Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approvement
2.2. Study Participants
2.3. Study Design
2.4. Sample Collection
2.5. Sample Preparation and NMR Spectroscopy Analysis
2.6. Pre-Processing and Statistical Analyses
2.7. Metabolite Identification
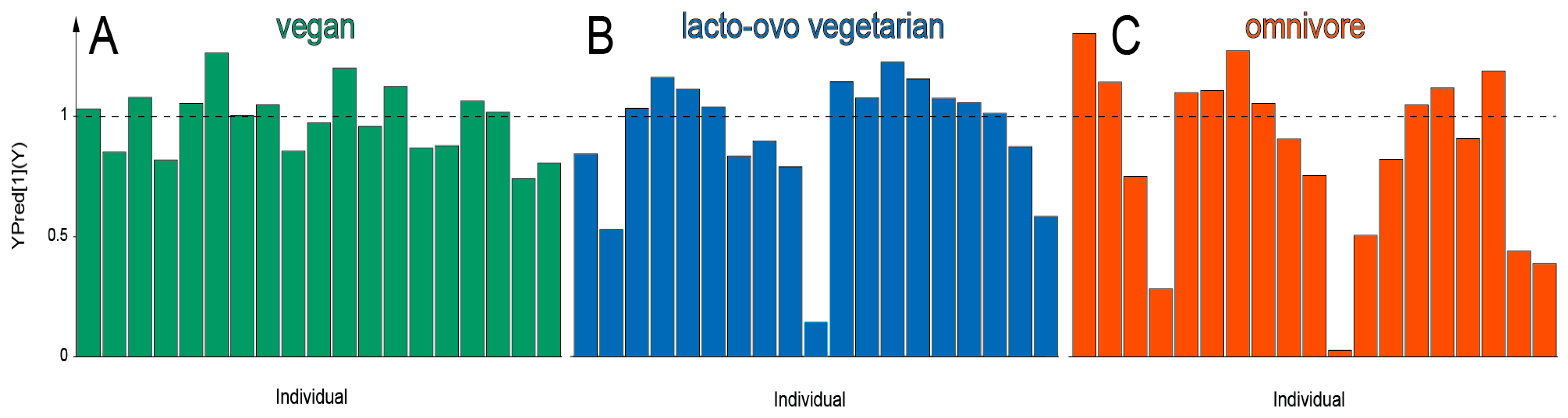
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuit, A.J.; van Loon, A.J.M.; Tijhuis, M.; Ocké, M.C. Clustering of lifestyle risk factors in a general adult population. Prev. Med. 2002, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Larsson, C.L.; Klock, K.S.; Nordrehaug Astrom, A.; Haugejorden, O.; Johansson, G. Lifestyle-related characteristics of young low-meat consumers and omnivores in sweden and norway. J. Adolesc. Health 2002, 31, 190–198. [Google Scholar] [CrossRef]
- Baines, S.; Powers, J.; Brown, W.J. How does the health and well-being of young australian vegetarian and semi-vegetarian women compare with non-vegetarians? Public Health Nutr. 2007, 10, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Panagiotakos, D.B.; Pitsavos, C.; Tsetsekou, E.; Fappa, E.; Papageorgiou, C.; Stefanadis, C. Eating habits in relations to anxiety symptoms among apparently healthy adults. A pattern analysis from the attica study. Appetite 2008, 51, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Brennan, L.; Manach, C.; Andres-Lacueva, C.; Dragsted, L.O.; Draper, J.; Rappaport, S.M.; van der Hooft, J.J.; Wishart, D.S. The food metabolome: A window over dietary exposure. Am. J. Clin. Nutr. 2014, 99, 1286–1308. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.B.; Rinnan, A.; Manach, C.; Poulsen, S.K.; Pujos-Guillot, E.; Larsen, T.M.; Astrup, A.; Dragsted, L.O. Untargeted metabolomics as a screening tool for estimating compliance to a dietary pattern. J. Proteome Res. 2014, 13, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.; Keski-Rahkonen, P.; Assi, N.; Ferrari, P.; Freisling, H.; Rinaldi, S.; Slimani, N.; Zamora-Ros, R.; Rundle, M.; Frost, G.; et al. A metabolomic study of biomarkers of meat and fish intake. Am. J. Clin. Nutr. 2017. [Google Scholar] [CrossRef] [PubMed]
- Draper, C.F.; Vassallo, I.; Di Cara, A.; Milone, C.; Comminetti, O.; Monnard, I.; Godin, J.P.; Scherer, M.; Su, M.; Jia, W.; et al. A 48-hour vegan diet challenge in healthy women and men induces a branch-chain amino acid related, health associated, metabolic signature. Mol. Nutr. Food Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Pellis, L.; van Erk, M.J.; van Ommen, B.; Bakker, G.C.; Hendriks, H.F.; Cnubben, N.H.; Kleemann, R.; van Someren, E.P.; Bobeldijk, I.; Rubingh, C.M.; et al. Plasma metabolomics and proteomics profiling after a postprandial challenge reveal subtle diet effects on human metabolic status. Metabolomcs 2012, 8, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Karimpour, M.; Surowiec, I.; Wu, J.; Gouveia-Figueira, S.; Pinto, R.; Trygg, J.; Zivkovic, A.M.; Nording, M.L. Postprandial