The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Treatments
- Placebo (coloured maltodextrin Ph. Eur.)
- 475 mg Sideritis scardica extract
- 950 mg Sideritis scardica extract
- 240 mg Ginkgo biloba extract Ph. Eur. (representing an active control)
2.3. Cognitive and Mood and Blood Pressure (BP) Assessment
3. Near-Infrared Spectroscopy (NIRS) Analysis
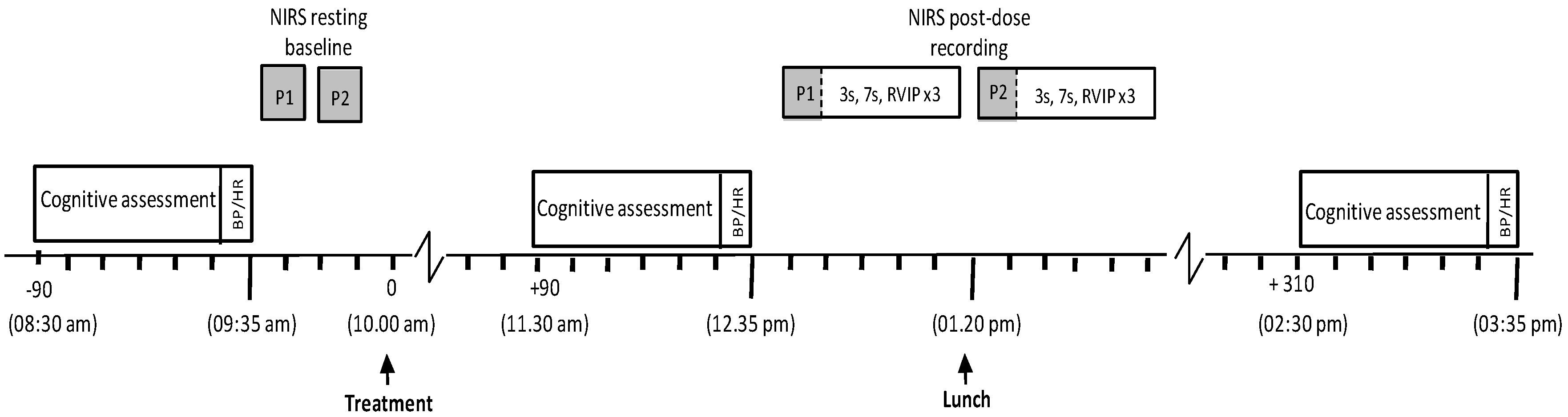
3.1. Procedure
3.2. Statistics
3.2.1. Cognitive, Mood (Bond-Lader) and Blood Pressure Assessment
3.2.2. Mood (State Trait Anxiety Inventory)
3.2.3. Near-Infrared Spectroscopy (NIRS) Assessment
4. Results
4.1. Compliance and Treatment Guess
4.2. Cognitive and Mood Assessment
4.2.1. Cognitive Performance
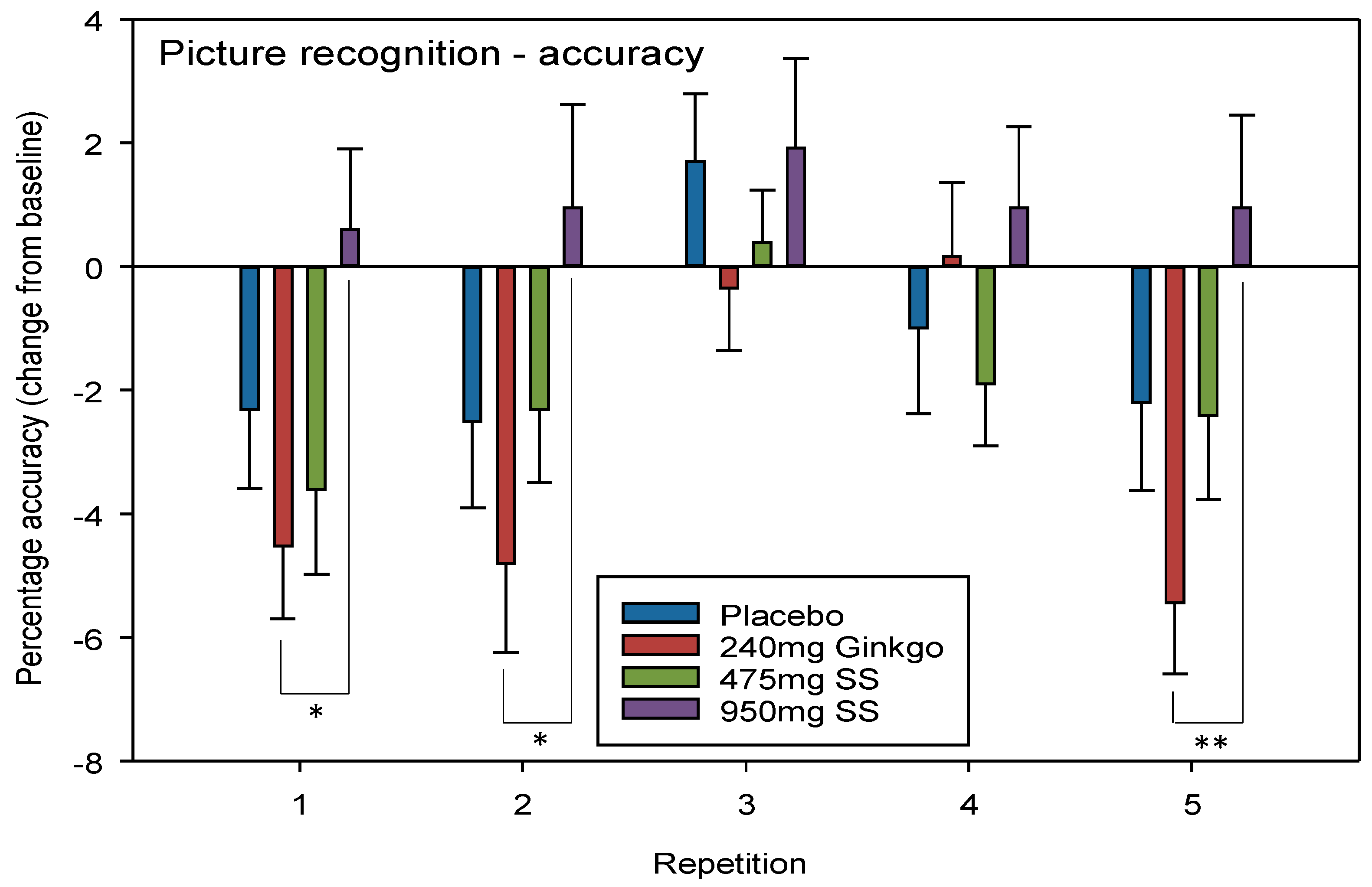
4.2.2. Picture Recognition Accuracy
4.2.3. Rapid Visual Information Processing (RVIP) False Alarms (FA)
4.2.4. Speed of Attention
4.2.5. Mood–Bond–Lader Visual Analogue Scales (VAS)
4.2.6. Mood—State-Trait Anxiety Inventory (STAI)
4.3. Blood Pressure (BP)
4.4. Near-Infrared Spectroscopy (NIRS)
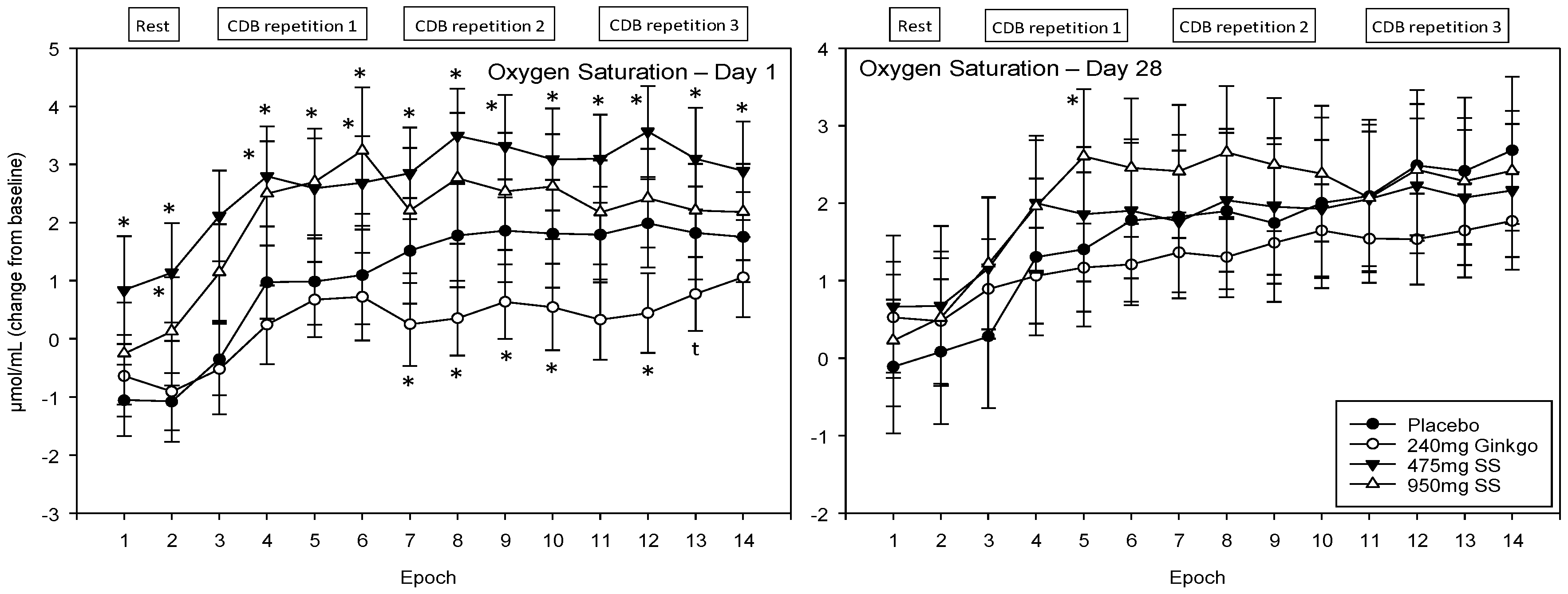
4.4.1. Oxygen Saturation (Ox%)
4.4.2. Total Haemoglobin (THb)
4.4.3. Oxygenated Haemoglobin (HbO)
4.4.4. Deoxygenated Haemoglobin (Hb)
5. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yaneva, I.; Balabanski, V. History of the uses of Pirin mountain tea (Sideritis scardica Griseb) in Bulgaria. Bulg. J. Public Health 2013, 5, 48–57. [Google Scholar]
- Todorova, M.; Trendafilova, A. Sideritis scardica Griseb, an endemic species of Balkan peninsula: Traditional uses, cultivation, chemical composition, biological activity. J. Ethnopharmacol. 2014, 152, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Heiner, F.; Feistel, B.; Wink, M. Sideritis scardica extracts inhibit aggregation and toxicity of amyloid-β in Caenorhabditis elegans used as a model for Alzheimer’s disease. PeerJ 2018, 6, e4683. [Google Scholar] [CrossRef] [PubMed]
- Knörle, R. Extracts of Sideritis scardica as triple monoamine reuptake inhibitors. J. Neural Transm. 2012, 119, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Qin, G.W.; Wang, J.; Chu, W.J.; Guo, L.H. Functional activation of monoamine transporters by luteolin and apigenin isolated from the fruit of Perilla frutescens (L.) Britt. Neurochem. Int. 2010, 56, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, I.; Schneider, I.; Schuchardt, J.P.; Bitterlich, N.; Hahn, A. Effect of an herbal extract of Sideritis scardica and B-vitamins on cognitive performance under stress: A pilot study. Int. J. Phytomed. 2016, 8, 95–103. [Google Scholar]
- Dimpfel, W.; Biller, A.; Suliman, S.; Chiegoua Dipah, G.N. Psychophysiological Effects of a Combination of Sideritis and Bacopa Extract (memoLoges®) in 32 Subjects Suffering from Mild Cognitive Impairment. A Double-Blind, Randomized, Placebo-Controlled, 2-Armed Study with Parallel Design. Adv. Alzheimer’s Dis. 2016, 5, 103–125. [Google Scholar] [CrossRef]
- Cheng, D.; Kong, H.; Pang, W.; Yang, H.; Lu, H.; Huang, C.; Jiang, Y. B vitamin supplementation improves cognitive function in the middle aged and elderly with hyperhomocysteinemia. Nutr. Neurosci. 2016, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Gregory, W.L.; Leo, M.; Kraemer, D.; Bone, K.; Oken, B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: A randomized, double-blind, placebo-controlled trial. J. Altern. Complement. Med. 2008, 14, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Silber, B.Y.; Scholey, AB.; Nolidin, K.; Goh, A.; Stough, C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 2013, 8, e82897. [Google Scholar] [CrossRef] [PubMed]
- Koh, P.-O. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab. Anim. Res. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P. The Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. In press.
