Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity
Abstract
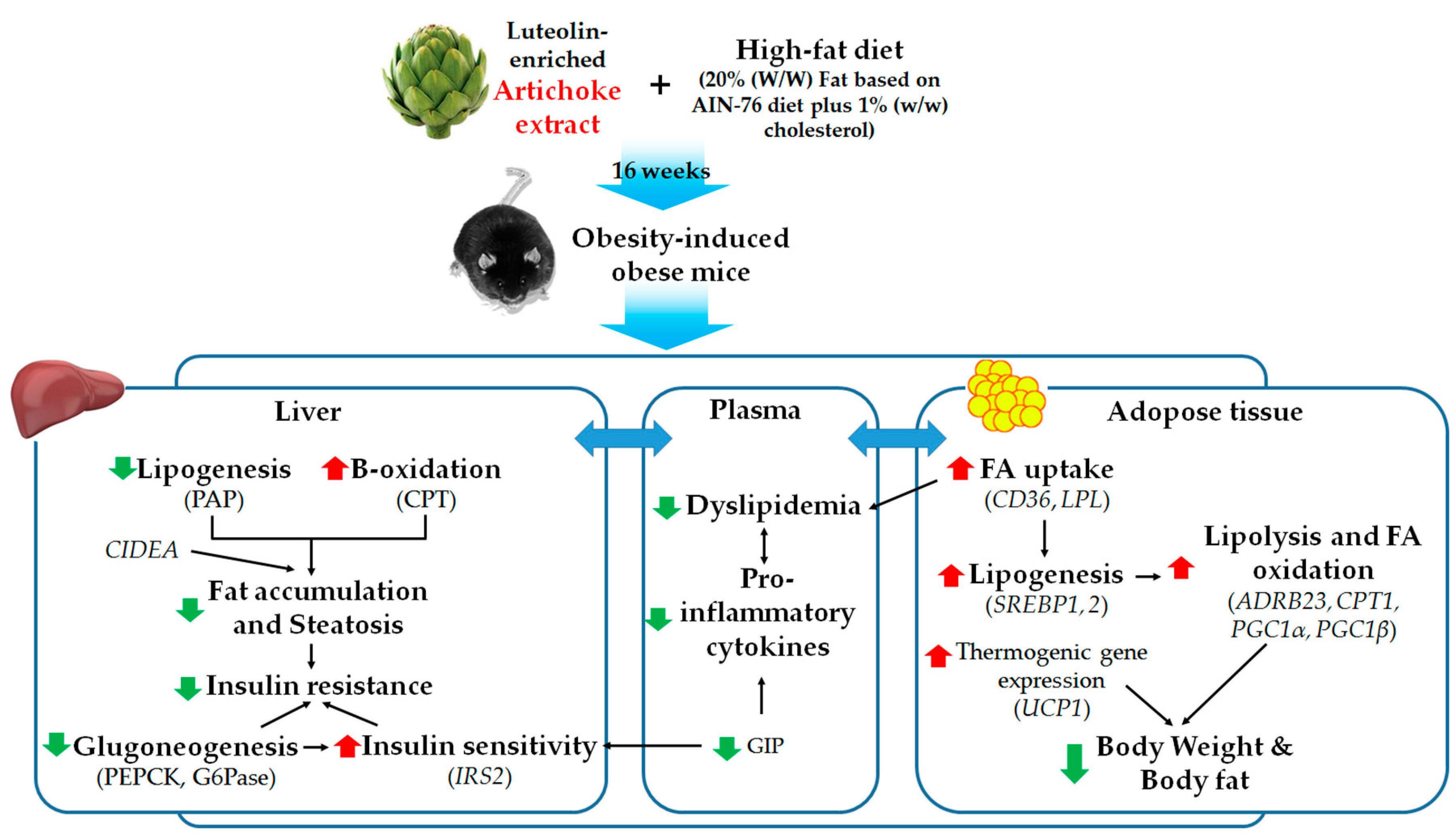
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Diets
2.2. Morphology of the Liver and Fat Tissues
2.3. Plasma Biomarkers
2.4. Fasting Blood Glucose, Intraperitoneal Glucose Tolerance Test, and Homeostatic Index of Insulin Resistance
2.5. Hepatic and Fecal Lipid Contents
2.6. Preparation of Hepatic Subcellular Fractions
2.7. Lipid-Regulating and Glucose-Regulating Enzymatic Activities
2.8. Analysis of Gene Expression
2.9. Statistical Analysis
3. Results
3.1. Supplementation with AR and LU Lowered the Body Weight Gain and Adipose Tissue Weight by Regulating Lipid Metabolism-Related Adipocyte Gene Expression in Mice with DIO
3.2. Supplementation with AR and LU Improved the Plasma Lipid Levels in Mice with DIO
3.3. Supplementation with AR and LU Lowered the Hepatic Lipid Levels by Modulating Hepatic Lipid-Regulating Enzyme Activities and Gene Expression and Increasing Fecal Lipid Levels in Mice with DIO
3.4. Supplementation with AR and LU Lowered Insulin Resistance and Glucose Tolerance by Modulating Hepatic Glucose-Regulating Enzymes in Mice with DIO
4. Discussions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG | ATP-binding cassette subfamily G member |
| ADRB3 | adrenoreceptor beta 3 |
| AI | atherogenic index |
| Apo | apolipoprotein |
| AR | artichoke |
| BW | body weight |
| CD36 | CD antigen 36 |
| CIDEA | cell death-inducing DFFA-like effector A |
| COX8B | cytochrome c oxidase subunit 8B |
| CPT | carnitine palmitoyltransferase |
| DIO | diet-induced obesity |
| FFA | free fatty acid |
| G6Pase | glucokinase, glucose-6-phosphatase |
| GIP | gastric inhibitory polypeptide |
| HFD | high-fat diet |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| IL | interleukin |
| IPGTT | intraperitoneal glucose tolerance test |
| IRS2 | insulin receptor substrate 2 |
| LPL | lipoprotein lipase |
| LU | luteolin |
| ME | malic enzyme |
| ND | normal diet |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAP | phosphatidate phosphohydrolase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| PGC1 | PPAR-gamma coactivator 1 |
| PPAR | peroxisome proliferator-activated receptor |
| SREBP | sterol regulatory element-binding protein |
| UCP1 | uncoupling protein 1 |
| WAT | white adipose tissue |
References
- Visscher, T.L.; Seidell, J.C. The public health impact of obesity. Annu. Rev. Public Health 2001, 22, 355–375. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizaion. Fact Sheet: Obesity and Overweight. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 February 2018).
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. Obesity and Dyslipidemia; MDText.com Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: a functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jauregui, O.; Lamuela-Raventós, R.M.; Bastida, J.; Viladomat, F.; Codina, C. Identification of phenolic compounds in artichoke waste by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2003, 1008, 57–72. [Google Scholar] [CrossRef]
