Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review
Abstract
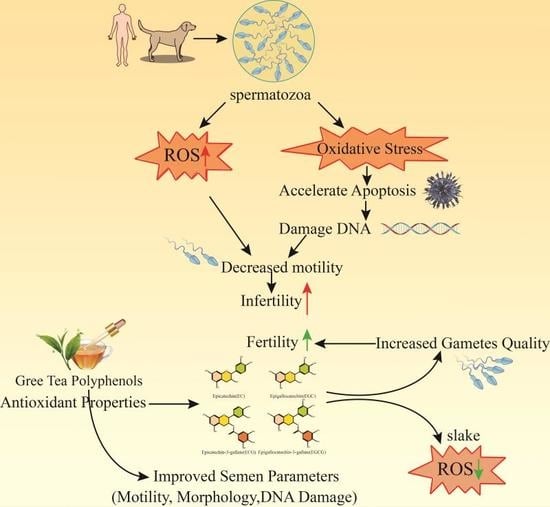
:1. Introduction
2. Factors Affecting Fertility
3. Antioxidants and Infertility
3.1. Vitamin E
3.2. L-Carnitine
3.3. Co-Enzymes
3.4. Superoxide Dismutase
3.5. Selenium
4. Green Tea Polyphenols Improve Fertility
5. Possible Combination of GrTPs with Different Extracts to Improve Fertility
5.1. Aspalathus Linearis
5.2. Vitex Agnus Castus
5.3. Pu-Erh
6. Conclusions and Future Perspective
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sharlip, I.D.; Jarow, J.P.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. J. Urol. 2002, 167, 2138–2144. [Google Scholar] [CrossRef]
- Sharpe, R.M. Environmental causes of testicular dysfunction. In Male Hypogonadism. Contemporary Endocrinology; Huhtaniemi, W.S., Ed.; Humana Press: Cham, The Netherlands, 2017; pp. 281–304. [Google Scholar]
- Bieniek, J.M.; Drabovich, A.P.; Lo, K.C. Seminal biomarkers for the evaluation of male infertility. Asian J. Androl. 2016, 18, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Barrat, C.L.R.; Bjorndahl, L.; Lamb, D.J.; Osorio, M.F.; Mclachlan, R.; Oates, R.D.; Sigman, M.; Sokol, R.; John, B.; Sigman, M.; et al. The diagnosis of male infetility: An analysis of the evidence to support the development of global WHO guidence-challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A. Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 2017, 5, 188. [Google Scholar] [CrossRef]
- David, J.M. Male infertility: Lifestyle factors and holistic, complementary, and alternative therapies. Asian J. Androl. 2016, 18, 410–418. [Google Scholar]
- Devi, A. Oxidative stress on male reproductive toxicitys. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 143–147. [Google Scholar]
- Tremellen, K. Oxidative Stress and Male Infertility: A Clinical Perspective. Hum. Reprod. Update 2012, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R.; Schill, W.B. Sperm separation in patients with urogenital infections. Andrologia 1998, 30, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Schuppe, H.C.; Meinhardt, A.; Allam, J.P.; Bergmann, M.; Weidner, W.; Haidl, G. Chronic orchitis: A neglected cause of male infertility? Andrologia 2008, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Sanocka-Maciejewska, D.; Ciupińska, M.; Kurpisz, M. Bacterial infection and semen quality. J. Reprod. Immunol. 2005, 67, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Syan, N.; Mathur, P.; Choudhary, S. Pharmacological profile of green tea and its polyphenols: A review. Med. Chem. Res. 2012, 21, 3347–3360. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liang, X.; Liang, J.; Zhang, C.; Yang, J.; Wang, C.; Kong, D.; Sun, H. ROS-Responsive Capsules Engineered from Green Tea Polyphenol-Metal Networks for Anticancer Drug Delivery. J. Mater. Chem. B 2018, 6, 1000–1010. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posadino, A.M.; Phu, H.T.; Cossu, A.; Giordo, R.; Fois, M.; Dtb, T.; Piga, A.; Sotgia, S.; Zinellu, A.; Carru, C.; et al. Oxidative stress-induced Akt downregulation mediates green tea toxicity towards prostate cancer cells. Toxicol In Vitro 2017, 42, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Alleviation of Metabolic Syndrome by Green Tea Polyphenol EGCG. Master’s Thesis, The State University of New Jersey, Rutgers, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Jin, D.; Hui, W.; Zhen-Biao, W.; Jie, Z.; Shun, Z.; Wei, L. Protection of murine spermatogenesis against ionizing radiation-induced testicular injury by a green tea polyphenol. Biol. Reprod. 2015, 92, 1–13. [Google Scholar]
- Lee, A.H.; Su, D.; Pasalich, M.; Binns, C.W. Tea consumption reduces ovarian cancer risk. Cancer Epidemiol. 2013, 37, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Lin, T.Y.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Improvement of green tea polyphenol with milk on skin with respect to antioxidation in healthy adults: A double-blind placebo-controlled randomized crossover clinical trial. Food Funct. 2016, 7, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A. Role of oxidants in male infertility: Rationale, significance, and treatment. Urol. Clin. N. Am. 2002, 29, 817–827. [Google Scholar] [CrossRef]