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Bryan, J.; Kunz, I.; Howe, P.R. Chronic resveratrol consumption improves brachial flow-mediated dilatation in healthy obese adults. J. Hypertens. 2013, 31, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Berry, N.M.; Coates, A.M.; Buckley, J.D.; Howe, P.R. Sustained Improvement of Vasodilator Function by Resveratrol in Obese Adults. J. Hypertens. 2012, 30, e70. [Google Scholar] [CrossRef]
- Wong, R.H.; Howe, P.R.; Buckley, J.D.; Coates, A.M.; Kunz, I.; Berry, N.M. Acute resveratrol supplementation improves flow-mediated dilatation in overweight/obese individuals with mildly elevated blood pressure. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Sorond, F.A.; Lipsitz, L.A.; Hollenberg, N.K.; Fisher, N.D. Cerebral blood flow response to flavanol-rich cocoa in healthy elderly humans. Neuropsychiatr. Dis. Treat. 2008, 4, 433–440. [Google Scholar] [PubMed]
- Wightman, E.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in humans: A randomised, double-blind, placebo-controlled, crossover investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vicente, A.; Cabral, P.D.; Garvin, J.L. Resveratrol increases nitric oxide production in the rat thick ascending limb via Ca2+/calmodulin. PLoS ONE 2014, 9, e110487. [Google Scholar] [CrossRef] [PubMed]
- Taubert, D.; Roesen, R.; Lehmann, C.; Jung, N.; Schömig, E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: A randomized controlled trial. JAMA 2007, 298, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.B.; French, S.J.; Morris, P.J.; Kennedy, D.O.; Milne, A.L.; Haskell, C.F. Consumption of cocoa flavanols results in acute improvements in mood and cognitive performance during sustained mental effort. J. Psychopharmacol. 2010, 24, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance through Cocoa Flavanol Consumption in Elderly Subjects with Mild Cognitive Impairment: The Cocoa, Cognition, and Aging (CoCoA) Study. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Jackson, P.A.; Haskell, C.F.; Scholey, A.B. Modulation of cognitive performance following single doses of 120 mg Ginkgo biloba extract administered to healthy young volunteers. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Harris, A.; Kristinsson, J.K.; Ciulla, T.A.; Kagemann, C.; Ritch, R. Ginkgo biloba extract increases ocular blood flow velocity. J. Ocul. Pharmacol. Ther. 1999, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Mashayekh, A.; Pham, D.L.; Yousem, D.M.; Dizon, M.; Barker, P.B.; Lin, D.D. Effects of Ginkgo biloba on cerebral blood flow assessed by quantitative MR perfusion imaging: A pilot study. Neuroradiology 2011, 53, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Forster, J.; Khan, J.; Haskell-Ramsay, C.F.; Jackson, P.A. Cognitive and Mood Effects of a Nutrient Enriched Breakfast Bar in Healthy Adults: A Randomised, Double-Blind, Placebo-Controlled, Parallel Groups Study. Nutrients 2017, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.; Lader, M. The use of analogue scales in rating subjective feelings. Psychol. Psychother. Theory Res. Pract. 1974, 47, 211–218. [Google Scholar] [CrossRef]
- Speilberger, C.D.; Gorsuch, R.L.; Lushene, R.E. The State Trait Anxiety Inventory Manual; Consulting Psychologists Press: Palo Alto, CA, USA, 1969. [Google Scholar]