metabolomics: A pilot mass spectrometry and nmr study of the human plasma metabolome in response to a challenge meal. Anal. Chim. Acta 2016, 908, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Fazelzadeh, P.; Hangelbroek, R.W.J.; Joris, P.J.; Schalkwijk, C.G.; Esser, D.; Afman, L.; Hankemeier, T.; Jacobs, D.M.; Mihaleva, V.V.; Kersten, S.; et al. Weight loss moderately affects the mixed meal challenge response of the plasma metabolome and transcriptome of peripheral blood mononuclear cells in abdominally obese subjects. Metabolomics 2018, 14, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministers, Nordic Council Ministers. Nordic Nutrition Recommendations, 5th ed.; Nordic Council of Ministers: Copenhagen, Denmark, 2014; Available online: https://books.google.com.hk/books?hl=zhTW&lr=&id=9_MblCPv5GcC&oi=fnd&pg=PA9&dq=Nordic+nutrition+recommendations+2012:+Integrating+nutrition+and+physical+activity%EF%BC%9B2014&ots=M7h_ndbEcZ&sig=5xbHmrVGfrkkYeerPXaw5cfcZT0&redir_esc=y#v=onepage&q&f=falseg (accessed on 15 July 2018).
- Trygg, J. O2-pls for qualitative and quantitative analysis in multivariate calibration. J. Chemom. 2002, 16, 283–293. [Google Scholar] [CrossRef]
- Jonsson, P.; Wuolikainen, A.; Thysell, E.; Chorell, E.; Stattin, P.; Wikstrom, P.; Antti, H. Constrained randomization and multivariate effect projections improve information extraction and biomarker pattern discovery in metabolomics studies involving dependent samples. Metabolomics 2015, 11, 1667–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. Hmdb 4.0: The human metabolome database for 2018. Nucl. Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. The metabolic signature associated with the western dietary pattern: A cross-sectional study. Nutr. J. 2013, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Rinaldi, S.; Scalbert, A.; Ferrari, P.; Achaintre, D.; Gunter, M.J.; Appleby, P.N.; Key, T.J.; Travis, R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the epic-oxford cohort. Eur. J. Clin. Nutr. 2016, 70, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Lombard, K.A.; Olson, A.L.; Nelson, S.E.; Rebouche, C.J. Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am. J. Clin. Nutr. 1989, 50, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Krajcovicova-Kudlackova, M.; Simoncic, R.; Bederova, A.; Babinska, K.; Beder, I. Correlation of carnitine levels to methionine and lysine intake. Physiol. Res. 2000, 49, 399–402. [Google Scholar] [PubMed]
- Demarquoy, J.; Georges, B.; Rigault, C.; Royer, M.-C.; Clairet, A.; Soty, M.; Lekounoungou, S.; Le Borgne, F. Radioisotopic determination of l-carnitine content in foods commonly eaten in western countries. Food Chem. 2004, 86, 137–142. [Google Scholar] [CrossRef]