- Smith, R.; Vantman, D.; Ponce, J.; Escobar, J.; Lissi, E. Andrology: Total antioxidant capacity of human seminal plasma. Hum. Reprod. 1996, 11, 1655–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukcharoen, N.; Keith, J.; Irvine, D.S.; Aitken, R.J. Predicting the fertilizing potential of human sperm suspensions in vitro: Importance of sperm morphology and leukocyte contamination. Fertil. Steril. 1995, 63, 1293–1300. [Google Scholar] [CrossRef]
- Aitken, R.J.; Irvine, D.S.; Wu, F.C. Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am. J. Obstet. Gynecol. 1991, 164, 542–551. [Google Scholar] [CrossRef]
- Hosen, M.B.; Islam, M.R.; Begum, F.; Kabir, Y.; Howlader, M.Z.H. Oxidative stress induced sperm DNA damage, a possible reason for male infertility. Iran. J. Reprod. Med. 2015, 13, 525–532. [Google Scholar] [PubMed]
- Hashim, F.; Tvrdá, E.; Massányi, P.; Stawarz, R.; Lukáč, N. Effects of biological active substances to the spermatozoa quality. J. Microbial. Biotechnol. Food Sci. 2016, 5, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Mckay, D.L.; Blumberg, J.B. The Role of Tea in Human Health: An Update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, B.H.; Jeong, J.M. Antioxidant, Antimutagenic and Chemopreventive Activities of a Phyto-extract Mixture Derived from Various Vegetables, Fruits, and Oriental Herbs. Food Sci. Biotechnol. 2003, 12, 631–638. [Google Scholar]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.; Heber, D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates With the Antioxidant Capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Yeung, S.Y.; Chang, Q.; Huang, Y.; Chen, Z.Y. Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br. J. Nutr. 2004, 91, 873–881. [Google Scholar] [PubMed]
- Higdon, J.V.; Frei, B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003, 43, 89–143. [Google Scholar] [CrossRef] [PubMed]
- Erba, D.; Riso, P.; Bordoni, A.; Foti, P.; Biagi, P.L.; Testolin, G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J. Nutr. Biochem. 2005, 16, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Benjamin, J.C. Redox regulation of human sperm function: From the physiological control of sperm capacitation to the etiology of infertility and DNA damage in the germ line. Antioxid. Redox Signal. 2011, 14, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Sikka, S.C.; Rajasekaran, M.; Hellstrom, W.J. Role of oxidative stress and antioxidants in male infertility. J. Androl. 1995, 180, 464–468. [Google Scholar]
- Moazzam, A. Oxidative Stress Induced Infertility in Varicocele. Andrology 2016, 5, 2167–2250. [Google Scholar]
- Showell, M.G.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2011, 1, CD007411. [Google Scholar]
- Balogun, A.M.; Charles-Davies, M.A.; Chikezie, I.C.; Okoli, S.U. Relationship between testosterone, oxidative stress biomarkers and antioxidant levels in male auto-mechanics in Ibadan, Nigeria. Afr. J. Biomed. Res. 2016, 19, 191–197. [Google Scholar]
- Ciattei, A.P. Micronutrients and reduction of oxidative stress in spermatozoas. Int. J. Nutrol. 2016, 9, 153–159. [Google Scholar]
- Desai, N.R.; Mahfouz, R.; Sharma, R.; Gupta, S.; Agarwal, A. Reactive oxygen species levels are independent of sperm concentration, motility, and abstinence in a normal, healthy, proven fertile man: A longitudinal study. Fertil. Steril. 2010, 94, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.K.; Agarwal, A. Role of reactive oxygen species in male infertility. Urology 1996, 48, 835–850. [Google Scholar] [CrossRef]