- Villringer, A.; Planck, J.; Hock, C.; Schleinkofer, L.; Dimagl, U. Near infrared spectroscopy (NIRS): A new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci. Lett. 1993, 154, 101–104. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.; Stuart, R.C.; Okello, E.J.; Watson, A.W. Cognitive and mood improvements following acute supplementation with purple grape juice in healthy young adults. Eur. J. Nutr. 2017, 56, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.W.; Kennedy, D.O.; Haskell, C.F.; Scheepens, A. A double blind placebo controlled study measuring the effect of two berry fruit extracts on mood, cognition and monoamine oxidase B inhibition in healthy young adults. Appetite 2012, 59, 636. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.; Head, K.; Morris, P.G.; Macdonald, I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006, 47, S215–S220. [Google Scholar] [CrossRef] [PubMed]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Scholey, A.B.; Wesnes, K.A. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology 2000, 151, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, J.; Beck, T.; Seibert, A. Influence of an extract of Ginkgo biloba on cerebral blood flow and metabolism. Life Sci. 1986, 39, 2327–2334. [Google Scholar] [CrossRef]
- Kleijnen, J.; Knipschild, P. Ginkgo biloba for cerebral insufficiency. Br. J. Clin. Pharmacol. 1992, 34, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Ruge, H.; Schindler, C.; Burkart, M.; Miller, R.; Kirschbaum, C.; Goschke, T. Effects of Ginkgo biloba extract EGb 761® on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment—A randomized double-blind placebo-controlled trial. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B.; Wesnes, K.A. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol. Behav. 2002, 75, 739–751. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Haskell, C.F.; Mauri, P.L.; Scholey, A.B. Acute cognitive effects of standardised Ginkgo biloba extract complexed with phosphatidylserine. Hum. Psychopharmacol. Clin. Exp. 2007, 22, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Jackson, P.A.; Haskell, C.F.; Scholey, A.B. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef] [PubMed]








| Measure | ANOVAs | Post-Hoc Comparisons | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Pure Chronic ANOVA | 2. Acute Effects within Day 1 and Day 28 ANOVA | ||||||||
| Effect | F | P | Effect | F | P | Treatment Comparisons | P | Effect Size | |
| Picture Recognition Accuracy (% correct) | D | 2.60 | 0.11 | R | 11.66 | <0.001 * | G V 950 mg Rep 1 G V 950 mg Rep 2 G V 950 mg Rep 5 | 0.02 0.03 0.01 | 0.68 0.61 0.79 |
| D*T | 0.94 | 0.42 | R*T | 1.84 | 0.04 * | ||||
| Rapid Visual Information Processing False Alarms (number) | D | 2.41 | 0.12 | R | 3.01 | 0.03 * | P V 950 mg Rep 4 | 0.02 | 0.66 |
| D*T | 2.16 | 0.10 | R*T | 2.08 | 0.03 * | ||||
| Speed of Attention (milliseconds) | D | 31.10 | <0.001 * | R | 2.47 | 0.05 t | G V 475 mg Rep 1 G V 950 mg Rep 1 G V 475 mg Rep 2 G V 950 mg Rep 2 G V 475 mg Rep 5 G V 950 mg Rep 5 | 0.01 0.05 t 0.09 t 0.02 0.02 0.01 | 0.66 0.54 0.51 0.63 0.59 0.74 |
| D*T | 0.51 | 0.68 | R*T | 1.65 | 0.09 t | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients 2018, 10, 955. https://doi.org/10.3390/nu10080955
Wightman EL, Jackson PA, Khan J, Forster J, Heiner F, Feistel B, Suarez CG, Pischel I, Kennedy DO. The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients. 2018; 10(8):955. https://doi.org/10.3390/nu10080955
Chicago/Turabian StyleWightman, Emma L., Philippa A. Jackson, Julie Khan, Joanne Forster, Felix Heiner, Bjoern Feistel, Cynthia G. Suarez, Ivo Pischel, and David O. Kennedy. 2018. "The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans" Nutrients 10, no. 8: 955. https://doi.org/10.3390/nu10080955
APA StyleWightman, E. L., Jackson, P. A., Khan, J., Forster, J., Heiner, F., Feistel, B., Suarez, C. G., Pischel, I., & Kennedy, D. O. (2018). The Acute and Chronic Cognitive and Cerebral Blood Flow Effects of a Sideritis scardica (Greek Mountain Tea) Extract: A Double Blind, Randomized, Placebo Controlled, Parallel Groups Study in Healthy Humans. Nutrients, 10(8), 955. https://doi.org/10.3390/nu10080955