- Cederblad, G. Effect of diet on plasma carnitine levels and urinary carnitine excretion in humans. Am. J. Clin. Nutr. 1987, 45, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Dragsted, L.O. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010, 84, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Heymsfield, S.; Lohman, T.; Wang, Z.; Going, S. Human Body Composition, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2005. [Google Scholar]
- Brosnan, J.T.; da Silva, R.P.; Brosnan, M.E. The metabolic burden of creatine synthesis. Amino Acids 2011, 40, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, F.; Ericson, U.; Almgren, P.; Nilsson, J.; Magnusson, M.; Fernandez, C.; Melander, O. Postprandial levels of branch chained and aromatic amino acids associate with fasting glycaemia. J. Amino Acids 2016, 2016, 8576730. [Google Scholar] [CrossRef] [PubMed]
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The human serum metabolome. PLOS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed]
- Zar, T.; Graeber, C.; Perazella, M.A. Recognition, treatment, and prevention of propylene glycol toxicity. Semin. Dial. 2007, 20, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography-mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 1523–1548. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.A.; Younger, J.G.; McHugh, C.; Yeomans, L.; Finkel, M.A.; Puskarich, M.A.; Jones, A.E.; Trexel, J.; Karnovsky, A. Whole blood reveals more metabolic detail of the human metabolome than serum as measured by 1 h-nmr spectroscopy: Implications for sepsis metabolomics. Shock 2015, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Nordlund, E.; Mattila, I.; Katina, K.; Aura, A.M.; Kolehmainen, M.; Oresic, M.; Mykkanen, H.; Poutanen, K. Postprandial differences in the plasma metabolome of healthy finnish subjects after intake of a sourdough fermented endosperm rye bread versus white wheat bread. Nutr. J. 2011, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Mullner, E.; Poutanen, K.; Mykkanen, H.; Moazzami, A.A. Metabolic changes in serum metabolome in response to a meal. Eur. J. Nutr. 2017, 56, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Svelander, C.; Undeland, I.; Pinto, R.; Sandberg, A.S. Herring and beef meals lead to differences in plasma 2-aminoadipic acid, beta-alanine, 4-hydroxyproline, cetoleic acid, and docosahexaenoic acid concentrations in overweight men. J. Nutr. 2015, 145, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.L.; Barr, S.I. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more ‘health conscious’. Int. J. Behav. Nutr. Phys. Act. 2005, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appleby, P.N.; Thorogood, M.; Mann, J.I.; Key, T.J. The oxford vegetarian study: An overview. Am. J. Clin. Nutr. 1999, 70, 525s–531s. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Wickenberg, J.; Fahlstrom, R.; Bjorgell, O.; Almer, L.O.; Darwiche, G. Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: A randomized blinded crossover trial. Nutr. J. 2007, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Goetze, O.; Steingoetter, A.; Menne, D.; van der Voort, I.R.; Kwiatek, M.A.; Boesiger, P.; Weishaupt, D.; Thumshirn, M.; Fried, M.; Schwizer, W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am. J. Physiol. Gastrointest Liver Physiol. 2007, 292, G11–G17. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheflin, A.M.; Melby, C.L.; Carbonero, F.; Weir, T.L. Linking dietary patterns with gut microbial composition and function. Gut Microbes 2016. [Google Scholar] [CrossRef] [PubMed]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The long-term stability of the human gut microbiota. Science 2013. [Google Scholar] [CrossRef] [PubMed]


| Model Set | Prediction Set 1 | |||
|---|---|---|---|---|
| Males (n = 11) | Females (n = 9) | Males (n = 5) | Females (n = 7) | |
| Characteristics | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Age (year) | 27.0 ± 6.6 | 25.9 ± 10.1 | 33.2 ± 13.2 | 31.9 ± 8.2 |
| Height (cm) | 184.1 ± 5.5 | 168.4 ± 4.7 | 183.0 ± 4.4 | 170.9 ± 3.8 |
| Body weight (kg) | 79.0 ± 11.0 | 62.0 ± 5.2 | 74.5 ± 4.4 | 60.0 ± 5.4 |
| BMI (kg/m2) | 23.3 ± 2.5 | 21.9 ± 1.9 | 22.2 ± 0.7 | 20.5 ± 1.6 |
| Fat mass (%) | 14.7 ± 5.4 | 23.5 ± 3.8 | 15.1 ± 3.0 | 21.8 ± 5.5 |
| Breakfast 1 | |||||
|---|---|---|---|---|---|
| Vegan | Lacto-Ovo Vegetarian | Omnivore | |||
| Food | g | Food | g | Food | g |
| Rye bred | 90 | Rye bred | 90 | Rye bred | 90 |
| Cashew nut butter | 22 | Hard cheese 28% | 24 | Liver pâté | 25 |
| Soy yoghurt blueberries | 100 | Fruit yoghurt 1.7% | 100 | Smoked ham | 30 |
| Olive oil 2 | 2 | Cottage cheese 4% | 47 | Egg | 54 |
| Lentils green (dry weight) 2 | 11 | Butter and margarine mix 75% | 12 | Butter and margarine mix 75% | 12 |
| Red bell pepper 2 | 9 | Apple | 20 | Red bell pepper | 22 |
| Green bell pepper | 25 | Tomato | 25 | Cucumber | 20 |
| Banana | 30 | Tea | 150 | Red caviar | 10 |
| Tea | 150 | Milk (1.5%) | 51 | Tea | 150 |
| Oat milk | 50 | Milk (1.5%) | 51 | ||
| Models | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VE 1 Breakfast | LOV 2 Breakfast | OM 3 Breakfast | LOV vs. VE Breakfast | LOV vs. OM Breakfast | ||||||||||
| Characteristic foods | Soy-yoghurt, cashew butter, lentils | Yoghurt, hard cheese, cottage cheese | Liver pâté, smoked ham, egg | |||||||||||
| Metabolite | Δconc. | p-value 4 | Δconc. | p-value | Δconc. | p-value | LOV Δconc. | VE Δconc. | p-value | Fold change | LOV Δconc. | OM Δconc. | p-value | Fold change |
| 3-Hydroxyisobutyrate | ↑ | 0.0001 | ↑ | 0.0004 | ↑ | <0.0001 | 1.83 | ↑ | 0.004 | 1.28 | ||||
| Acetate | ↓ | 0.0009 | ↓ | <0.0001 | ↓ | 0.0002 | ||||||||
| Acetoacetate | ↑ | <0.0001 | 1.45 | |||||||||||
| Acetone | ↓ | 0.02 | ||||||||||||
| Alanine | ↑ | 0.0004 | ↑ | 0.03 | 1.11 | |||||||||
| Glucose (alfa, beta) | ↓ | 0.03 | ↓ | 0.0006 | ||||||||||
| Arginine & Lysine 5 | ↑ | 0.003 | ↑ | <0.0001 | ↑ | 0.0002 | ||||||||
| Ascorbate | ↑ | 0.02 | 0.88 | |||||||||||