- Bansal, A.K.; Bilaspuri, G.S. Effect of ferrous sulphate and ascorbic acid on motility, viability and lipid peroxidation of crossbred cattle bull spermatozoa. Animal 2008, 2, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 40, 183. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab. J. Urol. 2017, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.E.; Agarwal, A.; Mahfouz, R.; Plessis, S.S.D.; Schatzl, G. The association between leukocytes and sperm quality is concentration dependent. Reprod. Biol. Endocrinol. 2010, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pasqualotto, F.F.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation. Fertil. Steril. 2000, 73, 459–464. [Google Scholar] [CrossRef]
- Luderer, U. Ovarian toxicity from reactive oxygen species. In Vitamins & Hormones; Academic Press: New York, NY, USA, 2014; Volume 94, pp. 99–127. [Google Scholar]
- Guérin, P.; El, M.S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camlin, N.J.; Sobinoff, A.P.; Sutherland, J.M.; Beckett, E.L.; Jarnicki, A.G.; Vander, R.L.; Hansbro, P.M.; Mclaughlin, E.A.; Hold, J.E. Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biol. Reprod. 2016, 94, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advance sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, G.; Chen, M.; Zuo, T.; Xu, W.; Liu, X. The Role of Antioxidant Enzymes in the Ovaries. Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Qureshi, M.S.; Akhtar, S.; Ali, I. Fertility improvement in cross breed dairy cows through supplementation of vitamin E as antioxidant. Pak. J. Zool. 2016, 48, 923–930. [Google Scholar]
- Aitken, R.J.; Gordon, E.; Harkiss, D.; Twigg, J.P.; Milne, P.; Jennings, Z.; Irvine, D.S. Relative Impact of Oxidative Stress on the Functional Competence and Genomic Integrity of Human Spermatozoa. Biol. Reprod. 1998, 59, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Hashim, F.; Tvrdá, E.; Greifová, H.; Lukáč, N. Effect of vitamins on the quality of insemination doses of bulls. J. Microbial. Biotech. Food Sci. 2018, 7, 242–247. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Nashtaei, M.S.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mazjoub, A.; Agarwal, A.; Esteves, S.C. Antioxidant for elevated sperm DNA fragmentation: A mini review. Trans. Androl. Urol. 2017, 6, S649–S653. [Google Scholar]
- Askari, H.A.; Check, J.H.; Peymer, N.; Bollendorf, A. Effect of natural antioxidants tocopherol and ascorbic acids in maintenance of sperm activity during freeze-thaw process. Arch. Androl. 1994, 33, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Parinaud, J.; Le, L.D.; Vieitez, G.; Griveau, J.F.; Milhet, P.; Richoilley, G. Enhancement of motility by treating spermatozoa with an antioxidant solution (Sperm-Fit) following ejaculation. Hum. Reprod. 1997, 12, 2434–2436. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, A.; Sgrò, P.; Salacone, P.; Paoli, D.; Gilio, B.; Lombardo, F.; Santulli, M.; Agarwal, A.; Gandini, L. A placebo-controlled double-blind randomized trial of the use of combined l-carnitine and l-acetyl-carnitine treatment in men with asthenozoospermia. Fertil. Steril. 2004, 81, 1578–1584. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Maiorino, M.; Roverato, A.; Roveri, A.; Ursini, F.; Foresta, C. Oral carnitine supplementation increases sperm motility in asthenozoospermic men with normal sperm phospholipid hydroperoxide glutathione peroxidase levels. Fertil. Steril. 2005, 83, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.M.; Lu, X.; Wang, Y.W.; Sun, J.; Tao, J.W.; Yin, F.H.; Cheng, H.J. Short-term medication of L-carnitine before intracytoplasmic sperm injection for infertile men with oligoasthenozoospermia. Zhonghua Nan Ke Xue 2012, 18, 253–256. [Google Scholar] [PubMed]
- Hong, C.Y.; Lee, M.F.; Lai, L.J.; Wang, C.P. Effect of lipid peroxidation on beating frequency of human sperm tail. Andrologia 1994, 26, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.S.; Rajasekaran, M.; Hellstrom, W.J.; Sikka, S.C. Antioxidant potential of human serum albumin: Role in the recovery of high quality human spermatozoa for assisted reproductive technology. J. Androl. 1998, 19, 412–419. [Google Scholar] [PubMed]
