortlepp, C.; Savage, K. Elastic proteins: Biological roles and mechanical properties. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 121–132. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Test | Placebo | ||
|---|---|---|---|---|
| Content (mg) | Content (%) | Content (mg) | Content (%) | |
| Low-molecular-weight Collagen peptide | 1000 | 2 | 0 | 0 |
| Vitamin C | 100 | 0.2 | 100 | 0.2 |
| Fruit concentrate mix | 3000 | 6.0 | 3000 | 6.0 |
| Flavor mix | 200 | 0.4 | 200 | 0.4 |
| Excipients | 1900 | 3.8 | 1900 | 3.8 |
| Sweetener | 12.5 | 0.025 | 12.5 | 0.025 |
| Water | 43,787.50 | 87.575 | 44,787.50 | 89.575 |
| Total | 50,000 | 100 | 50,000 | 100 |
| Inclusion Criteria |
|
| Exclusion Criteria |
|
| Variable | Test Group (n = 33) | Placebo Group (n = 31) | p-Value † | ||
|---|---|---|---|---|---|
| Mean (SD) | Min, Max | Mean (SD) | Min, Max | ||
| Age (years) | 48.00 (4.50) | 40, 59 | 48.35 (4.32) | 43, 57 | 0.749 |
| Weight (kg) | 54.45 (5.25) | 44.3, 70.5 | 56.73 (4.46) | 48.1, 65.3 | 0.067 |
| Systolic bp a (mmHg) | 117.61 (13.87) | 98, 140 | 114.52 (11.41) | 95, 140 | 0.336 |
| Diastolic bp a (mmHg) | 71.79 (10.29) | 48, 95 | 69.35 (9.32) | 46, 91 | 0.326 |
| Crow’s-feet visual grade | 3.21 (0.89) | 2, 6 | 3.19 (0.98) | 2, 5 | 0.937 |
| Time-Point | Test Group | Placebo Group | Test/Placebo p-Value † | |||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value * | Mean (SD) | p-Value * | |||
| Moisture | 0 W | 47.79 (12.48) | 48.43 (12.52) | |||
| 6 W | 60.00 (9.91) | 0.000 *** | 50.12 (11.33) | 0.273 | 0.000 ††† | |
| 12 W | 61.14 (11.31) | 0.000 *** | 53.02 (13.59) | 0.001 *** | 0.003 †† | |
| Evaluation Parameter | Time-Point | Test Group | Placebo Group | Test/Placebo p-Value † | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value * | Mean (SD) | p-Value * | |||
| Crow’s feet Visual grade | 0 W | 3.23 (0.95) | 3.19 (1.00) | |||
| 6 W | 3.19 (1.02) | 0.574 | 3.15 (1.03) | 0.574 | 0.988 | |
| 12 W | 2.81 (0.85) | 0.000 *** | 3.15 (1.03) | 0.746 | 0.013† | |
| Evaluation Parameter | Time-Point | Test Group | Placebo Group | Test/Placebo p-Value † | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value * | Mean (SD) | p-Value * | |||
| R1 | 0 W | 0.42 (0.09) | 0.43 (0.08) | |||
| 6 W | 0.42 (0.08) | 0.854 | 0.42 (0.07) | 0.301 | 0.475 | |
| 12 W | 0.40 (0.07) | 0.004 ** | 0.43 (0.09) | 0.685 | 0.043† | |
| R2 | 0 W | 0.28 (0.04) | 0.28 (0.04) | |||
| 6 W | 0.27 (0.04) | 0.066 | 0.28 (0.04) | 0.104 | 0.802 | |
| 12 W | 0.27 (0.04) | 0.055 | 0.28 (0.04) | 0.485 | 0.391 | |
| R3 | 0 W | 0.19 (0.03) | 0.19 (0.02) | |||
| 6 W | 0.19 (0.03) | 0.044 * | 0.19 (0.03) | 0.349 | 0.384 | |
| 12 W | 0.18 (0.02) | 0.001 *** | 0.20 (0.03) | 0.866 | 0.025 † | |
| R4 | 0 W | 0.07 (0.02) | 0.07 (0.02) | |||
| 6 W | 0.07 (0.02) | 1.000 | 0.07 (0.02) | 0.894 | 0.926 | |
| 12 W | 0.06 (0.02) | 0.008 ** | 0.07 (0.02) | 0.130 | 0.004 †† | |
| R5 | 0 W | 0.18 (0.05) | 0.18 (0.05) | |||
| 6 W | 0.18 (0.05) | 0.519 | 0.18 (0.04) | 1.000 | 0.690 | |
| 12 W | 0.17 (0.04) | 0.035 * | 0.19 (0.05) | 0.388 | 0.053 | |
| Evaluation Parameter | Time-Point | Test Group | Placebo Group | Test/Placebo p-Value † | ||
|---|---|---|---|---|---|---|
| Mean (SD) | p-Value * | Mean (SD) | p-Value * | |||
| R2 | 0 W | 0.74 (0.07) | 0.75 (0.07) | |||
| 6 W | 0.75 (0.08) | 0.287 | 0.74 (0.06) | 0.638 | 0.261 | |
| 12 W | 0.76 (0.06) | 0.088 | 0.73 (0.09) | 0.156 | 0.025 † | |
| R5 | 0 W | 0.64 (0.14) | 0.63 (0.13) | |||
| 6 W | 0.65 (0.15) | 0.293 | 0.62 (0.13) | 0.517 | 0.262 | |
| 12 W | 0.71 (0.16) | 0.002 ** | 0.64 (0.15) | 0.328 | 0.027 † | |
| R7 | 0 W | 0.36 (0.05) | 0.36 (0.05) | |||
| 6 W | 0.37 (0.05) | 0.722 | 0.35 (0.04) | 0.409 | 0.368 | |
| 12 W | 0.38 (0.04) | 0.149 | 0.35 (0.06) | 0.452 | 0.139 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-U.; Chung, H.-C.; Choi, J.; Sakai, Y.; Lee, B.-Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2018, 10, 826. https://doi.org/10.3390/nu10070826
Kim D-U, Chung H-C, Choi J, Sakai Y, Lee B-Y. Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2018; 10(7):826. https://doi.org/10.3390/nu10070826
Chicago/Turabian StyleKim, Do-Un, Hee-Chul Chung, Jia Choi, Yasuo Sakai, and Boo-Yong Lee. 2018. "Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study" Nutrients 10, no. 7: 826. https://doi.org/10.3390/nu10070826
APA StyleKim, D.-U., Chung, H.-C., Choi, J., Sakai, Y., & Lee, B.-Y. (2018). Oral Intake of Low-Molecular-Weight Collagen Peptide Improves Hydration, Elasticity, and Wrinkling in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients, 10(7), 826. https://doi.org/10.3390/nu10070826