| Asparagine | ↑ | 0.02 | ||||||||||||
| Betaine | ↑ | 0.0003 | ↑ | <0.0001 | ↑ | 0.02 | 0.84 | |||||||
| Carnitine & Acetoacetate 5 | ↑ | <0.0001 | ↑ | 0.0002 | ↑ | <0.0001 | 1.24 | ↑ | 0.007 | 1.18 | ||||
| Choline | ↑ | 0.0001 | ↑ | 0.003 | 0.80 | |||||||||
| Creatinine | ↓ | 0.009 | ||||||||||||
| Creatinine & Creatine & Creatine phosphate 5 | ↓ | 0.004 | ↑ | <0.0001 | 1.12 | ↑ | 0.003 | 0.93 | ||||||
| Isoleucine | ↑ | 0.0001 | ↑ | <0.0001 | 1.21 | ↑ | 0.08 | 0.90 | ||||||
| Lactate | ↑ | 0.06 | 1.22 | |||||||||||
| Leucine | ↓ | 0.05 | ↑ | <0.0001 | 1.31 | |||||||||
| Leucine & Arginine 5 | ↓ | 0.09 | ↑ | 0.002 | ↑ | 0.002 | ↑ | <0.0001 | 1.48 | |||||
| Lipids/FFA | ↑ | <0.0001 | ↑ | 0.002 | ↑ | <0.0001 | 0.69 | ↑ | 0.003 | 0.76 | ||||
| Lysine | ↑ | 0.0002 | ↑ | <0.0001 | 1.51 | ↑ | 0.3 | 0.94 | ||||||
| Mannose | ↓ | 0.0004 | ↓ | <0.0001 | ↓ | <0.0001 | ||||||||
| Methionine | ↓ | 0.001 | ↑ | 0.0002 | ↑ | <0.0001 | 1.65 | |||||||
| myo-Inositol | ↑ | 0.0007 | ↑ | 0.0006 | ||||||||||
| N-Acetylcysteine & Proline & Glutamate 5 | ↑ | <0.0001 | ↑ | <0.0001 | 1.37 | ↑ | 0.005 | 1.37 | ||||||
| O-Phosphocholine & 3-Hydroxybutyrate 5 | ↓ | 0.005 | ||||||||||||
| Ornithine | ↑ | 0.0001 | ↑ | <0.0001 | ↑ | 0.0001 | ||||||||
| Proline | ↑ | <0.0001 | ↑ | 0.0001 | ↑ | <0.0001 | 1.41 | ↑ | 0.003 | 1.29 | ||||
| Proline & Glutamate & Unknown 5 | ↑ | 0.1 | ↑ | <0.0001 | ↑ | 0.0001 | ↑ | <0.0001 | 1.76 | ↑ | 0.003 | 1.48 | ||
| Propylene glycol | ↑ | <0.0001 | ↑ | 0.0004 | 1.42 | ↑ | 0.02 | 1.38 | ||||||
| Pyruvate | ↑ | 0.2 | 1.28 | |||||||||||
| Serine & Tyrosine 5 | ↑ | 0.001 | 0.86 | |||||||||||
| Succinic acid | ↓ | 0.0005 | ↑ | 0.2 | 1.26 | |||||||||
| Threonine | ↓ | 0.004 | ↑ | <0.0001 | 1.33 | |||||||||
| Tyrosine | ↑ | <0.0001 | ↑ | 0.0003 | ↑ | <0.0001 | 1.42 | ↑ | 0.005 | 1.13 | ||||
| Valine | ↑ | <0.0001 | 0.0001 | ↑ | <0.0001 | 1.22 | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rådjursöga, M.; Lindqvist, H.M.; Pedersen, A.; Karlsson, B.G.; Malmodin, D.; Ellegård, L.; Winkvist, A. Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients 2018, 10, 1063. https://doi.org/10.3390/nu10081063
Rådjursöga M, Lindqvist HM, Pedersen A, Karlsson BG, Malmodin D, Ellegård L, Winkvist A. Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients. 2018; 10(8):1063. https://doi.org/10.3390/nu10081063
Chicago/Turabian StyleRådjursöga, Millie, Helen M. Lindqvist, Anders Pedersen, B. Göran Karlsson, Daniel Malmodin, Lars Ellegård, and Anna Winkvist. 2018. "Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets" Nutrients 10, no. 8: 1063. https://doi.org/10.3390/nu10081063
APA StyleRådjursöga, M., Lindqvist, H. M., Pedersen, A., Karlsson, B. G., Malmodin, D., Ellegård, L., & Winkvist, A. (2018). Nutritional Metabolomics: Postprandial Response of Meals Relating to Vegan, Lacto-Ovo Vegetarian, and Omnivore Diets. Nutrients, 10(8), 1063. https://doi.org/10.3390/nu10081063