- Negro, D.; Montesano, V.; Grieco, S.; Crupi, P.; Sarli, G.; De Lisi, A.; Sonnante, G. Polyphenol compounds in artichoke plant tissues and varieties. J. Food Sci. 2012, 77, C244–C252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Jung, U.J.; Park, T.; Yun, J.W.; Choi, M.S. Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in diet-induced obese mice. Diabetes 2015, 64, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Han, Y.J.; Zhang, X.; Wang, X.; Bao, B.; Qu, W.; Liu, J. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia 2016, 59, 2219–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Zhang, L.; Dong, J.; Zhang, X.; Chen, Y.G.; Bao, B.; Liu, J. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol. Nutr. Food Res. 2014, 58, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Davidson, A.L.; Arion, W.J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch. Biochem. Biophys. 1987, 253, 156–167. [Google Scholar] [CrossRef]
- Ochoa, S.; Mehler, A.H.; Kornberg, A. Biosynthesis of dicarboxylic acids by carbon dioxide fixation; isolation and properties of an enzyme from pigeon liver catalyzing the reversible oxidative decarboxylation of 1-malic acid. J. Biol. Chem. 1948, 174, 979–1000. [Google Scholar] [PubMed]
- Walton, P.A.; Possmayer, F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: Development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal. Biochem. 1985, 151, 479–486. [Google Scholar] [CrossRef]
- Markwell, M.A.; McGroarty, E.J.; Bieber, L.L.; Tolbert, N.E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J. Biol. Chem. 1973, 248, 3426–3432. [Google Scholar] [PubMed]
- Bentle, L.A.; Lardy, H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976, 251, 2916–2921. [Google Scholar] [PubMed]
- Alegre, M.; Ciudad, C.J.; Fillat, C.; Guinovart, J.J. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 1988, 173, 185–189. [Google Scholar] [CrossRef]
- Magielse, J.; Verlaet, A.; Breynaert, A.; Keenoy, B.M.Y.; Apers, S.; Pieters, L.; Hermans, N. Investigation of the in vivo antioxidative activity of Cynara scolymus (artichoke) leaf extract in the streptozotocin-induced diabetic rat. Mol. Nutr. Food Res. 2014, 58, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Küskü-Kiraz, Z.; Mehmetçik, G.; Doǧru-Abbasoǧlu, S.; Uysal, M. Artichoke leaf extract reduces oxidative stress and lipoprotein dyshomeostasis in rats fed on high cholesterol diet. Phytother. Res. 2010, 24, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Z.; Lee, S.O.; Ye, Z.; Wu, X.; Hendrich, S. Artichoke extract lowered plasma cholesterol and increased fecal bile acids in Golden Syrian hamsters. Phytother. Res. 2012, 26, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Morash, A.J.; Bureau, D.P.; McClelland, G.B. Effects of dietary fatty acid composition on the regulation of carnitine palmitoyltransferase (CPT) I in rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 152, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Esser, V.; Britton, C.H.; Weis, B.C.; Foster, D.W.; McGarry, J.D. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J. Biol. Chem. 1993, 268, 5817–5822. [Google Scholar] [PubMed]
- Masuo, K. Roles of beta2-and beta3-adrenoceptor polymorphisms in hypertension and metabolic syndrome. Int. J. Hypertens. 2010, 2010, 832821. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.R.; Gennemark, P.; O’Mahony, G.; Bartesaghi, S. Unlock the thermogenic potential of adipose tissue: Pharmacological modulation and implications for treatment of diabetes and obesity. Front. Endocrinol. 2015, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Townsend, K.L.; Tseng, Y.H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 2014, 25, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samuel, V.T.; Shulman, G.I. Mechanisms for insulin resistance: Common threads and missing links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef] [PubMed]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Maples, J.M.; Brault, J.J.; Witczak, C.A.; Park, S.; Hubal, M.J.; Weber, T.M.; Houmard, J.A.; Shewchuk, B.M. Differential epigenetic and transcriptional response of the skeletal muscle carnitine palmitoyltransferase 1B (CPT1B) gene to lipid exposure with obesity. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E345–E356. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, L.; Ye, J.; Li, D.; Wang, W.; Li, X.; Wu, L.; Wang, H.; Guan, F.; Li, P. Cidea promotes hepatic steatosis by sensing dietary fatty acids. Hepatolog 2012, 56, 95–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withers, D.J.; Gutierrez, J.S.; Towery, H.; Burks, D.J.; Ren, J.M.; Previs, S.; Zhang, Y.; Bernal, D.; Pons, S.; Shulman, G.I.; Bonner-Weir, S.; White, M.F. Disruption of IRS-2 causes type 2 diabetes in mice. Nature 1998, 391, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Singh, N.; Akanksha; Jayendra; Maurya, R.; Srivastava, A.K. Coagulanolide modulates hepatic glucose metabolism in C57BL/KsJ-db/db mice. Hum. Exp. Toxicol. 2012, 31, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Ueki, K.; Kahn, R. Complementary roles of IRS-1 and IRS-2 in the hepatic regulation of metabolism. J. Clin. Investig. 2005, 115, 718–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kammoun, H.L.; Kraakman, M.J.; Febbraio, M.A. Adipose tissue inflammation in glucose metabolism. Rev. Endocr. Metab. Disord. 2014, 15, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Negrin, K.A.; Flach, R.J.R.; DiStefano, M.T.; Matevossian, A.; Friedline, R.H.; Jung, D.; Kim, J.K.; Czech, M.P. IL-1 signaling in obesity-induced hepatic lipogenesis and steatosis. PLoS ONE 2014, 9, e107265. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Fuller, G.M.; Fuentes, N.L.; Moser, A.H.; Staprans, I.; Grunfeld, C.; Feingold, K.R. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 1995, 136, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Van Nhieu, J.T.; Jardel, C.; Bruckert, E.; Grimaldi, A.; Robert, J.J.; Capeau, J.; Hainque, B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J. Clin. Endocrinol. Metab. 2002, 87, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Maedler, K.; Dharmadhikari, G.; Schumann, D.M.; Størling, J. Interleukin-1 beta targeted therapy for type 2 diabetes. Expert Opin. Biol. Ther. 2009, 9, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- De Taeye, B.; Smith, L.H.; Vaughan, D.E. Plasminogen activator inhibitor-1: A common denominator in obesity, diabetes and cardiovascular disease. Curr. Opin. Pharmacol. 2005, 5, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Nasteska, D.; Harada, N.; Suzuki, K.; Yamane, S.; Hamasaki, A.; Joo, E.; Iwasaki, K.; Shibue, K.; Harada, T.; Inagaki, N. Chronic reduction of GIP secretion alleviates obesity and insulin resistance under high-fat diet conditions. Diabetes 2014, 63, 2332–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, V.; Vasu, S.; Flatt, P.R.; Irwin, N. Effects of chronic exposure of clonal β-cells to elevated glucose and free fatty acids on incretin receptor gene expression and secretory responses to GIP and GLP-1. Diabetes Obes. Metab. 2014, 16, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Okahara, F.; Osaki, N.; Shimotoyodome, A. Increased GIP signaling induces adipose inflammation via a HIF-1α-dependent pathway and impairs insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E414–E425. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Direction | Primer Sequence |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | Forward | 5′-CAAGTTCAACGGCACAGTCAAGG-3′ |
| Reverse | 5′-ACATACTCAGCACCAGCATCACC-3′ | |
| ATP-binding cassette subfamily G member 5 (ABCG5) | Forward | 5′-TCTTCCCTGAGCCTAGGGGG-3′ |
| Reverse | 5′-CGATTAGCTCTTCCACCCGT-3′ | |
| ABCG8 | Forward | 5′-TTCACGCTCATAGTCGCTGGATAG-3′ |
| Reverse | 5′-TGGTTCAATTCTCTTGGACACATCTTC-3′ | |
| Cell death-inducing DFFA-like effector A (CIDEA) | Forward | 5′-TTTCAAACCATGACCGAAGTAGC-3′ |
| Reverse | 5′-CCTCCAGCACCAGCGTAACC-3′ | |
| Peroxisome proliferator-activated receptor alpha (PPARα) | Forward | 5′-CCTGAACATCGAGTGTCGAATAT-3′ |
| Reverse | 5′-GGTCTTCTTCTGAATCTTGCAGCT-3′ | |
| PPAR-gamma coactivator 1alpha (PGC1α) | Forward | 5′-AAGTGTGGAACTCTCTGGAACTG-3′ |
| Reverse | 5′-GGGTTATCTTGGTTGGCTTTATG-3′ | |
| PGC1β | Forward | 5′-GGTCCCTGGCTGACATTCAC-3′ |
| Reverse | 5′-GGCACATCGAGGGCAGAG-3′ | |