- Lewin, A.; Lavon, H. The effect of coenzyme Q10 on sperm motility and function. Mol. Asp. Med. 1997, 18, 213–219. [Google Scholar] [CrossRef]
- Baker, H.W.G.; Brindle, J.; Irvine, D.S.; Aitken, R.J. Protective effect of antioxidants on the impairment of sperm motility by activated polymorphonuclear leukocytes. Fertil. Steril. 1996, 65, 411–419. [Google Scholar] [CrossRef]
- Thakur, A.S.; Littarru, G.P.; Funahashi, I.; Painkara, U.S.; Dange, N.S.; Chauhan, P. Effect of Ubiquinol Therapy on Sperm Parameters and Serum Testosterone Levels in Oligoasthenozoospermic Infertile Men. J. Clin. Diagn. Res. 2015, 9, BC01. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, R.; Gonzálezcomadrán, M.; Solà, I.; López, G.; Brassesco, M.; Carreras, R.; Checa, M.A. Coenzyme Q10 and male infertility: A meta-analysis. J. Assist. Reprod. Genet. 2013, 30, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Miyazaki, T.; Natori, M.; Nozawa, S. Protective role of superoxide dismutase in human sperm motility: Superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991, 6, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Negri, L.; Benaglia, R.; Monti, E.; Morenghi, E.; Pizzocaro, A.; Levi, P.S. Effect of superoxide dismutase supplementation on sperm DNA fragmentation. Arch. Ital. Urol. Androl. 2017, 89, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, C.; Iwasaki, A.; De, L.E.; Kovalski, N. Reactive oxygen species and human spermatozoa. Ann. N. Y. Acad. Sci. 1991, 637, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kovalski, N.N.; De, L.E.; Gagnon, C. Reactive oxygen species generated by human neutrophils inhibit sperm motility: Protective effect of seminal plasma and scavengers. Fertil. Steril. 1992, 58, 809–816. [Google Scholar] [CrossRef]
- Flohe, L. Selenium in mammalian spermiogenesis. Biol. Chem. 2007, 388, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, E2209. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Safarinejad, S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J. Urol. 2008, 181, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Rezaeian, Z.; Yazdekhasti, H.; Nasri, S.; Rajabi, Z.; Fallahi, P.; Amidi, F. Effects of selenium on human sperm parameters after freezing and thawing procedures. Asian Pac. J. Reprod. 2016, 5, 441–446. [Google Scholar] [CrossRef]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003, 49, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Nenkova, G.; Petrov, L.; Alexandrova, A. Role of Trace Elements for Oxidative Status and Quality of Human Sperm. Balkan Med. J. 2017, 34, 343. [Google Scholar] [CrossRef] [PubMed]
- Calogero, A.E.; Condorelli, R.A.; Russo, G.I.; Vignera, S. Conservative Nonhormonal Options for the Treatment of Male Infertility: Antibiotics, Anti-Inflammatory Drugs, and Antioxidants. Biomed. Res. Int. 2017, 2017, 4650182. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, D.; Hu, Z.; Zhao, S.; Zheng, Z.; Wei, L. Protective Effects of Green Tea Polyphenol Against Renal Injury Through ROS-Mediated JNK-MAPK Pathway in Lead Exposed Rats. Mol. Cells 2016, 39, 508–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.U.; Li, Y.; Huang, Y.; Zhu, L.; Feng, S.; Wu, J.; Wang, X. Treatment of inflammatory bowel disease via green tea polyphenols: Possible application and protective approaches. Inflammopharmacology 2018, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, K.F.; Marinovic, M.P.; Morandi, A.C.; Bolin, A.P.; Otton, R. Green tea polyphenol extract in vivo attenuates inflammatory features of neutrophils from obese rats. Eur. J. Nutr. 2016, 55, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Zhou, F.M.; Su, C.J.; Liu, T.T.; Zhou, Y.; Fan, L.; Wang, Z.H.; Liu, X.; Huang, Y.; Liu, T.; et al. Epigallocatechin-3-gallate attenuates acute and chronic psoriatic itch in mice: Involvement of antioxidant, anti-inflammatory effects and suppression of ERK and Akt signaling pathways. Biochem. Biophys. Res. Commun. 2018, 496, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hatasa, Y.; Chikazawa, M.; Furuhashi, M.; Nakashima, F.; Shibata, T.; Kondo, T.; Akagawa, M.; Hamagami, H.; Tanaka, H.; Tachibana, H.; et al. Oxidative Deamination of Serum Albumins by (−)-Epigallocatechin-3-O-Gallate: A Potential Mechanism for the Formation of Innate Antigens by Antioxidants. PLoS ONE 2016, 11, e0153002. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieiankopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01. [Google Scholar] [CrossRef] [PubMed]