| Sterol regulatory element-binding transcription factor 1a (SREBP1a) | Forward | 5′-TAGTCCGAAGCCGGGTGGGCGCCGGCGCCAT-3′ |
| Reverse | 5′-GATGTCGTTCAAAACCGCTGTGTGTCCAGTTC-3′ | |
| SREBP2 | Forward | 5′-CACAATATCATTGAAAAGCGCTACCGGTCC-3′ |
| Reverse | 5′-TTTTTCTGATTGGCCAGCTTCAGCACCATG-3′ | |
| Acetyl-CoA carboxylase (ACC) | Forward | 5′-GCCTCTTCCTGACAAACGAG-3′ |
| Reverse | 5′-TGACTGCCGAAACATCTCTG-3′ | |
| Fatty acid synthase (FAS) | Forward | 5′-GCTGCGGAAACTTCAGGAAAT-3′ |
| Reverse | 5′-AGAGACGTGTCACTCCTGGACTT-3′ | |
| Lipoprotein lipase (LPL) | Forward | 5′-GACTCGCTCTCAGATGCCCTAC-3′ |
| Reverse | 5′-GCCTGGTTGTGTTGCTTGCC-3′ | |
| CD antigen 36 (CD36) | Forward | 5′-TGGTGGATGGTTTCCTAGCCTTTC-3′ |
| Reverse | 5′-TCGCCAACTCCCAGGTACAATC-3′ | |
| Adrenoreceptor beta 3 (ADRB3) | Forward | 5′-ACCAACGTGTTCGTGACT-3′ |
| Reverse | 5′-ACAGCTAGGTAGCGGTCC-3′ | |
| CPT1 | Forward | 5′-ATCTGGATGGCTATGGTCAAGGTC-3′ |
| Reverse | 5′-GTGCTGTCATGCGTTGGAAGTC-3′ | |
| CPT2 | Forward | 5′-GCCTGCTGTTGCGTGACTG-3′ |
| Reverse | 5′-TGGTGGGTACGATGCTGTGC-3′ | |
| Cytochrome c oxidase subunit 8B (COX8B) | Forward | 5′-TGTGGGGATCTCAGCCATAGT-3′ |
| Reverse | 5′-AGTGGGCTAAGACCCATCCTG-3′ | |
| Uncoupling protein 1 (UCP1) | Forward | 5′-AGATCTTCTCAGCCGGAGTTT-3′ |
| Reverse | 5′-CTGTACAGTTTCGGCAATCCT-3′ |
| ND | HFD | AR | LU | |
|---|---|---|---|---|
| FFA (mmol/L) | 1.12 ± 0.01 | 1.16 ± 0.02 c | 0.98 ± 0.02 a | 1.07 ± 0.03 b |
| TG (mmol/L) | 0.94 ± 0.06 ** | 1.20 ± 0.05 b | 1.00 ± 0.04 a | 1.13 ± 0.02 ab |
| TC (mmol/L) | 6.28 ± 0.22 *** | 8.70 ± 0.32 b | 6.90 ± 0.44 a | 8.07 ± 0.58 ab |
| HDL-C (mmol/L) | 1.64 ± 0.06 ** | 1.98 ± 0.09 | 1.81 ± 0.16 | 1.99 ± 0.11 |
| Non HDL-C (mmol/L) | 4.64 ± 0.17 *** | 6.72 ± 0.29 b | 5.09 ± 0.30 a | 6.08 ± 0.51 ab |
| HTR | 26.20 ± 0.55 ** | 22.87 ± 0.93 | 26.08 ± 0.97 | 24.91 ± 1.39 |
| AI | 2.83 ± 0.08 * | 3.41 ± 0.19 | 2.86 ± 0.14 | 3.06 ± 0.22 |
| ApoA-I (mg/dL) | 18.64 ± 0.22 * | 17.74 ± 0.20 ab | 18.20 ± 0.16 b | 17.47 ± 0.29 a |
| ApoB100 (mg/dL) | 7.50 ± 0.55 | 10.12 ± 1.30 | 7.51 ± 0.97 | 8.15 ± 1.27 |
| ApoA-I/ApoB100 | 2.57 ± 0.20 | 1.90 ± 0.20 | 2.76 ± 0.48 | 2.30 ± 0.34 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, E.-Y.; Kim, S.Y.; Choi, M.-S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients 2018, 10, 979. https://doi.org/10.3390/nu10080979
Kwon E-Y, Kim SY, Choi M-S. Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients. 2018; 10(8):979. https://doi.org/10.3390/nu10080979
Chicago/Turabian StyleKwon, Eun-Young, So Young Kim, and Myung-Sook Choi. 2018. "Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity" Nutrients 10, no. 8: 979. https://doi.org/10.3390/nu10080979
APA StyleKwon, E.-Y., Kim, S. Y., & Choi, M.-S. (2018). Luteolin-Enriched Artichoke Leaf Extract Alleviates the Metabolic Syndrome in Mice with High-Fat Diet-Induced Obesity. Nutrients, 10(8), 979. https://doi.org/10.3390/nu10080979