- Sreejaya, P.; Nirmala, P. Evaluation of anti-oxidant effect of green tea extract in cryo preserved human semen samples. Int. J. Curr. Res. 2016, 8, 27270–27274. [Google Scholar]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and Polyphenols: Concentrations and Relation to Male Infertility and Treatment Success. Oxid. Med. Cell. Longev. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Henkel, R. The impact of oxidants on sperm function. Andrologia 2005, 37, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, D.; Parlaktas, B.; Uluocak, N.; Gencten, Y.; Erdemir, F.; Ozyurt, H.; Erkorkmaz, U.; Aslan, H. Pomegranate (Punica granatum) juice reduces oxidative injury and improves sperm concentration in a rat model of testicular torsion-detorsion. Exp. Ther. Med. 2014, 8, 478–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Chakrabarty, S.; Sinha, D.; Bhattacharya, R.K.; Siddiqi, M. Anticlastogenic, antigenotoxic and apoptotic activity of epigallocatechin gallate: A green tea polyphenol. Mutat. Res. 2003, 523–524, 33–41. [Google Scholar] [CrossRef]
- Wittayarat, M.; Ito, A.; Kimura, T.; Namula, Z.; Luu, V.V.; Do, L.T.K.; Sato, Y.; Taniguchi, M.; Otoi, T. Effects of green tea polyphenol on the quality of canine semen after long-term storage at 5 degrees C. Reprod. Biol. 2013, 13, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Bucci, D.; Spinaci, M.; Mislei, B.; Gadani, B.; Rizzato, G.; Love, C.; Tamanini, C.; Galeati, G.; Mari, G. Epigallocatechin-3-gallate (EGCG) and green tea polyphenols do not improve stallion semen parameters during cooling at 4 °C. Reprod. Domest. Anim. 2017, 52, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, M.M.; Khatoon, N.; Azmi, M.A.; Rajput, M.T.; Zaidi, S.I.; Azmi, M.A.; Perveen, R.; Naqvi, S.N.; Rashid, M. Report: Effects of Camellia sinensis L. (green tea) extract on the body and testicular weight changes in adult Wistar rate. Pak. J. Pharm. Sci. 2015, 28, 249. [Google Scholar] [PubMed]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Cocuzza, M.; Sikka, S.C.; Athayde, K.S.; Agarwal, A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: An evidence based analysis. Int. Braz. J. Urol. 2007, 33, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Morriss, A.; Khairy, M.; Khalaf, Y.; Braude, P.; Coomarasamy, A.; El-Toukhy, T. A systematic review of the effect of oral antioxidants on male infertility. Reprod. Biomed. Online 2010, 20, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Sabanegh, E.S. The Role of Over-the-Counter Supplements for the Treatment of Male Infertility—Fact or Fiction? J. Androl. 2012, 33, 292–308. [Google Scholar] [CrossRef] [PubMed]
- Amicis, F.D.; Santoro, M.; Guido, C.; Russo, A.; Aquila, S. Epigallocatechin gallate affects survival and metabolism of human sperm. Mol. Nutr. Food Res. 2012, 56, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Plaza, D.M.; Bucci, D.; Galeati, G.; Peña, F.J.; Mari, G.; Giaretta, E.; Tamanini, C.; Spinaci, M. Epigallocatechin-3-Gallate (EGCG) Reduces Rotenone Effect on Stallion Sperm-Zona Pellucida Heterologous Binding. Reprod. Domest. Anim. 2015, 50, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Gadani, B.; Bucci, D.; Spinaci, M.; Tamanini, C.; Galeati, G. Resveratrol and Epigallocatechin-3-gallate addition to thawed boar sperm improves in vitro fertilization. Theriogenology 2017, 90, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Awoniyi, D.O.; Aboua, Y.G.; Marnewick, J.; Brooks, N. The effects of rooibos (Aspalathus linearis), green tea (Camellia sinensis) and commercial rooibos and green tea supplements on epididymal sperm in oxidative stress-induced rats. Phytother. Res. 2012, 26, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, M.M.; Manfredini, V.; Brum, D.D.S.; Vargas, L.M.; Spiazzi, C.C.; Soares, M.B.; Izaguirry, A.P.; Santos, F.W. Green tea infusion improves cyclophosphamide-induced damage on male mice reproductive system. Toxicol. Rep. 2015, 2, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Mann, T.; Sherins, R.J. Adverse effects of peroxidized lipid on human spermatozoa. Proc. R. Soc. Lond. B 1978, 201, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A.; Hafez, T.; Comhaire, F. Evaluation of the role of reactive oxygen species in male infertility. Hum. Reprod. 1995, 10, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Lu, S.C.; Chen, A. Epigallocatechin-3-gallate inhibits growth of activated hepatic stellate cells by enhancing the capacity of glutathione synthesis. Mol. Pharmacol. 2008, 73, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Kitaji, H.; Ookutsu, S.; Sato, M.; Miyoshi, K. Preincubation with green tea polyphenol extract is beneficial for attenuating sperm injury caused by freezing-thawing in swine. Anim. Sci. J. 2015, 86, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; Jurisicova, A.; Sun, J.G.; Casper, R.F. Reactive oxygen species: Potential cause for DNA fragmentation in human spermatozoa. J. Urol. 1998, 160, 896–900. [Google Scholar] [CrossRef]
- Mahmood, S.; Jawd, S.M.; Jwad, S.M. The Ethanolic Extract of Green Tea Ameliorates Oxidative Stress Parameters and Female Reproductive Performance Regression Induced by Indomethacin in Pregnant Rats. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 549–563. [Google Scholar]
- Gaskins, A.J.; Chavarro, J.E. Diet and Fertility: A Review. Am. J. Obstet. Gynecol. 2018, 218, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Seidlova-Wuttke, D.; Wuttke, W. The premenstrual syndrome, premenstrual mastodynia, fibrocystic mastopathy and infertility have often common roots: Effects of extracts of chasteberry (Vitex agnus castus) as a solution. Clin. Phytosci. 2017, 3, 6. [Google Scholar] [CrossRef]
- Batool, H.R.; Maryam, N. Effects of Vitex agnus-castus extract on the secretory function of pituitary-gonadal axis and pregnancy rate in patients with premature ovarian aging (POA). J. Herb. Med. 2017, 10, 24–30. [Google Scholar]
- Di, W.; Jie, M.; Hui, G.; Kunlong, X.; Rong, X.; Ying, Z.; Xiao, L.; Ping, Y.; Hong, Y.; Liu, L. Evaluation of reproductive and developmental toxicities of Pu-erh black tea (Camellia sinensis var. assamica) extract in Sprague Dawley rats. J. Ethnopharmacol. 2013, 148, 190–198. [Google Scholar]
- Twigg, J.; Fulton, N.; Gomez, E.; Irvine, D.S.; Aitken, R.J. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: Lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum. Reprod. 1998, 13, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.; De, V.W.J.S. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.S.; Mcclain, C.J.; de Villiers, W.J. Antioxidants as novel therapy in a murine model of colitis. J. Nutr. Biochem. 2005, 16, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Ebersole, J.L. Application of prodrugs to inflammatory diseases of the gut. Molecules 2008, 13, 452–474. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.U.; Huang, Y.; Zhu, L.; Feng, S.; Khan, I.M.; Wu, J.; Li, Y.; Wang, X. Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients 2018, 10, 834. https://doi.org/10.3390/nu10070834
Rahman SU, Huang Y, Zhu L, Feng S, Khan IM, Wu J, Li Y, Wang X. Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review. Nutrients. 2018; 10(7):834. https://doi.org/10.3390/nu10070834
Chicago/Turabian StyleRahman, Sajid Ur, Yingying Huang, Lei Zhu, Shibin Feng, Ibrar Muhammad Khan, Jinjie Wu, Yu Li, and Xichun Wang. 2018. "Therapeutic Role of Green Tea Polyphenols in Improving Fertility: A Review" Nutrients 10, no. 7: 834. https://doi.org/10.3390/nu10070834